Optical Method and Biochemical Source for the Assessment of the Middle-Molecule Uremic Toxin β2-Microglobulin in Spent Dialysate
Abstract
1. Introduction
2. Results
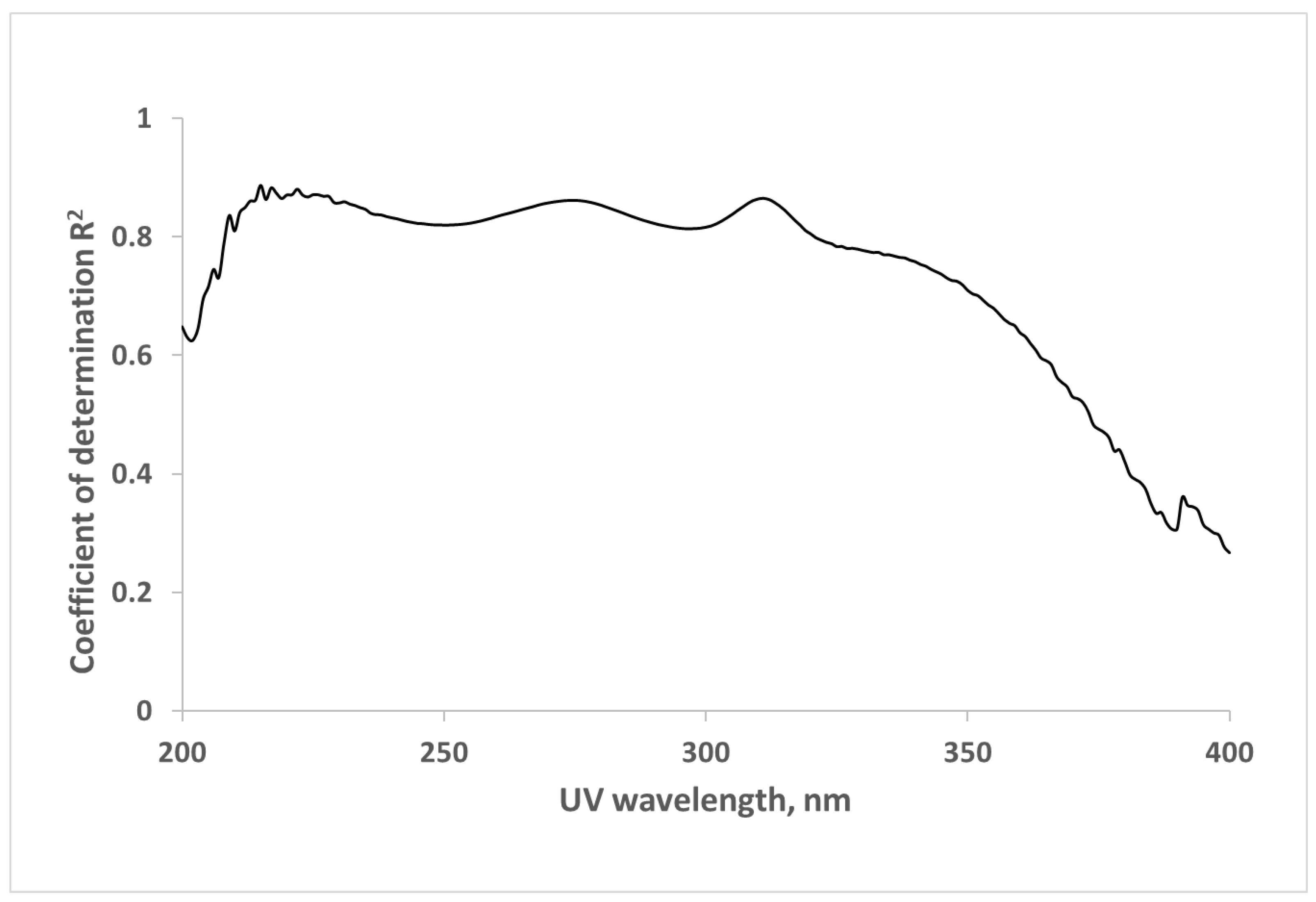
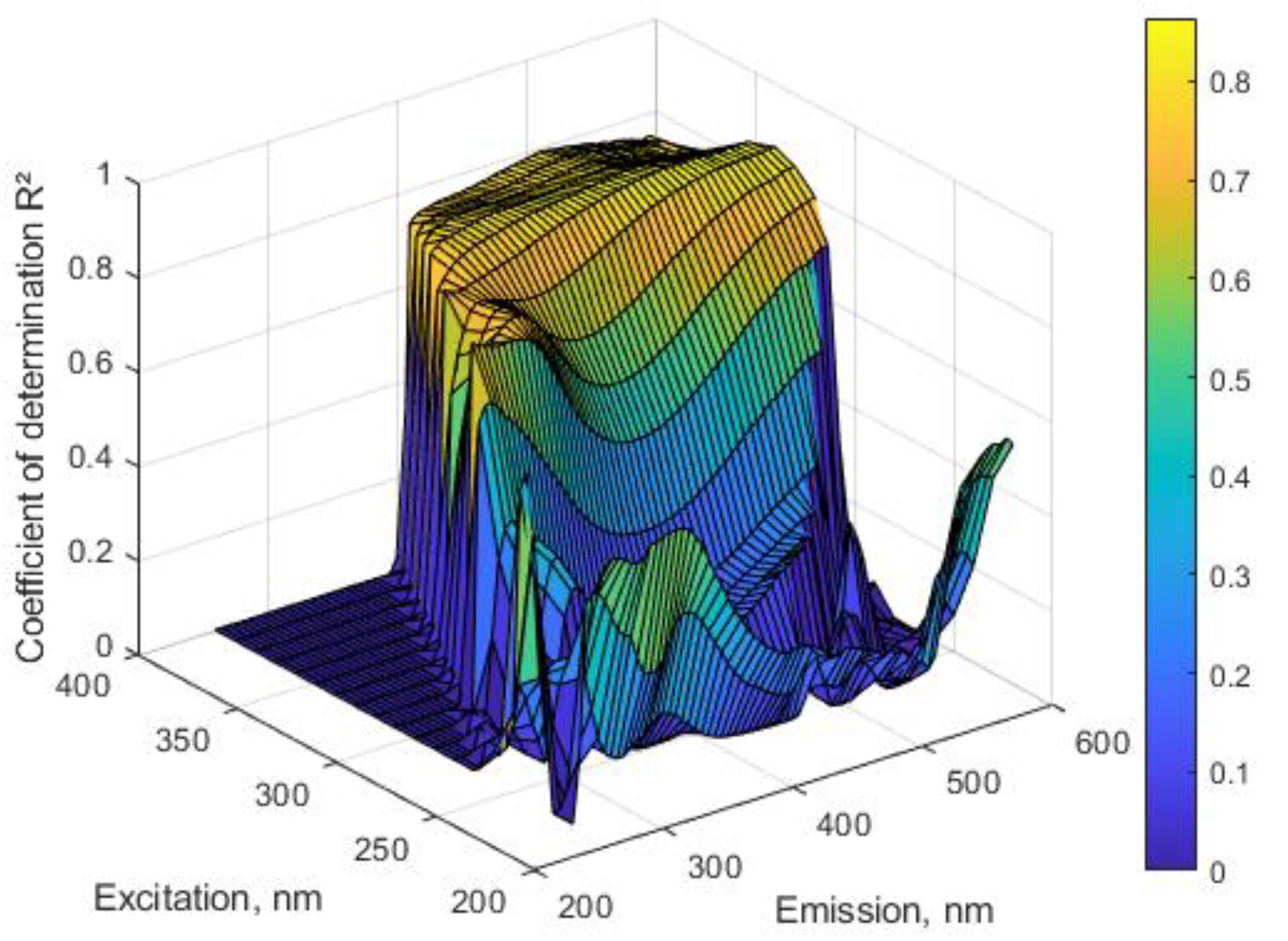
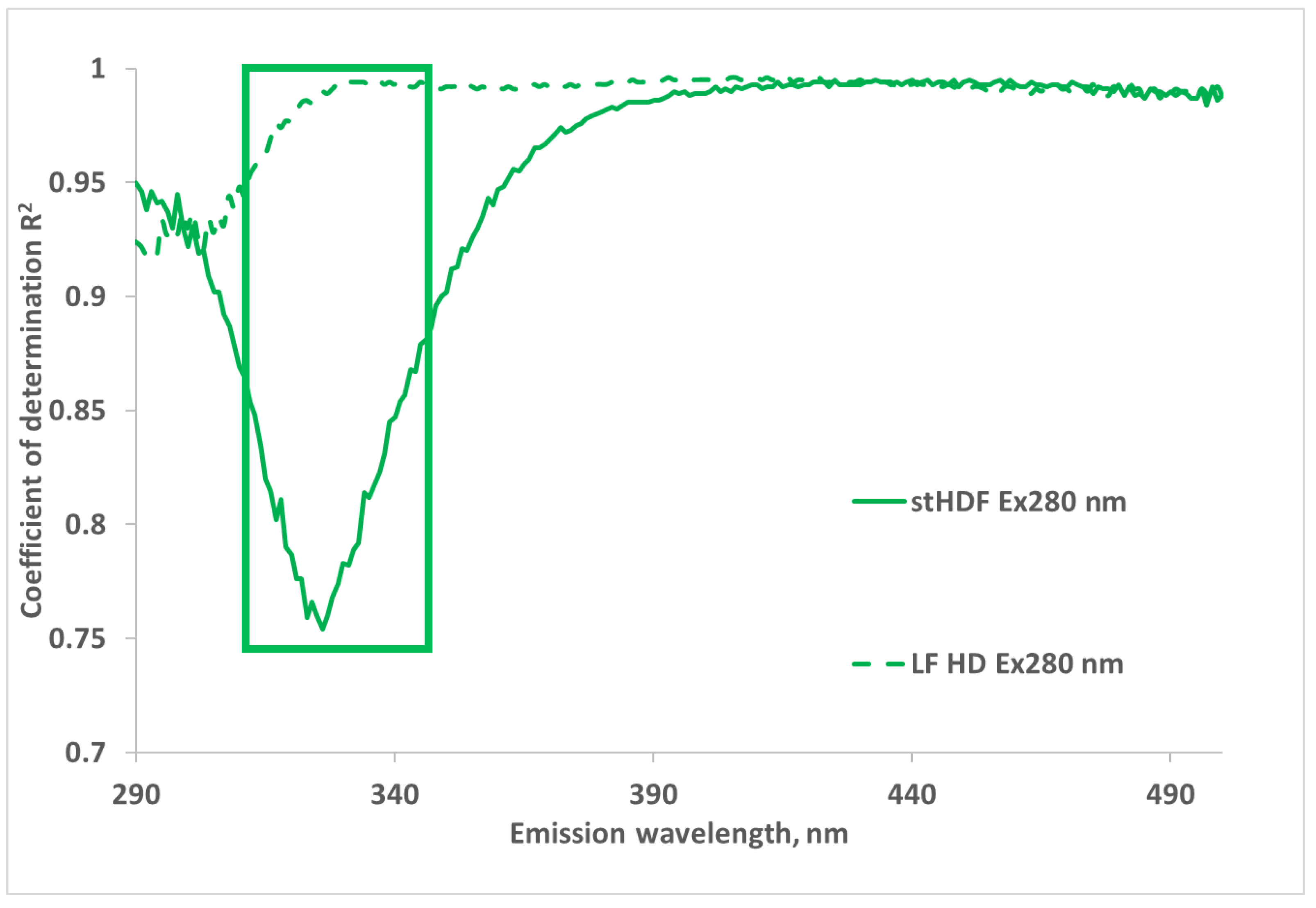
2.1. Correlations between Optical Data and Concentration of β2M in Dialysate
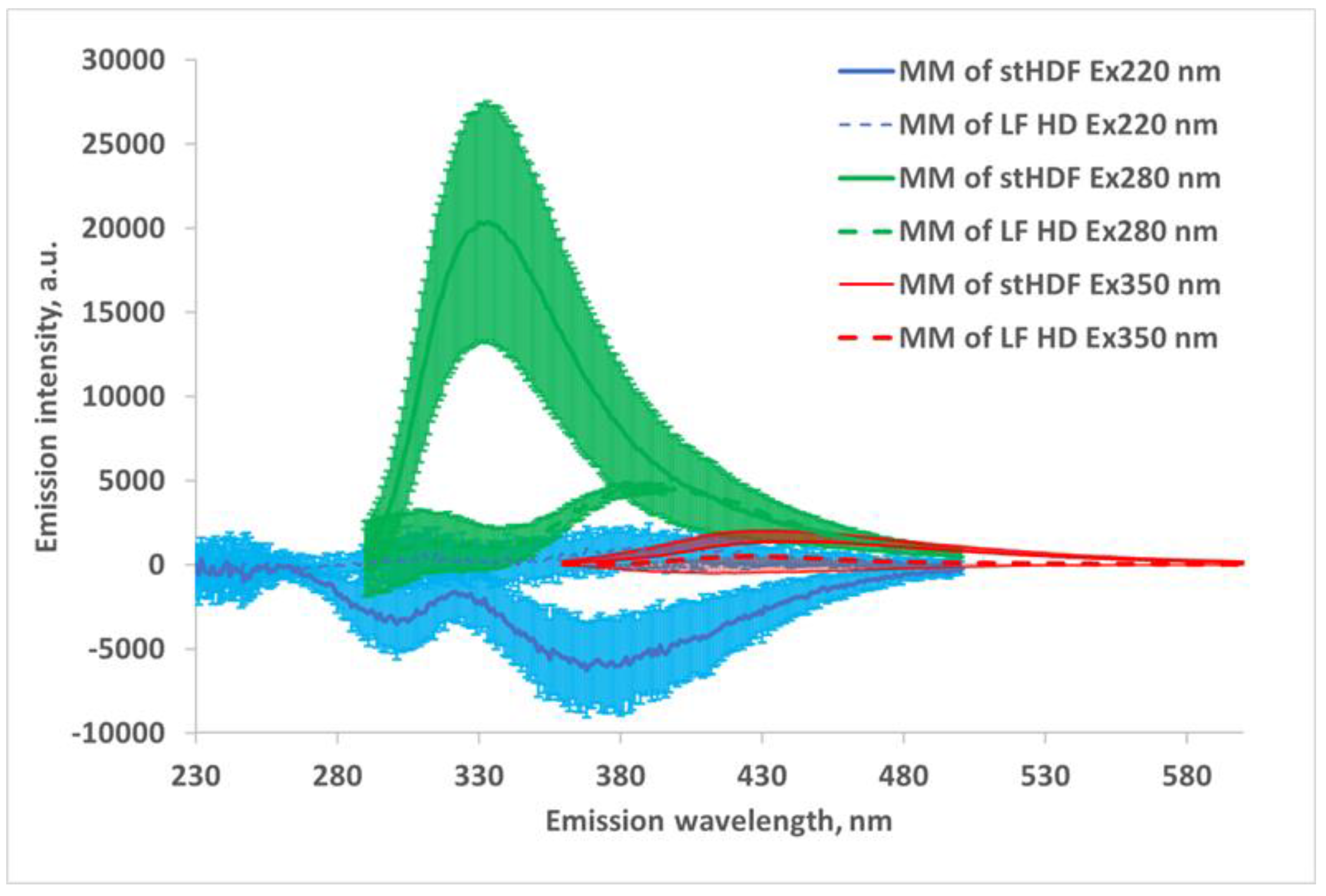
2.2. UV and Fluorescence Spectra of the MM Fraction
3. Discussion
4. Conclusions
5. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tattersall, J.; Martin-Malo, A.; Pedrini, L.; Basci, A.; Canaud, B.; Fouque, D.; Haage, P.; Konner, K.; Kooman, J.; Pizzarelli, F.; et al. EBPG guideline on dialysis strategies. Nephrol. Dial. Transplant. 2007, 22, ii5–ii21. [Google Scholar] [CrossRef]
- Tattersall, E.; Ward, R.; Canaud, B.; Blankestijn, P.J.; Bots, M.; Covic, A.; Davenport, A.; Grooteman, M.; Gura, V.; Hegbrant, J.; et al. Online haemodiafiltration: Definition, dose quantification and safety revisited. Nephrol. Dial. Transplant. 2013, 28, 542–550. [Google Scholar] [CrossRef]
- Daugirdas, J.T.; Depner, T.A.; Inrig, J.; Mehrotra, R.; Rocco, M.V.; Suri, R.S.; Weiner, D.E.; Greer, N.; Ishani, A.; MacDonald, R.; et al. KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 Update. Am. J. Kidney Dis. 2015, 66, 884–930. [Google Scholar] [CrossRef]
- Gál, G.; Gróf, J. Continuous Uv Photometric Monitoring of the Efficiency of Hemodialysis. Int. J. Artif. Organs 1980, 3, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Fridolin, I.; Magnusson, M.; Lindberg, L.-G. Measurement of solutes in dialysate using UV absorption. BiOS 2001 Int. Symp. Biomed. Opt. 2001, 4263, 40–48. [Google Scholar] [CrossRef]
- Fridolin, I.; Magnusson, M.; Lindberg, L.-G. On-Line Monitoring of Solutes in Dialysate Using Absorption of Ultraviolet Radiation: Technique Description. Int. J. Artif. Organs 2002, 25, 748–761. [Google Scholar] [CrossRef]
- Uhlin, F.; Fridolin, I.; Magnusson, M.; Lindberg, L.-G. Dialysis dose (Kt/V) and clearance variation sensitivity using measurement of ultraviolet-absorbance (on-line), blood urea, dialysate urea and ionic dialysance. Nephrol. Dial. Transplant. 2006, 21, 2225–2231. [Google Scholar] [CrossRef]
- Castellarnau, A.; Werner, M.; Günthner, R.; Jakob, M. Real-time Kt/V determination by ultraviolet absorbance in spent dialysate: Technique validation. Kidney Int. 2010, 78, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Umimoto, K.; Tatsumi, Y.; Kanaya, H.; Jokei, K. Analysis of Uremic Substances in Dialysate by Visible Ultraviolet Spectroscopy; Springer: Berlin, Germany, 2007; pp. 3205–3207. [Google Scholar]
- Umimoto, K.; Kanaya, Y.; Kawanishi, H.; Kawai, N. Measuring of Uremic Substances in Dialysate by Visible Ultraviolet Spectroscopy; Springer: Berlin, Germany, 2009; pp. 42–45. [Google Scholar]
- Vasilevskiy, A.M.; Konoplev, G.A. Polycomponental monitoring of process of effluent dialyzate by a method of uf of spectrometry. Biotekhnosfera 2009. Available online: https://scholar.google.com/scholar?cluster=6506178806057926161 (accessed on 23 December 2020).
- Arund, J.; Tanner, R.; Uhlin, F.; Fridolin, I. Do Only Small Uremic Toxins, Chromophores, Contribute to the Online Dialysis Dose Monitoring by UV Absorbance? Toxins 2012, 4, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Holmar, J.; Fridolin, I.; Uhlin, F.; Lauri, K.; Luman, M. Optical Method for Cardiovascular Risk Marker Uric Acid Removal Assessment during Dialysis. Sci. World J. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tomson, R.; Fridolin, I.; Uhlin, F.; Holmar, J.; Lauri, K.; Luman, M. Optical measurement of creatinine in spent dialysate. Clin. Nephrol. 2013, 79, 107–117. [Google Scholar] [CrossRef]
- Enberg, P.; Fridolin, I.; Holmar, J.; Fernström, A.; Uhlin, F. Utilization of UV Absorbance for Estimation of Phosphate Elimination during Hemodiafiltration. Nephron 2012, 121, c1–c9. [Google Scholar] [CrossRef]
- Holmar, J.; Uhlin, F.; Fernström, A.; Luman, M.; Jankowski, J.; Fridolin, I. An Optical Method for Serum Calcium and Phosphorus Level Assessment during Hemodialysis. Toxins 2015, 7, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Luman, M.; Jerotskaja, J.; Lauri, K.; Fridolin, I. Dialysis dose and nutrition assessment by optical on-line dialysis adequacy monitor. Clin. Nephrol. 2009, 72, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Uhlin, F.; Fridolin, I. Optical Monitoring of Dialysis Dose; Springer Science and Business Media LLC: New York, NY, USA, 2013; pp. 867–928. [Google Scholar]
- Adimea Real-Time Monitoring Process. Available online: https://www.bbraun.com/content/dam/catalog/bbraun/bbraunProductCatalog/S/AEM2015/en-01/b3/adimea.pdf (accessed on 23 December 2020).
- Kt/v Measurement. Dialysis Dose Monitor Measuring the Delivered Dialysis Dose. Available online: http://nikkisomedical.com/wp-content/uploads/2017/06/DDM_english_2013-03_vers04.pdf (accessed on 23 December 2020).
- Arund, J.; Luman, M.; Uhlin, F.; Tanner, R.; Fridolin, I. Is Fluorescence Valid to Monitor Removal of Protein Bound Uremic Solutes in Dialysis? PLoS ONE 2016, 11, e0156541. [Google Scholar] [CrossRef]
- Holmar, J.; Arund, J.; Uhlin, F.; Tanner, R.; Fridolin, I. Quantification of Indoxyl Sulphate in the Spent Dialysate Using Fluorescence Spectra. In Proceedings of the 1st World Congress on Electroporation and Pulsed Electric Fields in Biology, Medicine and Food & Environmental Technologies, Portorož, Slovenia, 6–10 September 2011; pp. 45–48. [Google Scholar]
- Holmar, J.; Uhlin, F.; Ferenets, R.; Lauri, K.; Tanner, R.; Arund, J.; Luman, M.; Fridolin, I. Estimation of removed uremic toxin indoxyl sulphate during hemodialysis by using optical data of the spent dialysate. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Institute of Electrical and Electronics Engineers (IEEE), Osaka, Japan, 3–7 July 2013; pp. 6707–6710. [Google Scholar]
- Masakane, I.; Sakurai, K. Current approaches to middle molecule removal: Room for innovation. Nephrol. Dial. Transplant. 2018, 33, 12–21. [Google Scholar] [CrossRef]
- Yamamoto, S. Molecular mechanisms underlying uremic toxin-related systemic disorders in chronic kidney disease: Focused on β2-microglobulin-related amyloidosis and indoxyl sulfate-induced atherosclerosis—Oshima Award Address 2016. Clin. Exp. Nephrol. 2019, 23, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.R.; Dehghani, N.L.; Narsimhan, V.; Ronco, C. Uremic Toxins and their Relation to Dialysis Efficacy. Blood Purif. 2019, 48, 299–314. [Google Scholar] [CrossRef]
- Blankestijn, P.J.; Fischer, I.K.; Barth, C.; Cromm, K.; Canaud, B.; Davenport, A.E.; Grobbee, D.; Hegbrant, J.; Roes, K.C.; Rose, M.; et al. Benefits and harms of high-dose haemodiafiltration versus high-flux haemodialysis: The comparison of high-dose haemodiafiltration with high-flux haemodialysis (CONVINCE) trial protocol. BMJ Open 2020, 10, e033228. [Google Scholar] [CrossRef]
- Kirsch, A.H.; Lyko, R.; Nilsson, L.-G.; Beck, W.; Amdahl, M.; Lechner, P.; Schneider, A.; Wanner, C.; Rosenkranz, A.R.; Krieter, D.H. Performance of hemodialysis with novel medium cut-off dialyzers. Nephrol. Dial. Transplant. 2016, 32, 165–172. [Google Scholar] [CrossRef]
- Lauri, K.; Luman, M.; Holmar, J.; Tomson, R.; Kalle, S.; Arund, J.; Uhlin, F.; Fridolin, I. Can Removal of Middle Molecular Uremic Retention Solutes be Estimated by UV-absorbance Measurements in Spent Dialysate? Springer: Toronto, ON, Canada, 2015; pp. 1297–1300. [Google Scholar]
- Uhlin, F.; Holmar, J.; Yngman-Uhlin, P.; Fernström, A.; Fridolin, I. Optical Estimation of Beta 2 Microglobulin during Hemodiafiltration‑Does It Work? Blood Purif. 2015, 40, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Lauri, K.; Arund, J.; Holmar, J.; Tanner, R.; Kalle, S.; Luman, M.; Fridolin, I. Removal of Urea, β2-Microglobulin, and Indoxyl Sulfate Assessed by Absorbance and Fluorescence in the Spent Dialysate During Hemodialysis. ASAIO J. 2019, 66, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Wolley, M.J.; Hutchison, C.A. Large uremic toxins: An unsolved problem in end-stage kidney disease. Nephrol. Dial. Transplant. 2018, 33, iii6–iii11. [Google Scholar] [CrossRef]
- Ward, A.R.; Greene, T.W.; Hartmann, B.; Samtleben, W. Resistance to intercompartmental mass transfer limits β2-microglobulin removal by post-dilution hemodiafiltration. Kidney Int. 2006, 69, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Brunati, C.C.M.; Gervasi, F.; Cabibbe, M.; Ravera, F.; Menegotto, A.; Querques, M.; Colussi, G. Single Session and Weekly Beta 2-Microglobulin Removal with Different Dialytic Procedures: Comparison between High-Flux Standard Bicarbonate Hemodialysis, Post-Dilution Hemodiafiltration, Short Frequent Hemodialysis with NxStage Technology and Automated Peritoneal Dialysis. Blood Purif. 2019, 48, 86–96. [Google Scholar] [CrossRef]
- Krochmal, M.; Schanstra, J.P.; Mischak, H. Urinary peptidomics in kidney disease and drug research. Expert Opin. Drug Discov. 2017, 13, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Mandal, I.; Singh, S.; Paul, A.; Mandal, B.; Venkatramani, R.; Swaminathan, R. Near UV-Visible electronic absorption originating from charged amino acids in a monomeric protein. Chem. Sci. 2017, 8, 5416–5433. [Google Scholar] [CrossRef]
- Rosenheck, K.; Doty, P. The Far Ultraviolet Absorption Spectra of Polypeptide and Protein Solutions and Their Dependence on Conformation. Proc. Natl. Acad. Sci. USA 1961, 47, 1775–1785. [Google Scholar] [CrossRef]
- Jerotskaja, J.; Lauri, K.; Tanner, R.; Luman, M.; Fridolin, I. Optical dialysis adequacy sensor: Wavelength dependence of the ultra violet absorbance in the spent dialysate to the removed solutes. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 2960–2963. [Google Scholar]
- Fridolin, I.; Lindberg, L.-G. On-line monitoring of solutes in dialysate using wavelength-dependent absorption of ultraviolet radiation. Med. Biol. Eng. Comput. 2003, 41, 263–270. [Google Scholar] [CrossRef]
- Fonin, A.V.; Sulatskaya, A.I.; Kuznetsova, I.M.; Turoverov, K.K. Fluorescence of Dyes in Solutions with High Absorbance. Inner Filter Effect Correction. PLoS ONE 2014, 9, e103878. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zeng, L.-H.; Li, D.-L. A review on the methods for correcting the fluorescence inner-filter effect of fluorescence spectrum. Appl. Spectrosc. Rev. 2017, 52, 883–908. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Fluorophores. In Principles of Fluorescence Spectroscopy, 3rd ed.; Lakowicz, J.R., Ed.; Springer US: Boston, MA, USA, 2006; pp. 63–95. [Google Scholar]
- Ulrich, P. Protein Glycation, Diabetes, and Aging. Recent Prog. Horm. Res. 2001, 56, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Stirban, A.; Gawlowski, T.; Roden, M. Vascular effects of advanced glycation endproducts: Clinical effects and molecular mechanisms. Mol. Metab. 2014, 3, 94–108. [Google Scholar] [CrossRef]
- Gugliucci, A. Formation of Fructose-Mediated Advanced Glycation End Products and Their Roles in Metabolic and Inflammatory Diseases. Adv. Nutr. 2017, 8, 54–62. [Google Scholar] [CrossRef]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxidative Med. Cell. Longev. 2020, 2020, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J.; Rabbani, N. Highlights and Hotspots of Protein Glycation in End-Stage Renal Disease. Semin. Dial. 2009, 22, 400–404. [Google Scholar] [CrossRef]
- Mallipattu, S.K.; Uribarri, J. Advanced glycation end product accumulation. Curr. Opin. Nephrol. Hypertens. 2014, 23, 547–554. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kazama, J.J.; Wakamatsu, T.; Takahashi, Y.; Kaneko, Y.; Goto, S.; Narita, I. Removal of uremic toxins by renal replacement therapies: A review of current progress and future perspectives. Ren. Replace. Ther. 2016, 2, 43. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Advanced glycation end products in the pathogenesis of chronic kidney disease. Kidney Int. 2018, 93, 803–813. [Google Scholar] [CrossRef]
- Stinghen, A.E.; Massy, Z.A.; Vlassara, H.; Striker, G.E.; Boullier, A. Uremic Toxicity of Advanced Glycation End Products in CKD. J. Am. Soc. Nephrol. 2015, 27, 354–370. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.A.; Thomas, M.C.; Fernando, D.; Macmillan, N.; Power, D.A.; Ierino, F.L. Low molecular weight advanced glycation end products predict mortality in asymptomatic patients receiving chronic haemodialysis. Nephrol. Dial. Transplant. 2006, 21, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Taneda, S.; Kawai, R.; Ueda, Y.; Horiuchi, S.; Hara, M.; Maeda, K.; Monnier, V.M. Identification of pentosidine as a native structure for advanced glycation end products in beta-2-microglobulin-containing amyloid fibrils in patients with dialysis-related amyloidosis. Proc. Natl. Acad. Sci. USA 1996, 93, 2353–2358. [Google Scholar] [CrossRef] [PubMed]
- Meerwaldt, R.; Links, T.; Graaff, R.; Thorpe, S.R.; Baynes, J.W.; Hartog, J.; Gans, R.; Smit, A. Simple Noninvasive Measurement of Skin Autofluorescence. Ann. N. Y. Acad. Sci. 2005, 1043, 290–298. [Google Scholar] [CrossRef]
- Kalle, S.; Tanner, R.; Luman, M.; Fridolin, I. Free Pentosidine Assessment Based on Fluorescence Measurements in Spent Dialysate. Blood Purif. 2018, 47, 85–93. [Google Scholar] [CrossRef]
- Schmitt, A.; Schmitt, J.; Münch, G.; Gasic-Milencovic, J. Characterization of advanced glycation end products for biochemical studies: Side chain modifications and fluorescence characteristics. Anal. Biochem. 2005, 338, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Tessier, F.; Obrenovich, M.; Monnier, V.M. Structure and Mechanism of Formation of Human Lens Fluorophore LM-1. J. Biol. Chem. 1999, 274, 20796–20804. [Google Scholar] [CrossRef]
- Miyata, T.; Oda, O.; Inagi, R.; Iida, Y.; Araki, N.; Yamada, N.; Horiuchi, S.; Taniguchi, N.; Maeda, K.; Kinoshita, T. beta 2-Microglobulin modified with advanced glycation end products is a major component of hemodialysis-associated amyloidosis. J. Clin. Investig. 1993, 92, 1243–1252. [Google Scholar] [CrossRef]
- Förster, T. Experimentelle und theoretische Untersuchung des zwischenmolekularen Übergangs von Elektronenanregungsenergie. Z. Nat. A 1949, 4, 321–327. [Google Scholar] [CrossRef]
- Förster, T. Delocalized Excitation and Excitation Transfer; Florida State University: Tallahassee, FL, USA, 1965. [Google Scholar]
- Donadio, C.; Calia, D.; Ghimenti, S.; Onor, M.; Colombini, E.; Fuoco, R.; Di Francesco, F. The Removal of β2-Microglobulin in Spent Dialysate Cannot Be Monitored by Spectrophotometric Analysis. Blood Purif. 2015, 40, 109–112. [Google Scholar] [CrossRef]
- Vivian, J.T.; Callis, P.R. Mechanisms of Tryptophan Fluorescence Shifts in Proteins. Biophys. J. 2001, 80, 2093–2109. [Google Scholar] [CrossRef]
- Narang, D.; Sharma, P.K.; Mukhopadhyay, S. Dynamics and dimension of an amyloidogenic disordered state of human β2-microglobulin. Eur. Biophys. J. 2013, 42, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Esbensen, K. Multivariate Data Analysis –in Practice. Introduction to Multivariate Data Analysis and Experimental Design, 5th ed.; CAMO Software: Oslo, Norway, 2009. [Google Scholar]
- Eloot, S.; Dhondt, A.; Vierendeels, J.; De Wachter, D.; Verdonck, P.; Vanholder, R. Temperature and concentration distribution within the Genius(R) dialysate container. Nephrol. Dial. Transplant. 2007, 22, 2962–2969. [Google Scholar] [CrossRef] [PubMed]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]




| Clinical Parameter | β2M Lab mean ± SD | β2M Opt mean ± SD | p | Accuracy (BIAS ± SE) | Pearson Correlation |
|---|---|---|---|---|---|
| RR (%, N = 31) | 73.37 ± 10.39 | 72.06 ± 7.77 | 0.35 | −1.31 ± 5.41 | 0.894 |
| TRS (mg, N = 33) | 234.5 ± 72.8 | 228.6 ± 83.9 | 0.35 | −5.95 ± 36.09 | 0.904 |
| Amino Acid | Excitation Wavelength (nm) | Emission Wavelength (nm) | Bandwidth (nm) | Quantum Yield |
|---|---|---|---|---|
| Tryptophan | 295 | 353 | 60 | 0.13 |
| Tyrosine | 275 | 304 | 34 | 0.14 |
| Phenylalanine | 260 | 282 | - | 0.02 |
| Entity of the Data | Specification |
|---|---|
| Cause of ESKD | Diabetes (4); Hypertension (8); Glomerulonephritis (3); Tubulointerstitial nephritis (3); Renal carcinoma (2); Other (2) |
| Age (years) | 55 ± 17 |
| Gender | M (17), F (=5) |
| Race, Caucasian (%) | 100 |
| BMI, kg/m2 a | 26.8 ± 5.8 |
| BW, kg a | 81.5 ± 21.3 |
| Ultrafiltration volume, mL | 2565 ± 1190 |
| Urinary volume, mL | 0 (14 patients) 700 (335–825) (8 patients) |
| Serum total protein, g/L | 62.8 ± 5.5 |
| Hematocrit, % a | 34.4 (3.5) |
| Serum calcium, mmol/L a | 2.25 (0.16) |
| Serum phosphorus, mmol/L a Serum parathyroid hormone, pmol/L a | 1.92 (1.63–2.29) 28.7 (16.8–41.9) |
| Dialysis access | native fistula (15); graft (7) |
| Dialysis vintage, months a | 23 (11–83) |
| spKt/Vurea a | 1.47 (1.23–1.67) |
| Entity of the Data | Standard HDF | Low Flux HD | Medium HDF | High HDF |
|---|---|---|---|---|
| Volume substituted (Vs, L) | 21.1 ± 3.1 | 0 ± 0 | 15.3 ± 1.4 | 25.3 ± 2.8 |
| Dialysis time, min. | 240 | 240 | 240 | 240 |
| Blood flow, mL/min (Qb) | 300.8 ± 12.7 | 200 ± 0 | 299.7 ± 1.0 | 364.2 ± 27.1 |
| Dialysate flow, ml/min (Qd) | 470.8 ± 105.4 | 300 ± 0 | 799.8 ± 0.9 | 800.0 ± 0.0 |
| Dialyzer area a, m2 | 2.0 ± 0.2 | 1.5 ± 0.0 | 2.2 ± 0.0 | 2.2 ± 0.0 |
| Number of dialyses (N) | 22 | 22 | 22 | 22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paats, J.; Adoberg, A.; Arund, J.; Fridolin, I.; Lauri, K.; Leis, L.; Luman, M.; Tanner, R. Optical Method and Biochemical Source for the Assessment of the Middle-Molecule Uremic Toxin β2-Microglobulin in Spent Dialysate. Toxins 2021, 13, 255. https://doi.org/10.3390/toxins13040255
Paats J, Adoberg A, Arund J, Fridolin I, Lauri K, Leis L, Luman M, Tanner R. Optical Method and Biochemical Source for the Assessment of the Middle-Molecule Uremic Toxin β2-Microglobulin in Spent Dialysate. Toxins. 2021; 13(4):255. https://doi.org/10.3390/toxins13040255
Chicago/Turabian StylePaats, Joosep, Annika Adoberg, Jürgen Arund, Ivo Fridolin, Kai Lauri, Liisi Leis, Merike Luman, and Risto Tanner. 2021. "Optical Method and Biochemical Source for the Assessment of the Middle-Molecule Uremic Toxin β2-Microglobulin in Spent Dialysate" Toxins 13, no. 4: 255. https://doi.org/10.3390/toxins13040255
APA StylePaats, J., Adoberg, A., Arund, J., Fridolin, I., Lauri, K., Leis, L., Luman, M., & Tanner, R. (2021). Optical Method and Biochemical Source for the Assessment of the Middle-Molecule Uremic Toxin β2-Microglobulin in Spent Dialysate. Toxins, 13(4), 255. https://doi.org/10.3390/toxins13040255





