Effects of Light on the Ochratoxigenic Fungi Aspergillus ochraceus and A. carbonarius
Abstract
1. Introduction
2. Results
2.1. Effect of Light on Fungal Growth and Morphology
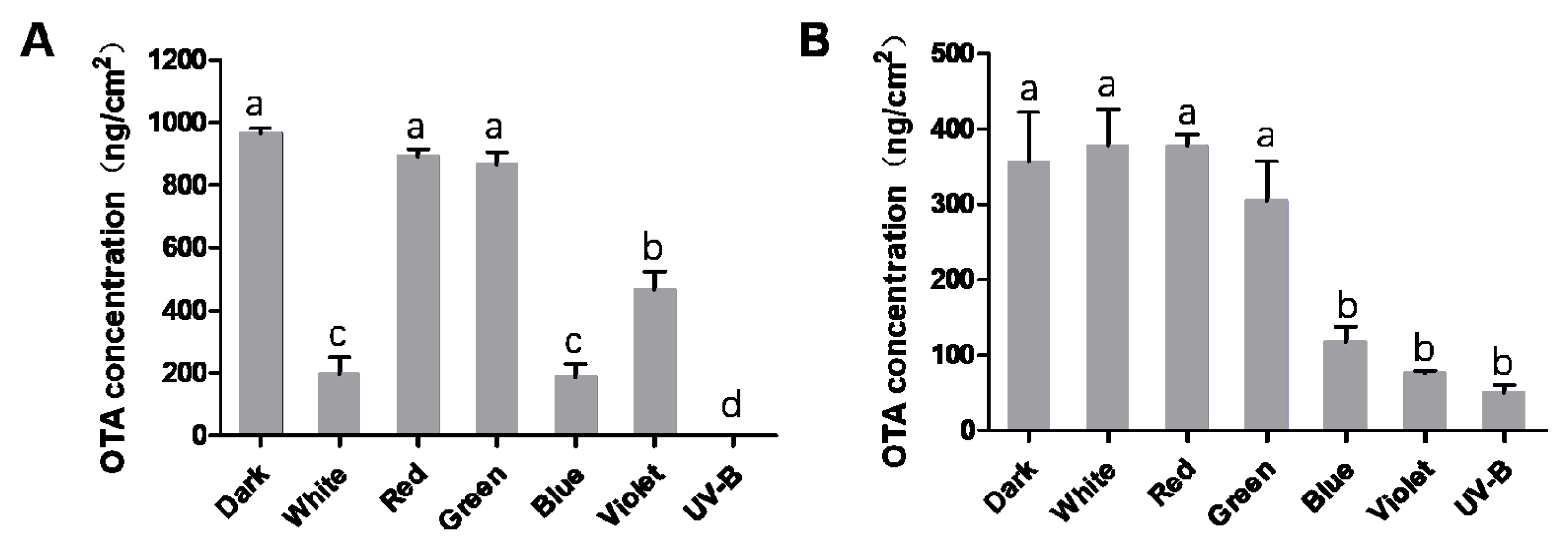
2.2. Analysis of OTA Production
2.3. OTA Biosynthetic Genes Expression of A. ochraceus and A. carbonarius Were Regulated by Light
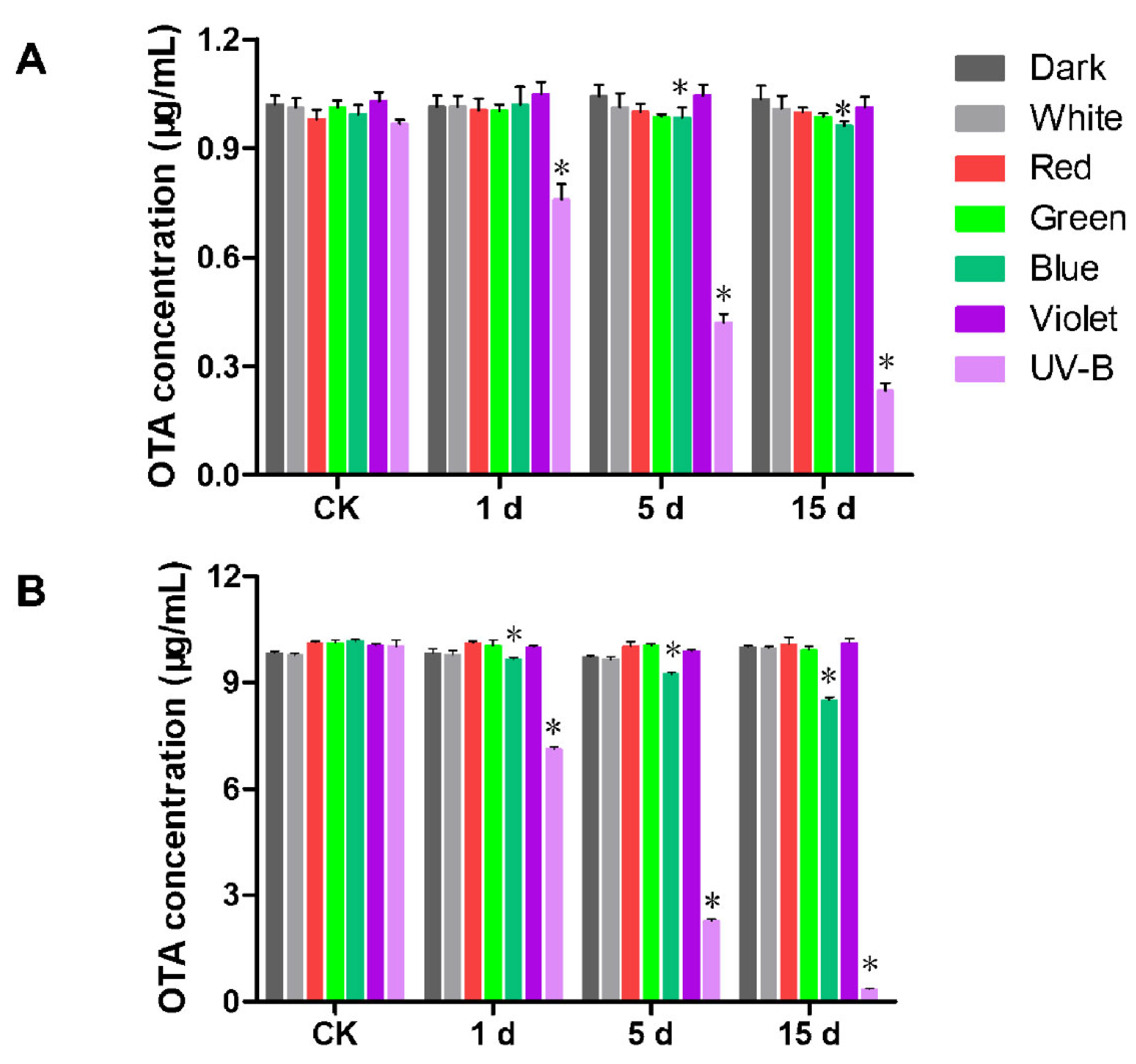
2.4. Degradation of OTA by Light
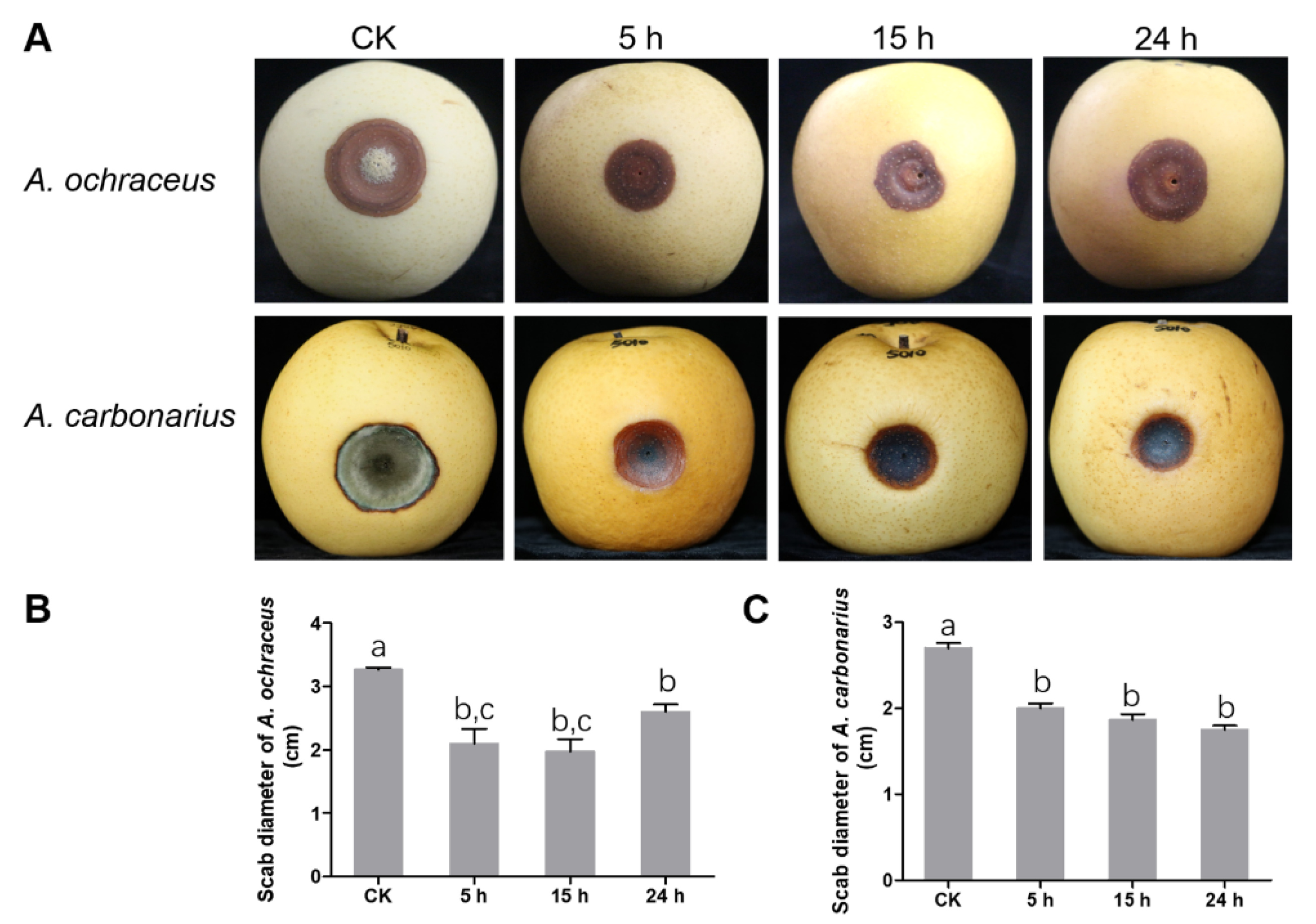
2.5. UV-B Inhibited the Pathogenicity of A. ochraceus and A. carbonarius
3. Discussion
4. Materials and Methods
4.1. Solvents and Reagents
4.2. Strains and Growth Conditions
4.3. The Light Incubation Conditions
4.4. Quantification of OTA by HPLC-FLD
4.5. Isolation of RNA
4.6. Quantitative Real-Time RT-PCR Reaction and Relative Gene Expression Determination
4.7. Degradation of OTA Standard Pure Solution Samples under Different Light Wavelengths
4.8. The Effect of UV-B on Pathogenicity
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krogh, P.; Hald, B.; Gjertsen, P.; Myken, F. Fate of ochratoxin A and citrinin during malting and brewing experiments. Appl. Microbiol. 1974, 28, 31–34. [Google Scholar] [CrossRef]
- Scott, P.M. Effects of processing and detoxification treatments on ochratoxin A: Introduction. Food Addit. Contam. 1996, 13, 19–21. [Google Scholar]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 Years of Research. Toxins 2016, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.G.; Saraiva, N.; Guerreiro, P.S.; Louro, H.; Silva, M.J.; Miranda, J.P.; Castro, M.; Batinic-Haberle, I.; Fernandes, A.S.; Oliveira, N.G. Ochratoxin A-induced cytotoxicity, genotoxicity and reactive oxygen species in kidney cells: An integrative approach of complementary endpoints. Food Chem. Toxicol. 2016, 87, 65–76. [Google Scholar] [CrossRef] [PubMed]
- IARC (International Agency for Research on Cancer). Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 1993; Volume 56, pp. 489–521.6. [Google Scholar]
- Cheong, K.K.; Strub, C.; Montet, D.; Durand, N.; Alter, P.; Meile, J.-C.; Galindo, S.S.; Fontana, A. Effect of different light wave lengths on the growth and ochratoxin A production in Aspergillus carbonarius and Aspergillus westerdijkiae. Fungal Biol. 2016, 120, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Perši, N.; Pleadin, J.; Kovačević, D.; Scortichini, G.; Milone, S. Ochratoxin A in raw materials and cooked meat products made from OTA-treated pigs. Meat Sci. 2014, 96, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Fischer, R. Light sensing and responses in fungi. Nat. Rev. Genet. 2019, 17, 25–36. [Google Scholar] [CrossRef]
- Fanelli, F.; Geisen, R.; Schmidt-Heydt, M.; Logrieco, A.; Mule, G. Light regulation of mycotoxin biosynthesis: New perspectives for food safety. World Mycotoxin J. 2016, 9, 129–146. [Google Scholar] [CrossRef]
- Schmidt-Heydt, M.; Rüfer, C.; Raupp, F.; Bruchmann, A.; Perrone, G.; Geisen, R. Influence of light on food relevant fungi with emphasis on ochratoxin producing species. Int. J. Food Microbiol. 2011, 145, 229–237. [Google Scholar] [CrossRef]
- Schmidt-Heydt, M.; Bode, H.; Raupp, F.; Geisen, R. Influence of light on ochratoxin biosynthesis by Penicillium. Mycotoxin Res. 2009, 26, 1–8. [Google Scholar] [CrossRef]
- Dunlap, J.C.; Loros, J.J. How fungi keep time: Circadian system in Neurospora and other fungi. Curr. Opin. Microbiol. 2006, 9, 579–587. [Google Scholar] [CrossRef]
- Schmidt-Heydt, M.; Cramer, B.; Graf, I.; Lerch, S.; Humpf, H.-U.; Geisen, R. Wavelength-Dependent Degradation of Ochra-toxin and Citrinin by Light in Vitro and in Vivo and Its Implications on Penicillium. Toxins 2012, 4, 1535–1551. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Wu, F.; Liu, F.; Wang, Q.; Zhang, X.; Selvaraj, J.N.; Zhao, Y.; Xing, F.; Yin, W.-B.; et al. A Consensus Ochratoxin A Biosynthetic Pathway: Insights from the Genome Sequence of Aspergillus ochraceus and a Comparative Genomic Analysis. Appl. Environ. Microbiol. 2018, 84, e01009-18. [Google Scholar] [CrossRef]
- Gallo, A.; Bruno, K.S.; Solfrizzo, M.; Perrone, G.; Mulè, G.; Visconti, A.; Baker, S.E. New insight into the ochratoxin A biosyn-thetic pathway through deletion of a nonribosomal peptide synthetase gene in Aspergillus carbonarius. Appl. Environ. Microbiol. 2012, 78, 8208–8218. [Google Scholar] [CrossRef]
- Gallo, A.; Knox, B.P.; Bruno, K.S.; Solfrizzo, M.; Baker, S.E.; Perrone, G. Identification and characterization of the polyketide synthase involved in ochratoxin A biosynthesis in Aspergillus carbonarius. Int. J. Food Microbiol. 2014, 179, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Massimo, F.; Giancarlo, P.; Lucia, G.; Filomena, E.; Michele, S.; Antonia, G. Identification of a Halogenase Involved in the Biosynthesis of Ochratoxin A in Aspergillus carbonarius. Appl. Environ. Microbiol. 2016, 82, 5631–5641. [Google Scholar]
- Lee, K.; Singh, P.; Chung, W.-C.; Ash, J.; Kim, T.S.; Hang, L.; Park, S. Light regulation of asexual development in the rice blast fungus, Magnaporthe oryzae. Fungal Genet. Biol. 2006, 43, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Purschwitz, J.; Müller, S.; Kastner, C.; Fischer, R. Seeing the rainbow: Light sensing in fungi. Curr. Opin. Microbiol. 2006, 9, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Purschwitz, J.; Müller, S.; Kastner, C.; Schöser, M.; Haas, H.; Espeso, E.A.; Atoui, A.; Calvo, A.M.; Fischer, R. Functional and Physical Interaction of Blue- and Red-Light Sensors in Aspergillus nidulans. Curr. Biol. 2008, 18, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Froehlich, A.C.; Liu, Y.; Loros, J.J.; Dunlap, J.C. White Collar-1, a Circadian Blue Light Photoreceptor, Binding to the frequency Promoter. Science 2002, 297, 815–819. [Google Scholar] [CrossRef]
- Fuller, K.K.; Ringelberg, C.S.; Loros, J.J.; Dunlap, J.C. The Fungal Pathogen Aspergillus fumigatus Regulates Growth, Metabolism, and Stress Resistance in Response to Light. mBio 2013, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Röhrig, J.; Kastner, C.; Fischer, R. Light inhibits spore germination through phytochrome in Aspergillus nidulans. Curr. Genet. 2013, 59, 55–62. [Google Scholar] [CrossRef] [PubMed]
- García-Cela, E.; Marin, S.; Sanchis, V.; Crespo-Sempere, A.; Ramos, A.J. Effect of ultraviolet radiation A and B on growth and mycotoxin production by Aspergillus carbonarius and Aspergillus parasiticus in grape and pistachio media. Fungal Biol. 2015, 119, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Ruger-Herreros, C.; Rodríguez-Romero, J.; Fernández-Barranco, R.; Olmedo, M.; Fischer, R.; Corrochano, L.M.; Canovas, D. Regulation of Conidiation by Light inAspergillus nidulans. Genetics 2011, 188, 809–822. [Google Scholar] [CrossRef]
- Chen, C.-H.; Ringelberg, C.S.; Gross, R.H.; Dunlap, J.C.; Loros, J.J. Genome-wide analysis of light-inducible responses reveals hierarchical light signalling in Neurospora. EMBO J. 2009, 28, 1029–1042. [Google Scholar] [CrossRef]
- Hu, Y.; He, J.; Wang, Y.; Zhu, P.; Zhang, C.; Lu, R.; Xu, L. Disruption of a phytochrome-like histidine kinase gene by homol-ogous recombination leads to a significant reduction in vegetative growth, sclerotia production, and the pathogenicity of Bo-trytis cinerea. Physiol Mol. Plant. Pathol. 2014, 85, 25–33. [Google Scholar] [CrossRef]
- Ulises, E.-N.E.; Mónica, G.-E.; Elizabeth, M.-C.; Alejandro, C.-P.V.; Luis, P.-A.J.; Fidel, L.-J.; Antonio, C.-C.J.; Alfredo, H.-E. A Trichoderma atroviride stress-activated MAPK pathway integrates stress and light signals. Mol. Microbiol. 2016, 100, 860–876. [Google Scholar]
- Wang, Y.; Wang, L.; Liu, F.; Wang, Q.; Selvaraj, J.N.; Xing, F.; Zhao, Y.; Liu, Y. Ochratoxin A Producing Fungi, Biosynthetic Pathway and Regulatory Mechanisms. Toxins 2016, 8, 83. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Kouadio, I.A.; Koffi, L.B.; Nemlin, J.G.; Dosso, M.B. Effect of Robusta (Coffea canephora P.) coffee cherries quantity put out for sun drying on contamination by fungi and Ochratoxin A (OTA) under tropical humid zone (Côte d‘Ivoire). Food Chem. Toxicol. 2012, 50, 1969–1979. [Google Scholar] [CrossRef]
- Toshikazu, I.; Yasuo, T.; Takao, A.; Kiyoshi, O.; Naoko, I.; Kazuo, E.; Masaharu, Y.; Kazutoshi, N. Ochratoxin A Contamination of Red Chili Peppers from Chile, Bolivia and Peru, Countries with a High Incidence of Gallbladder Cancer. Asian Pac. J. Cancer Prev. 2015, 16, 5987–5991. [Google Scholar]
- Canadian Food Inspection Agency. Ochratoxin A in Wheat Products, Oat Products, Rice Products and Other Grain Products April 1, 2018 to March 31, 2019. Available online: https://inspection.canada.ca/food-safety-for-industry/food-chemistry-and-microbiology/food-safety-testing-bulletin-and-reports/ochratoxin-a-in-wheat-products-oat-products-rice-p/eng/1593534314634/1593534315071 (accessed on 31 March 2021).
- Wang, Y.; Liu, F.; Wang, L.; Wang, Q.; Selvaraj, J.N.; Zhao, Y.; Wang, Y.; Xing, F.; Liu, Y. pH-Signaling Transcription Factor AopacC Regulates Ochratoxin A Biosynthesis in Aspergillus ochraceus. J. Agric. Food Chem. 2018, 66, 4394–4401. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, B.; Muhammad, H.; Geng, H.; Wang, G.; Zhang, C.; Gao, S.; Xing, F.; Liu, Y. A novel strain Lactobacillus brevis 8-2B inhibiting Aspergillus carbonarius growth and ochratoxin A production. LWT 2021, 136, 110308. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, H.; Wang, Y.; Liu, F.; Li, E.; Ma, J.; Yang, B.; Zhang, C.; Li, L.; Liu, Y. Requirement of LaeA, VeA, and VelB on Asexual Development, Ochratoxin A Biosynthesis, and Fungal Virulence in Aspergillus ochraceus. Front. Microbiol. 2019, 10, 2759. [Google Scholar] [CrossRef] [PubMed]
- Lappa, I.K.; Kizis, D.; Panagou, E.Z. Monitoring the Temporal Expression of Genes Involved in Ochratoxin A Production of Aspergillus carbonarius under the Influence of Temperature and Water Activity. Toxins 2017, 9, 296. [Google Scholar] [CrossRef] [PubMed]


| Primers (A. ochraceus) | Sequence (5′ to 3′) |
| GADPH-RT-F | CGGCAAGAAGGTTCAGTT |
| GADPH-RT-R | CTCGTTGGTGGTGAAGAC |
| AFotaA-RT-F | GGATCTTTATGACCGAATCAG |
| AFotaA-RT-R | CCTTGACCTGAAGAATGCT |
| AFotaB-RT-F | ATACCACCAGAGCTCCAAA |
| AFotaB-RT-R | GAGATGTTCGGTCTGTTCA |
| AFotaC-RT-F | CTTAATACGGTGGTCTACGA |
| AFotaC-RT-R | GAATGATAGGTCCGTATTTCT |
| AFotaD-RT-F | TATTCCCTAGATACCATATCGG |
| AFotaD-RT-R | GCTTCCTTCTGGTTGTTCA |
| AFotaR1-RT-F | GCTTTCAAATCGAATGATTCC |
| AFotaR1-RT-R | GATCGGTTGGAAGTGTAGAA |
| Primers (A. carbonarius) | Sequence (5′ to 3′) |
| tubβ-RT-F | CGCATGAACGTCTACTTCAACGAG |
| tubβ-RT-R | AGTTGTTACCAGCACCGGACT |
| ACotaA-RT-F | GTCAAGGTCGGGTGCTACAA |
| ACotaA-RT-R | TCGGAATGATACGCGACTTT |
| ACotaB-RT-F | CTCCACCCATCCTCCCGTTC |
| ACotaB-RT-R | AATCCATGTCCTCACCATCGC |
| ACotaC-RT-F | GTGGTTATCCCGCCCAATAC |
| ACotaC-RT-R | TGCCAGATTCATCCCGATAC |
| ACotaD-RT-F | GAACGCCAGTAGAGGGACAG |
| ACotaD-RT-R | ATGGAGGTGGTGTTGTTGTG |
| ACotaR1-RT-F | AATGGAACCAGCATTGATCTC |
| ACotaR1-RT-R | GACCCAAGCATTCGCTCTA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Wang, G.; Yang, Q.; Yang, X.; Zheng, Y.; Liu, Y.; Xing, F. Effects of Light on the Ochratoxigenic Fungi Aspergillus ochraceus and A. carbonarius. Toxins 2021, 13, 251. https://doi.org/10.3390/toxins13040251
Zhang H, Wang G, Yang Q, Yang X, Zheng Y, Liu Y, Xing F. Effects of Light on the Ochratoxigenic Fungi Aspergillus ochraceus and A. carbonarius. Toxins. 2021; 13(4):251. https://doi.org/10.3390/toxins13040251
Chicago/Turabian StyleZhang, Haiyong, Gang Wang, Qingli Yang, Xu Yang, Yongquan Zheng, Yang Liu, and Fuguo Xing. 2021. "Effects of Light on the Ochratoxigenic Fungi Aspergillus ochraceus and A. carbonarius" Toxins 13, no. 4: 251. https://doi.org/10.3390/toxins13040251
APA StyleZhang, H., Wang, G., Yang, Q., Yang, X., Zheng, Y., Liu, Y., & Xing, F. (2021). Effects of Light on the Ochratoxigenic Fungi Aspergillus ochraceus and A. carbonarius. Toxins, 13(4), 251. https://doi.org/10.3390/toxins13040251







