High Levels of Tetrodotoxin (TTX) in Trumpet Shell Charonia lampas from the Portuguese Coast
Abstract
1. Introduction
2. Results
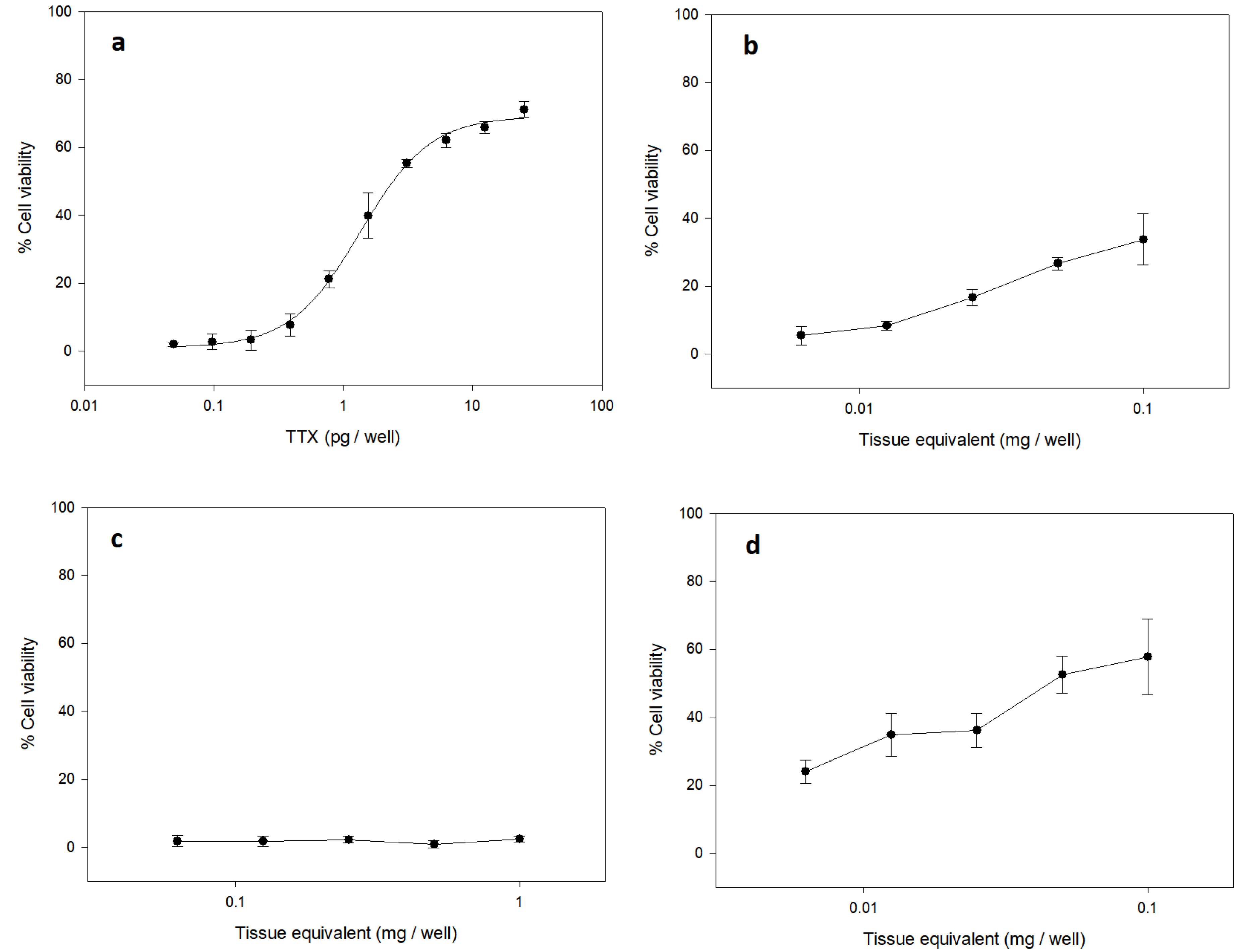
2.1. Detection of TTX by Cell Based Assay
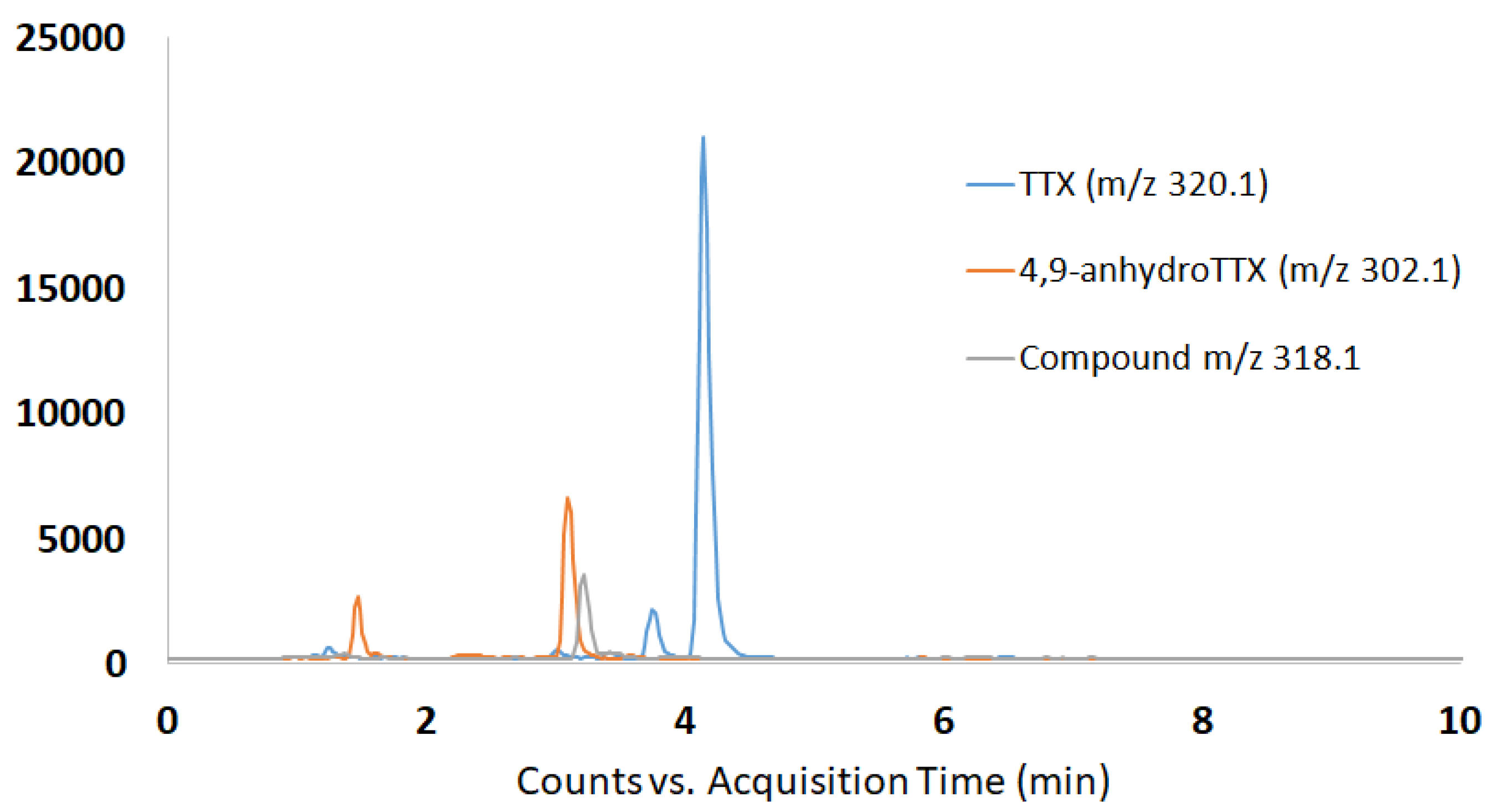
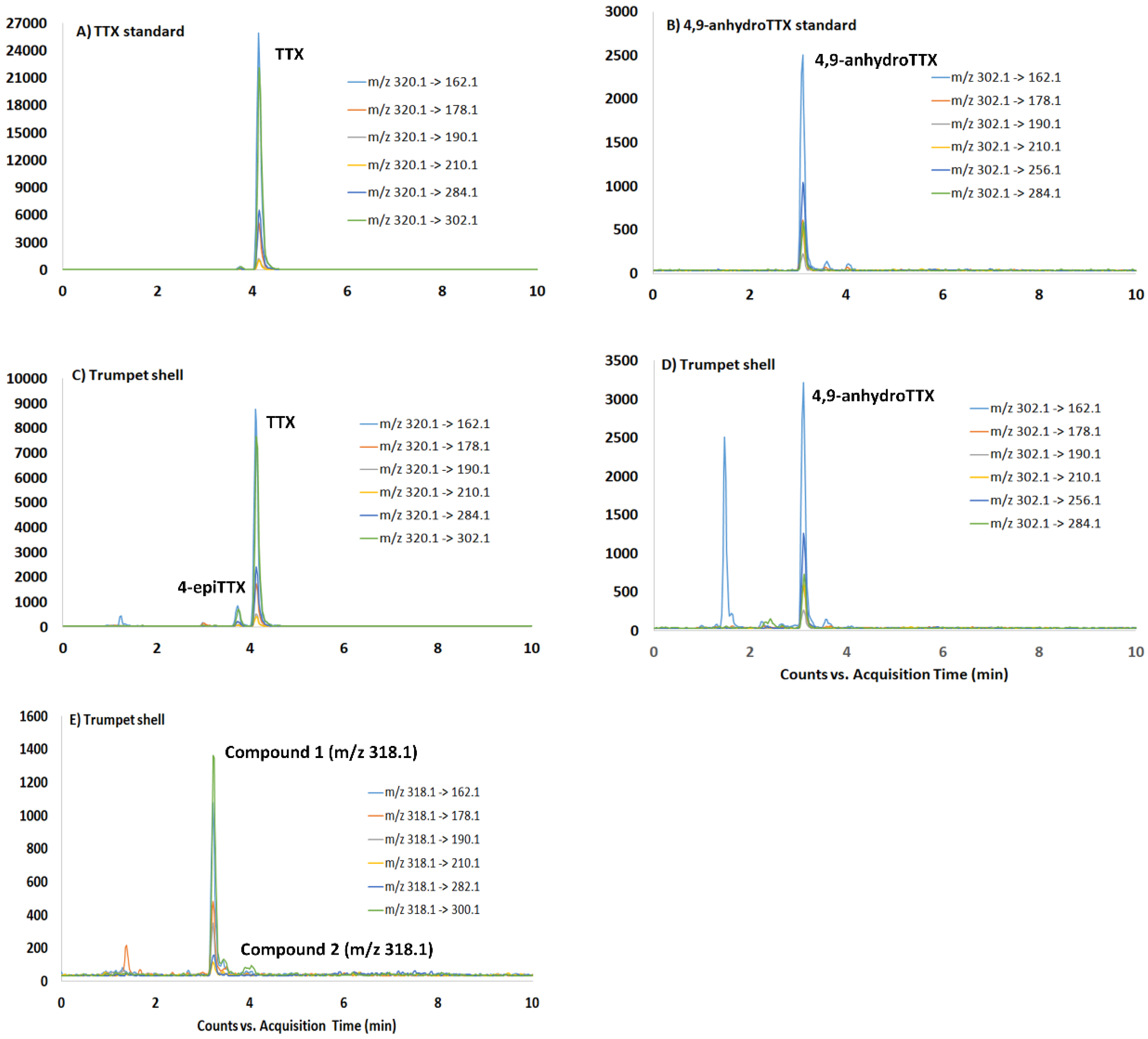
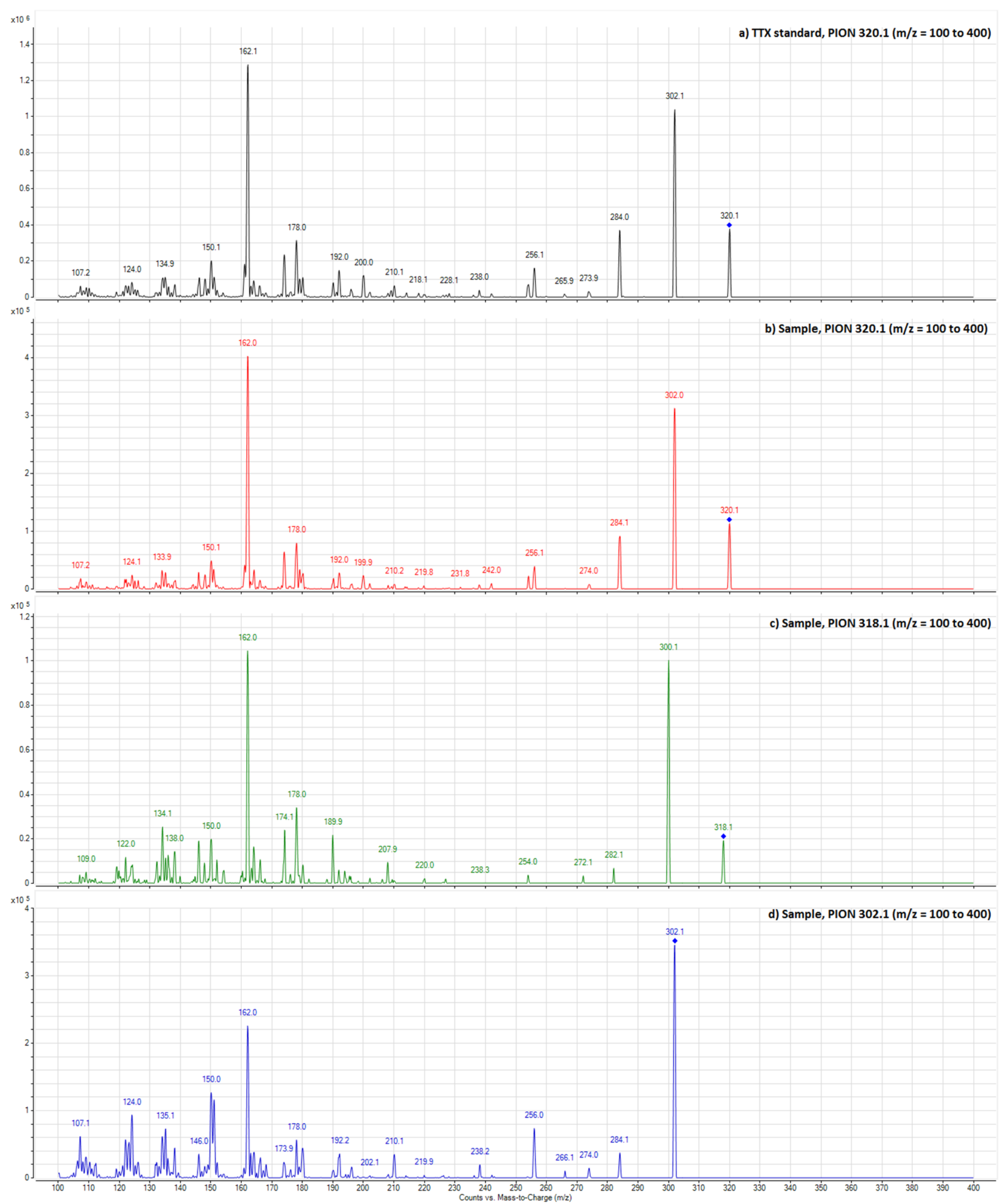
2.2. Determination of TTX and TTX Analogues by HILIC-MSMS
3. Discussion
4. Materials and Methods
4.1. Obtention and Preparation of Samples for TTX Extraction
4.2. TTX Analysis
4.2.1. Reagents and Standards
4.2.2. Cell-Based Assay (CBA)
4.2.3. HILIC-MS/MS Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Noguchi, T.; Onuki, K.; Arakawa, O. Tetrodotoxin poisoning due to pufferfish and gastropods, and their intoxication mechanism. ISRN Toxicol. 2011, 2011, 276939. [Google Scholar] [CrossRef]
- Narita, H.; Noguchi, T.; Maruyama, J.; Ueda, Y.; Hashimoto, K.; Watanabe, Y.; Hida, K. Occurrence of tetrodotoxin in a trumpet shell, “Boshubora” Charonia sauliae. Bull. Jpn. Soc. Sci. Fish. 1981, 47, 935–941. [Google Scholar] [CrossRef]
- Noguchi, T.; Narita, H.; Maruyama, J.; Hashimoto, K. Tetrodotoxin in the starfish Astropecten polyacanthus; in association with toxification of a trumpet shell, “boshubora” Charonia sauliae. Bull. Jap. Soc. Sci. Fish. 1982, 48, 1173–1177. [Google Scholar] [CrossRef]
- Guardone, L.; Maneschi, A.; Meucci, V.; Gasperetti, L.; Nucera, D.; Armani, A. A Global Retrospective Study on Human Cases of Tetrodotoxin (TTX) Poisoning after Seafood Consumption. Food Rev. Int. 2020, 36, 645–667. [Google Scholar] [CrossRef]
- Biessy, L.; Boundy, M.J.; Smith, K.; Harwood, D.T.; Hawes, I.; Wood, S.A. Tetrodotoxin in marine bivalves and edible gastropods: A mini-review. Chemosphere 2019, 236, 124404. [Google Scholar] [CrossRef]
- Narahashi, T.; Moore, J.W.; Scott, W. Tetrodotoxin blockage of sodium conductance increase in excitation. J. Gen. Physiol. 1964, 47, 965–974. [Google Scholar] [CrossRef]
- Narahashi, T.; Haas, H.G.; Therrien, E.F. Saxitoxin and tetrodotoxin: Comparison of nerve blocking mechanism. Science 1967, 157, 1438–1440. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.Y. Tetrodotoxin, saxitoxin and their significance in the study of excitation phenomenon. Pharm. Rev. 1966, 18, 997–1049. [Google Scholar]
- European Commission. Commission Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for on the hygiene of foodstuffs. Off. J. Eur. Union 2004, 139, 55–205. [Google Scholar]
- European Commission. Commission Regulation (EC) No 854/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific rules for the organisation of official controls on products of animal origin intended for human consumption. Off. J. Eur. Union 2004, 226, 83–127. [Google Scholar]
- Fernández-Ortega, J.F.; Morales-de los Santos, J.M.; Herrera-Gutiérrez, M.E.; Fernández-Sánchez, V.; Rodríguez, P.; Alfonso, A.; Téllez-Andrade, A. Seafood intoxication by tetrodotoxin: First case in Europe. J. Emerg. Med. 2010, 39, 612–617. [Google Scholar] [CrossRef]
- Rodríguez, P.; Alfonso, A.; Vale, C.; Alfonso, C.; Vale, P.; Tellez, A.; Botana, L.M. First toxicity report of tetrodotoxin and 5,6,11-trideoxyTTX in the trumpet shell Charonia lampas lampas in Europe. Anal. Chem. 2008, 80, 5622–5629. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Azevedo, J.; Rodriguez, P.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. New Gastropod Vectors and Tetrodotoxin Potential Expansion in Temperate Waters of the Atlantic Ocean. Mar. Drugs 2012, 10, 712–726. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.D.; Powell, A.; Schofield, A.; Lees, D.N.; Baker-Austin, C. Detection of the pufferfish toxin Tetrodotoxin in European bivalves, England, 2013 to 2014. Eurosurveillance 2015, 20, 2–8. [Google Scholar] [CrossRef]
- Vlamis, A.; Katikou, P.; Rodriguez, I.; Rey, V.; Alfonso, A.; Papazachariou, A.; Zacharaki, T.; Botana, A.M.; Botana, L.M. First detection of tetrodotoxin in Greek shellfish by UPLC-MS/MS potentially linked to the presence of the dinoflagellate Prorocentrum minimum. Toxins 2015, 7, 1779–1807. [Google Scholar] [CrossRef]
- Gerssen, A.; Bovee, T.H.F.; Klijnstra, M.D.; Poelman, M.; Portier, L.; Hoogenboom, R.L.A.P. First report on the occurrence of tetrodotoxins in bivalve molluscs in the Netherlands. Toxins 2018, 10, 450. [Google Scholar] [CrossRef]
- Bordin, P.; Dall’Ara, S.; Tartaglione, L.; Antonelli, P.; Calfapietra, A.; Varriale, F.; Guiatti, D.; Milandri, A.; Dell’Aversano, C.; Arcangeli, G.; et al. First Occurrence of Tetrodotoxins in Bivalve Mollusks from Northern Adriatic Sea (Italy). Food Control 2021, 120, 107510. [Google Scholar] [CrossRef]
- Hort, V.; Arnich, N.; Guérin, T.; Lavison-Bompard, G.; Nicolas, M. First Detection of Tetrodotoxin in Bivalves and Gastropods from the French Mainland Coasts. Toxins 2020, 12, 599. [Google Scholar] [CrossRef]
- EFSA. Scientific opinion on the risks for public health related to the presence of tetrodotoxin (TTX) and TTX analogues in marine bivalves and gastropods. EFSA J. 2017, 15, 4752. [Google Scholar]
- Leao, J.M.; Lozano-Leon, A.; Giráldez, J.; Vilariño, O.; Gago-Martínez, A. Preliminary results on the evaluation of the occurrence of tetrodotoxin associated to marine Vibrio spp in bivalves from the Galician Rias (Northwest Spain). Mar. Drugs 2018, 16, 81. [Google Scholar] [CrossRef]
- Blanco, L.; Lago, J.; González, V.; Paz, B.; Rambla-Alegre, M.; Cabado, A.G. Occurrence of tetrodotoxin in bivalves and gastropods from harvesting areas and other natural spaces in Spain. Toxins 2019, 11, 331. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Pinto, E.P.; Oliveira, P.; Pedro, S.; Costa, P.R. Evaluation of the occurrence of tetrodotoxin in bivalve mollusks from the Portuguese coast. J. Mar. Sci. Eng. 2019, 7, 232. [Google Scholar] [CrossRef]
- Pinto, E.P.; Rodrigues, S.M.; Gouveia, N.; Timóteo, V.; Costa, P.R. Tetrodotoxin and saxitoxin in two native species of puffer fish, Sphoeroides marmoratus and Lagocephalus lagocephalus, from NE Atlantic Ocean (Madeira Island, Portugal). Mar. Environ. Res. 2019, 151, 104780. [Google Scholar] [CrossRef]
- Silva, M.; Rodríguez, I.; Barreiro, A.; Kaufmann, M.; Neto, A.I.; Hassouani, M.; Sabour, B.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. Tetrodotoxins occurrence in non-traditional vectors of the North Atlantic waters (Portuguese maritime territory, and Morocco coast). Toxins 2019, 11, 306. [Google Scholar] [CrossRef]
- Noguchi, T.; Mahmud, Y. Current methodologies for detection of tetrodotoxin. J. Toxicol. Toxin Rev. 2001, 20, 35–50. [Google Scholar] [CrossRef]
- Kogure, K.; Tampline, M.; Simidu, U.; Colwell, R.R. A tissue culture assay for tetrodotoxin, saxitoxin and related toxins. Toxicon 1988, 26, 191–197. [Google Scholar] [CrossRef]
- Shoji, Y.; Yotsu-Yamashita, M.; Miyazawa, T.; Yasumoto, T. Electrospray ionization mass spectrometry of tetrodotoxin and its analogs: Liquid chromatography/mass spectrometry, tandem mass spectrometry, and liquid chromatography/tandem mass spectrometry. Anal. Biochem. 2001, 290, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Bane, V.; Brosnan, B.; Barnes, P.; Lehane, M.; Furey, A. High-resolution mass spectrometry analysis of tetrodotoxin (TTX) and its analogues in puffer fish and shellfish. Food Addit. Contam. A 2016, 33, 1468–1489. [Google Scholar] [CrossRef]
- Nakagawa, T.; Jang, J.; Yotsu-Yamashita, M. Hydrophilic interaction liquid chromatography-electrospray ionization mass spectrometry of tetrodotoxin and its analogs. Anal. Biochem. 2006, 352, 142–144. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Abe, Y.; Kudo, Y.; Ritson-Williams, R.; Paul, V.; Konoki, K.; Cho, Y.; Adachi, M.; Imazu, T.; Nishikawa, T. First identification of 5, 11-dideoxytetrodotoxin in marine animals, and characterization of major fragment ions of tetrodotoxin and its analogs by high resolution ESI-MS/MS. Mar. Drugs 2013, 11, 2799–2813. [Google Scholar] [CrossRef]
- Boundy, M.J.; Selwood, A.I.; Harwood, D.T.; McNabb, P.S.; Turner, A.D. Development of a sensitive and selective liquid chromatography-mass spectrometry method for high throughput analysis of paralytic shellfish toxins using graphitised carbon solid phase extraction. J. Chromatogr. A 2015, 1387, 1–12. [Google Scholar] [CrossRef]
- European Commission. Commission Decision of 15 March 2002 establishing special health checks for the harvesting and processing of certain bivalve mollusks with a level of amnesic shellfish poison (ASP) exceeding the limit laid down by Council Directive 91/492/EEC. Off. J. Eur. Comm. 2002, L 75, 65–66. [Google Scholar]
- Morton, B. Foregut anatomy and predation by Charonia lampas (gastropoda: Prosobranchia: Neotaenioglossa) attacking Ophidiaster ophidianus (asteroidea: Ophidiasteridae) in the Azores, with a review of triton feeding behavior. J. Nat. Hist. 2012, 46, 2621–2637. [Google Scholar] [CrossRef]
- Narita, H.; Matsubara, S.; Miwa, N.; Akahane, S.; Murakami, M.; Goto, T.; Nava, M.; Noguchi, T.; Saito, T.; Shida, Y.; et al. Vibrio alginolyticus, a TTX-producing bacterium isolated from the starfish Astropecten polyacanthus. Nippon Suisan Gakkaishi 1987, 53, 617–621. [Google Scholar] [CrossRef]
- Lin, S.-J.; Hwang, D.-F. Possible source of tetrodotoxin in the starfiish Astropecten scoparius. Toxicon 2001, 39, 573–579. [Google Scholar] [CrossRef]
- Costa, P.R.; Costa, S.T.; Braga, A.C.; Rodrigues, S.M.; Vale, P. Relevance and challenges in monitoring marine biotoxins in non-bivalve vectors. Food Control 2017, 76, 24–33. [Google Scholar] [CrossRef]
- EULRMB. Determination of Tetrodontoxin byHILIC-MS/MS, European Reference Laboratory for Marine Biotoxins, Ed 01062107. 2017. Available online: http://www.aecosan.msssi.gob.es/en/CRLMB/docs/docs/metodos_analiticos_de_desarrollo/HILIC-LCMSMS_SOP_for_TTX_in_mussels.pdf (accessed on 12 January 2021).
- Turner, A.D.; Dhanji-Rapkova, M.; Coates, L.; Bickerstaff, L.; Milligan, S.; O’Neill, A.; Faulkner, D.; McEneny, H.; Baker-Austin, C.; Lees, D.N.; et al. Detection of tetrodotoxin shellfish poisoning (tsp) toxins and causative factors in bivalve molluscs from the UK. Mar. Drugs 2017, 15, 277. [Google Scholar] [CrossRef]
- Manger, R.L.; Leja, L.S.; Lee, S.Y.; Hungerford, J.M.; Wekell, M.M. Tetrazolium-based cell bioassay for neurotoxins active on voltage-sensitive sodium channels: Semiautomated assay for saxitoxins, brevetoxins and ciguatoxins. Anal. Biochem. 1993, 214, 190–194. [Google Scholar] [CrossRef]
- Manger, R.L.; Leja, L.S.; Lee, S.Y.; Hungerford, J.M.; Hokama, Y.; Dickey, R.W.; Granade, H.R.; Lewis, R.; Yasumoto, T.; Wekell, M.M. Detection of sodium channel toxins: Directed cytotoxicity assays for purified ciguatoxins, brevetoxins, saxitoxins and seafood extracts. J. AOAC Int. 1995, 78, 521–527. [Google Scholar] [CrossRef]
| Sample | Toxins Concentration (µg kg−1) | |
|---|---|---|
| TTX | 4,9-anhydroTTX | |
| Edible muscle | ||
| Cl-1 | 15.0 | 35.4 |
| Cl-2 | <LOD | <LOD |
| Cl-3 | 31.3 | 88.0 |
| Non edible viscera | ||
| Cl-1 | 10,961.4 | 12,652.0 |
| Cl-2 | 8.5 | <LOD |
| Cl-3 | 42,163.0 | 56,325.5 |
| Charonia lampas (Cl) | Length (cm) | Weight of Soft Tissue (g) |
|---|---|---|
| Cl-1 | 21.5 | 169.0 |
| Cl-2 | 27.1 | 213.2 |
| Cl-3 | 25.2 | 191.3 |
| Compound | Precursor Ion | Product Ions | |
|---|---|---|---|
| TTX and 4-epi TTX | 320.1 | 302.1 | 162.1 |
| 11-deoxyTTX and 5-deoxy TTX | 304.1 | 286.1 | 162.1 |
| 4,9-anhydro TTX | 302.1 | 284.1 | 162.1 |
| 6, 11-dideoxy TTX | 288.1 | 270.1 | 162.1 |
| 5,6,11- trideoxy TTX | 272.1 | 254.1 | 162.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, P.R.; Giráldez, J.; Rodrigues, S.M.; Leão, J.M.; Pinto, E.; Soliño, L.; Gago-Martínez, A. High Levels of Tetrodotoxin (TTX) in Trumpet Shell Charonia lampas from the Portuguese Coast. Toxins 2021, 13, 250. https://doi.org/10.3390/toxins13040250
Costa PR, Giráldez J, Rodrigues SM, Leão JM, Pinto E, Soliño L, Gago-Martínez A. High Levels of Tetrodotoxin (TTX) in Trumpet Shell Charonia lampas from the Portuguese Coast. Toxins. 2021; 13(4):250. https://doi.org/10.3390/toxins13040250
Chicago/Turabian StyleCosta, Pedro Reis, Jorge Giráldez, Susana Margarida Rodrigues, José Manuel Leão, Estefanía Pinto, Lucía Soliño, and Ana Gago-Martínez. 2021. "High Levels of Tetrodotoxin (TTX) in Trumpet Shell Charonia lampas from the Portuguese Coast" Toxins 13, no. 4: 250. https://doi.org/10.3390/toxins13040250
APA StyleCosta, P. R., Giráldez, J., Rodrigues, S. M., Leão, J. M., Pinto, E., Soliño, L., & Gago-Martínez, A. (2021). High Levels of Tetrodotoxin (TTX) in Trumpet Shell Charonia lampas from the Portuguese Coast. Toxins, 13(4), 250. https://doi.org/10.3390/toxins13040250








