Venom Use in Eulipotyphlans: An Evolutionary and Ecological Approach
Abstract
1. Introduction
1.1. Natural Toxins
1.2. Venom Definition
1.3. Venomous Mammals
1.4. Purpose of This Review
2. Extant Venomous Eulipotyphlans
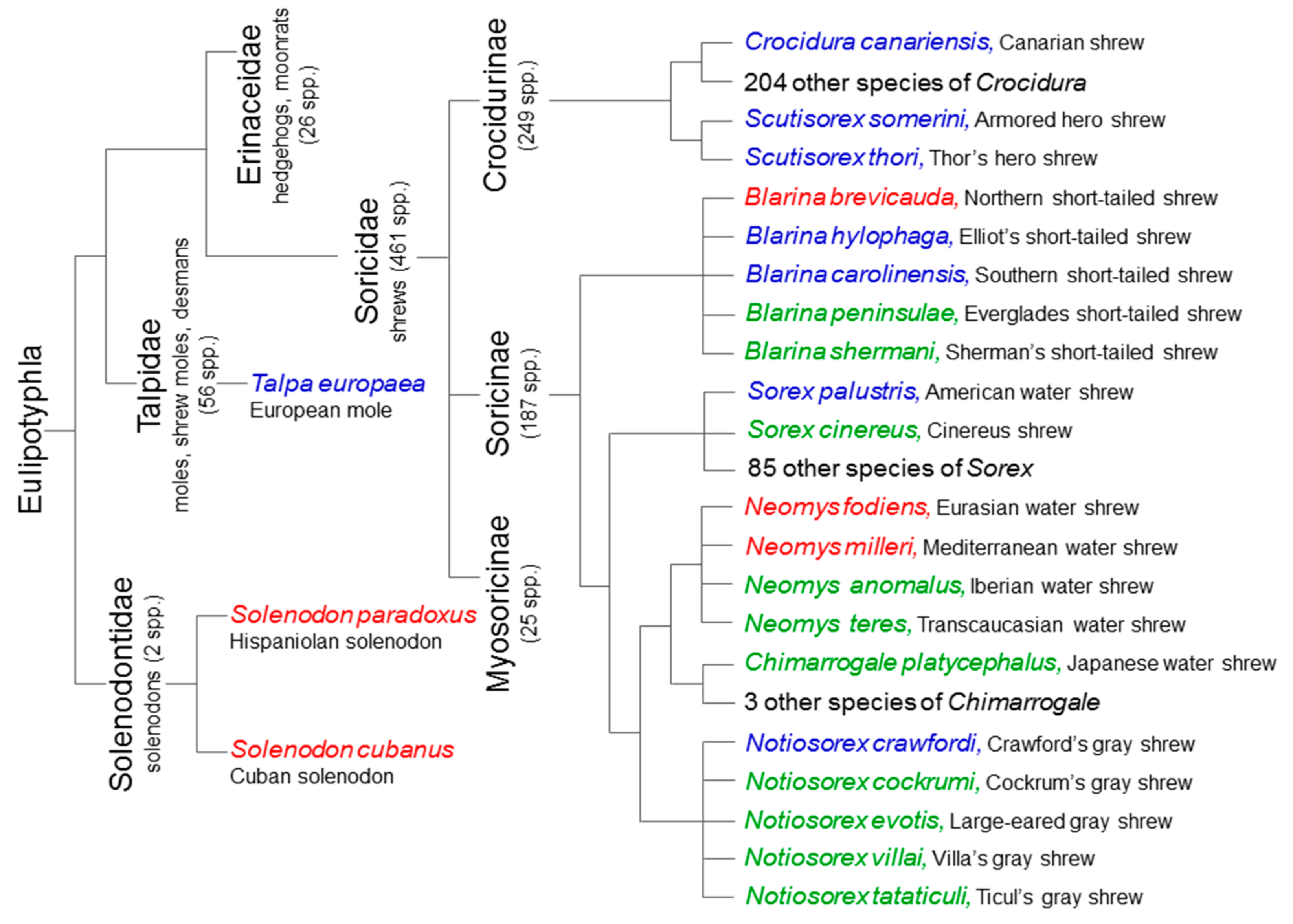
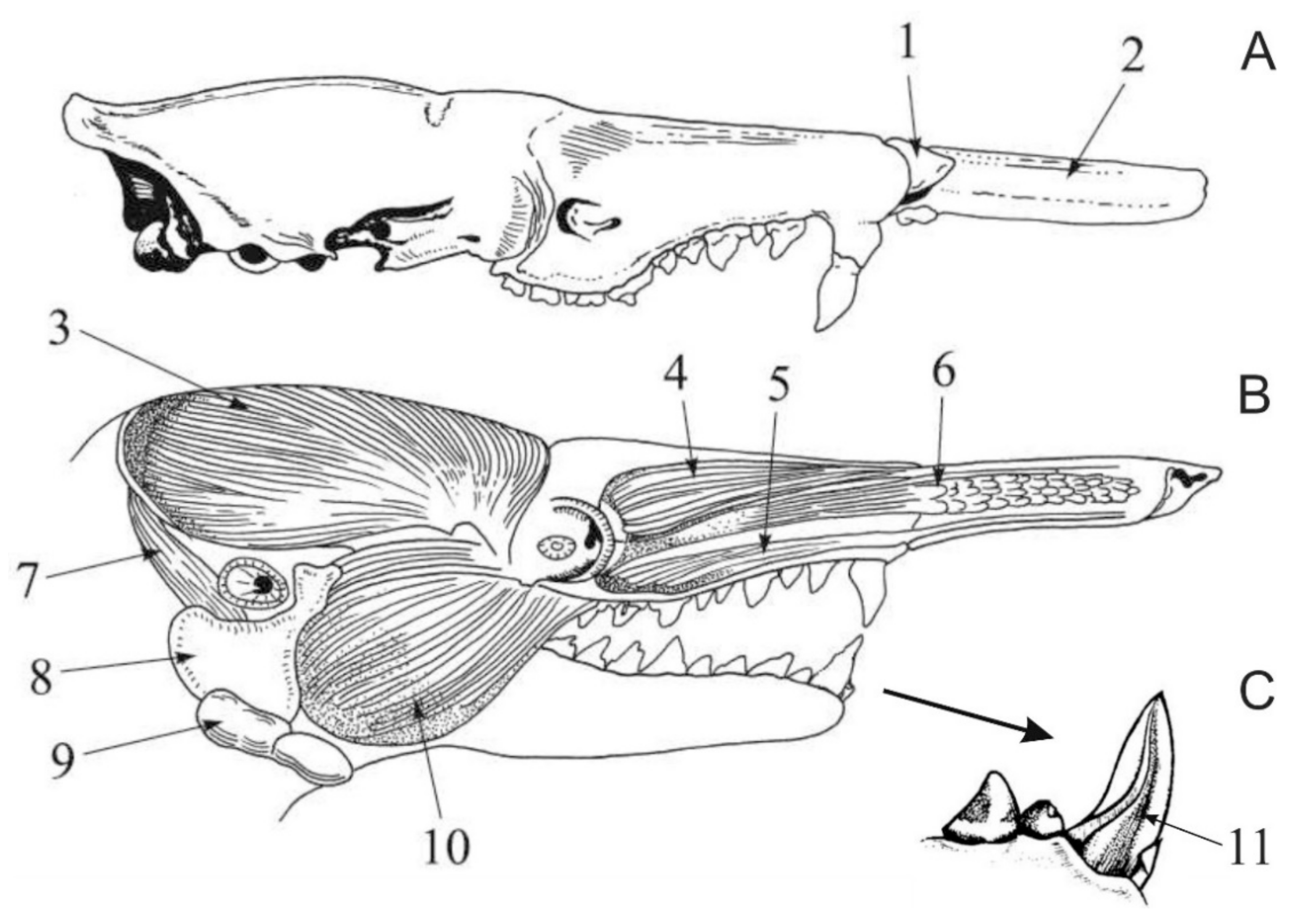
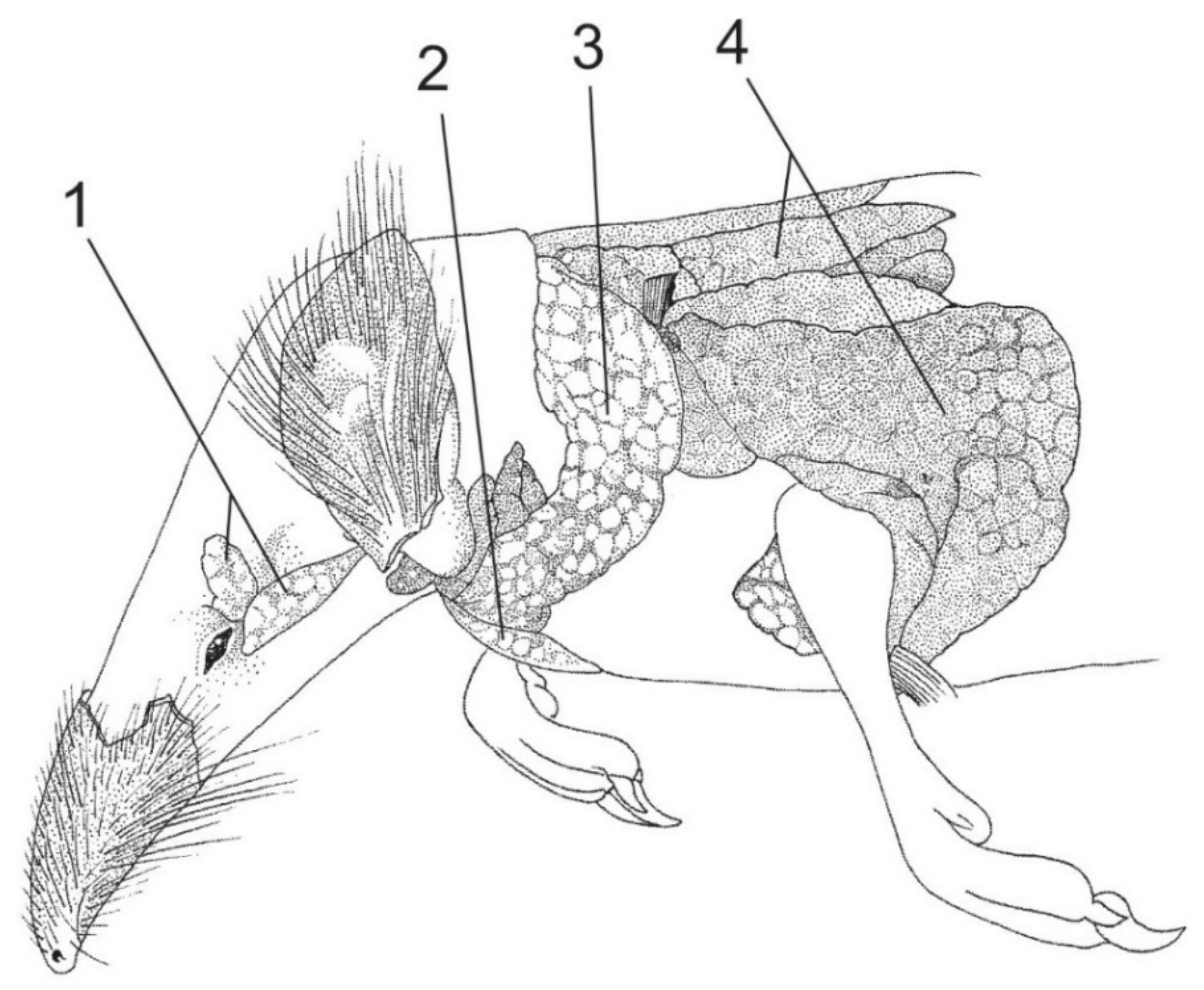
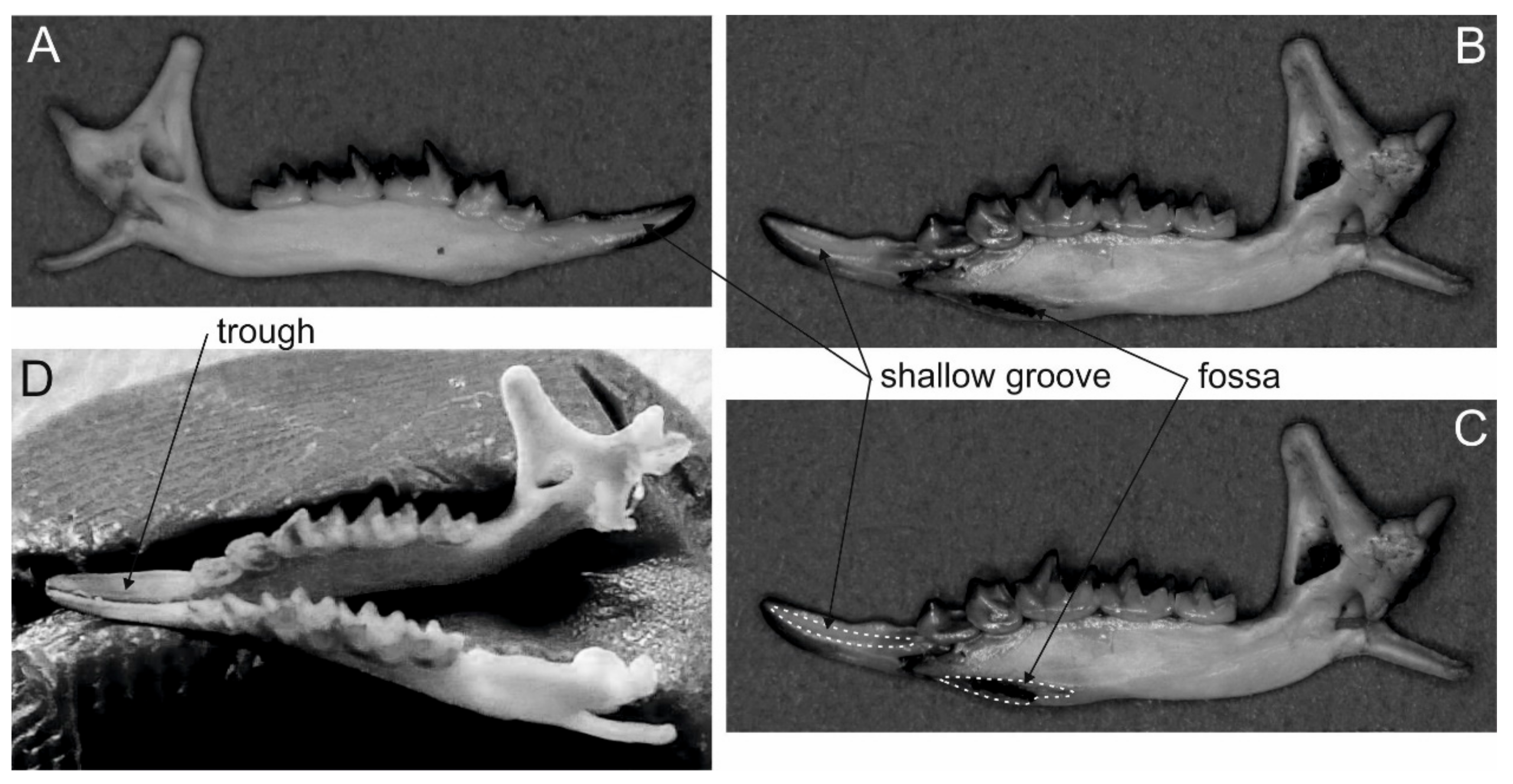
3. Extinct Venomous Eulipotyphlans
4. Toxicity of the Eulipotyphlan Venom
5. Biochemistry of the Eulipotyphlan Venom
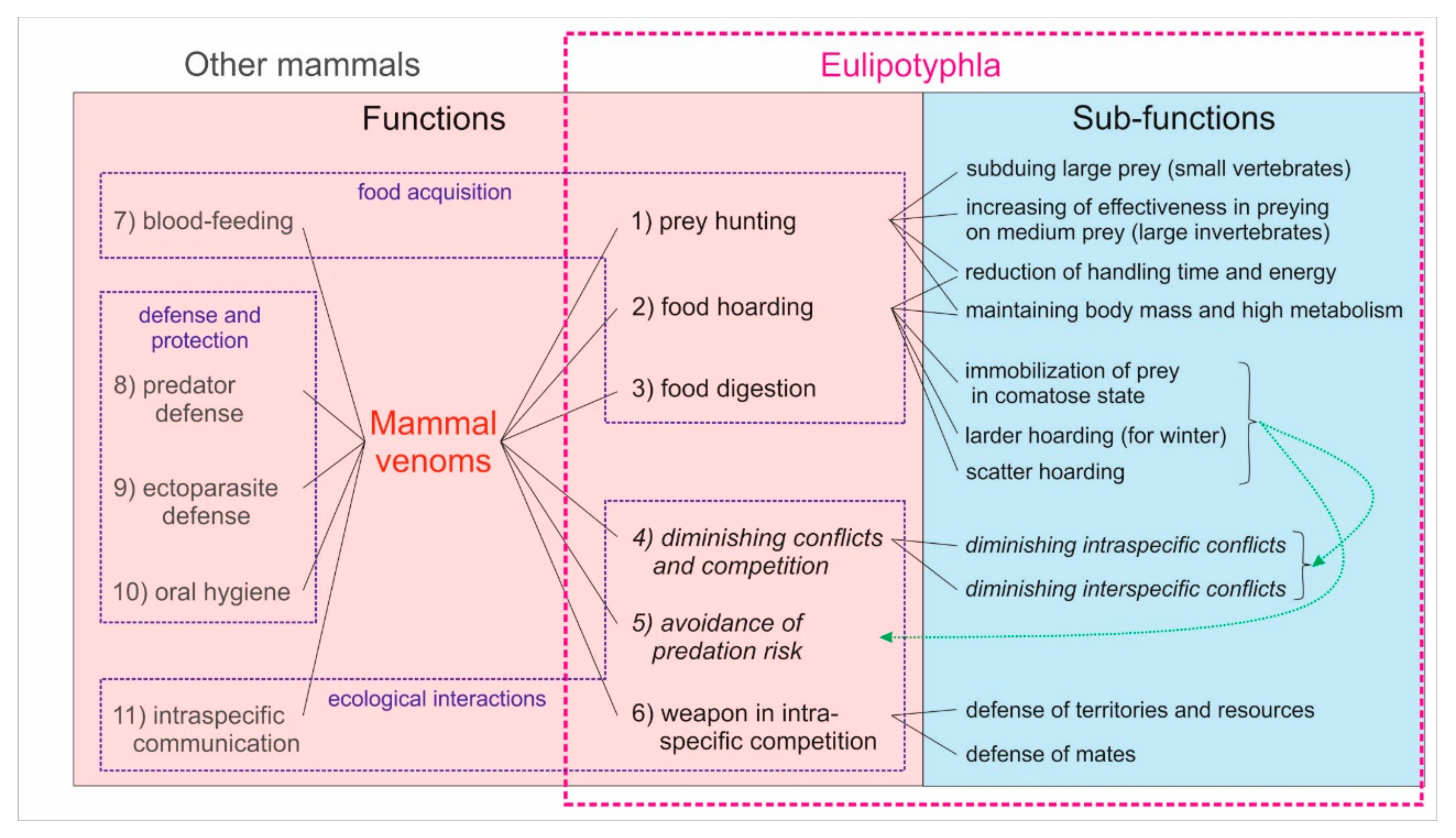
6. Ecological Functions of the Eulipotyphlan Venom
7. Why Are So Few Eulipotyphlans Venomous?
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calvete, J.J.; Juárez, P.; Sanz, L. Snake venomics. Strategy and applications. J. Mass Spectrom. 2007, 42, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Sanz, L.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M. Venoms, venomics, antivenomics. FEBS Lett. 2009, 583, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Escoubas, P.; King, G.F. Venomics as a drug discovery platform. Expert Rev. Proteom. 2009, 6, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Vetter, I.; Davis, J.L.; Rash, L.D.; Anangi, R.; Mobli, M.; Alewood, P.F.; Lewis, R.J.; King, G.F. Venomics: A new paradigm for natural products-based drug discovery. Amino Acids 2011, 40, 15–28. [Google Scholar] [CrossRef]
- Sunagar, K.; Morgenstern, D.; Reitzel, A.M.; Moran, Y. Ecological venomics: How genomics, transcriptomics and proteomics can shed new light on the ecology and evolution of venom. J. Proteom. 2016, 135, 62–72. [Google Scholar] [CrossRef]
- Ménez, A.; Bontems, F.; Roumestand, C.; Gilquin, B.; Toma, F. Structural basis for functional diversity of animal toxins. Proc. R. Soc. Edinb. Sect. B 1992, 99, 83–103. [Google Scholar] [CrossRef]
- Von Reumont, B.M.; Campbell, L.I.; Jenner, R.A. Quo vadis venomics? A road map to neglected venomous invertebrates. Toxins 2014, 6, 3488–3551. [Google Scholar] [CrossRef]
- King, G.F. Venoms as a platform for human drugs: Translating toxins into therapeutics. Expert Opin. Biol. Ther. 2011, 11, 1469–1484. [Google Scholar] [CrossRef]
- Takacs, Z.; Nathan, S. Animal venoms in medicine. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Elsevier Inc.; Academic Press: Waltham, MA, USA, 2014; Volume 1, pp. 252–259. [Google Scholar]
- Clark, G.C.; Casewell, N.R.; Elliott, C.T.; Harvey, A.L.; Jamieson, A.G.; Strong, P.N.; Turner, A.D. Friends or foes? Emerging impacts of biological toxins. Trends Biochem. Sci. 2019, 44, 365–379. [Google Scholar] [CrossRef]
- Holland, J.S. Jad który leczy [Venom that heals]. Natl. Geogr. Poland 2013, 2, 70–85. (In Polish) [Google Scholar]
- Brown, G.V.; Warrell, D.A. Venomous bites and stings in the tropical world. Med. J. Aust. 1993, 159, 773–779. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Lomonte, B.; León, G.; Alape-Girón, A.; Flores-Díaz, M.; Sanz, L.; Angulo, Y.; Calvete, J.J. Snake venomics and antivenomics: Proteomic tools in the design and control of antivenoms for the treatment of snakebite envenoming. J. Proteom. 2009, 72, 165–182. [Google Scholar] [CrossRef]
- Warrell, D.A. Snake bite. Lancet 2010, 375, 77–88. [Google Scholar] [CrossRef]
- Grow, N.B.; Wirdateti; Nekaris, K.A.I. Does toxic defence in Nycticebus ssp. relate to ectoparasites? The lethal effects of slow loris venom on arthropods. Toxicon 2015, 95, 1–5. [Google Scholar] [CrossRef]
- Rode-Margono, J.E.; Nekaris, K.A.I. Cabinet of curiosities: Venom systems and their ecological function in mammals, with a focus on primates. Toxins 2015, 7, 2639–2658. [Google Scholar] [CrossRef]
- Kowalski, K.; Rychlik, L. The role of venom in the hunting and hoarding of prey differing in body size by the Eurasian water shrew, Neomys fodiens. J. Mammal. 2018, 99, 351–362. [Google Scholar] [CrossRef]
- Nelsen, D.R.; Nisani, Z.; Cooper, A.M.; Fox, G.A.; Gren, E.C.K.; Corbit, A.G.; Hayes, W.K. Poisons, toxungens, and venoms: Redefining and classifying toxic biological secretions and the organisms that employ them. Biol. Rev. 2014, 89, 450–465. [Google Scholar] [CrossRef]
- Bücherl, W. Introduction. In Venomous Animals and Their Venoms; Bücherl, W., Buckley, E.E., Deulofeu, V., Eds.; Academic Press: New York, NY, USA, 1968; pp. ix–xii. [Google Scholar]
- Mebs, D. Venomous and Poisonous Animals; Medpharm: Stuttgart, Germany, 2002. [Google Scholar]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S.; et al. The toxicogenomic multiverse: Convergent recruitment of proteins into animal venoms. Annu. Rev. Genom. Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef]
- Basanova, A.V.; Baskova, I.P.; Zavalova, L.L. Vascular-platelet and plasma hemostasis regulators from bloodsucking animals. Biochemistry (Moscow) 2002, 67, 143–150. [Google Scholar] [CrossRef]
- Pournelle, G.H. Classification, biology, and description of the venom apparatus of insectivores of the genera Solenodon, Neomys, and Blarina. In Venomous Animals and Their Venoms; Bücherl, W., Buckley, E.E., Deulofeu, V., Eds.; Academic Press: New York, NY, USA, 1968; pp. 31–42. [Google Scholar]
- Fox, R.C.; Scott, C.S. First evidence of a venom delivery apparatus in extinct mammals. Nature 2005, 435, 1091–1093. [Google Scholar] [CrossRef]
- Cuenca-Bescós, G.; Rofes, J. First evidence of poisonous shrews with an envenomation apparatus. Die Nat. 2007, 94, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Peigné, S.; Chaimanee, Y.; Yamee, C.; Marandat, B.; Srisuk, P.; Jaeger, J.-J. An astonishing example of convergent evolution toward carnivory: Siamosorex debonisi n. gen., n. sp. (Mammalia, Lipotyphla, Soricomorpha, Plesiosoricidae) from the latest Oligocene of Thailand. Geodiversitas 2009, 31, 973–992. [Google Scholar] [CrossRef]
- Rofes, J.; Cuenca-Bescós, G. First record of Beremendia fissidens (Mammalia, Soricidae) in the Pleistocene of the Iberian Peninsula, with a review of the biostratigraphy, biogeography and palaeoecology of the species. Compt. Rend. Palevol. 2009, 8, 21–37. [Google Scholar] [CrossRef]
- Rofes, J.; Cuenca-Bescós, G. A new genus of red-toothed shrew (Mammalia, Soricidae) from the Early Pleistocene of Gran Dolina (Atapuerca, Burgos, Spain), and a phylogenetic approach to the Eurasiatic Soricinae. Zool. J. Linn. Soc. 2009, 155, 904–925. [Google Scholar] [CrossRef]
- Furió, M.; Agustí, J.; Mouskhelishvili, A.; Sanisidro, Ó.; Santos-Cubedo, A. The paleobiology of the extinct venomous shrew Beremendia (Soricidae, Insectivora, Mammalia) in relation to the geology and paleoenvironment of Dmanisi (Early Pleistocene, Georgia). J. Vertebr. Paleontol. 2010, 30, 928–942. [Google Scholar] [CrossRef]
- Kita, M.; Nakamura, Y.; Okumura, Y.; Ohdachi, S.D.; Oba, Y.; Yoshikuni, M.; Kido, H.; Uemura, D. Blarina toxin, a mammalian lethal venom from the short-tailed shrew Blarina brevicauda: Isolation and characterization. Proc. Natl. Acad. Sci. USA 2004, 101, 7542–7547. [Google Scholar] [CrossRef]
- Aminetzach, Y.T.; Srouji, J.R.; Kong, C.Y.; Hoekstra, H.E. Convergent evolution of novel protein function in shrew and lizard venom. Curr. Biol. 2009, 19, 1925–1931. [Google Scholar] [CrossRef]
- Whittington, C.M.; Koh, J.M.S.; Warren, W.C.; Papenfuss, A.T.; Torres, A.M.; Kuchel, P.W.; Belov, K. Understanding and utilising mammalian venom via a platypus venom transcriptome. J. Proteom. 2009, 72, 155–164. [Google Scholar] [CrossRef]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef]
- Dufton, M.J. Venomous mammals. Pharmacol. Ther. 1992, 53, 199–215. [Google Scholar] [CrossRef]
- Ligabue-Braun, R.; Verli, H.; Carlini, C.R. Venomous mammals: A review. Toxicon 2012, 59, 680–695. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, K.; Rychlik, L. Jadowite ssaki [Venomous mammals]. Kosmos 2014, 63, 643–655, (In Polish with English summary). [Google Scholar]
- O’Brien, S.J. The platypus genome unraveled. Cell 2008, 133, 9–11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Whittington, C.M.; Papenfuss, A.T.; Locke, D.P.; Mardis, E.R.; Wilso, R.K.; Abubucker, S.; Mitreva, M.; Wong, E.S.W.; Hsu, A.L.; Kuchel, P.W.; et al. Novel venom gene discovery in the platypus. Genome Biol. 2010, 11, R95. [Google Scholar] [CrossRef] [PubMed]
- Kita, M. Bioorganic studies on the venom from duckbill platypus. Pure Appl. Chem. 2012, 84, 1317–1328. [Google Scholar] [CrossRef]
- Whittington, C.M.; Belov, K. Platypus venom: A review. Aust. Mammal. 2007, 29, 57–62. [Google Scholar] [CrossRef]
- Whittington, C.M.; Belov, K. Tracing monotreme venom evolution in the genomics era. Toxins 2014, 6, 1260–1273. [Google Scholar] [CrossRef]
- Pucek, M. Jadowitość wśród ssaków [Venomousness in mammals]. Prz. Zool. 1959, 3, 106–115. (In Polish) [Google Scholar]
- Kowalski, K.; Marciniak, P.; Rosiński, G.; Rychlik, L. Evaluation of the physiological activity of venom from the Eurasian water shrew Neomys fodiens. Front. Zool. 2017, 14, 46. [Google Scholar] [CrossRef]
- Casewell, N.R.; Petras, D.; Cardd, D.C.; Suranse, V.; Mychajliw, A.M.; Richards, D.; Koludarov, I.; Albulescu, L.O.; Slagboom, J.; Hempel, B.F.; et al. Solenodon genome reveals convergent evolution of venom in eulipotyphlan mammals. Proc. Natl. Acad. Sci. USA 2019, 116, 25745–25755. [Google Scholar] [CrossRef]
- Hanf, Z.R.; Chavez, A.S. A comprehensive multi-omic approach reveals a relatively simple venom in a diet generalist, the northern short-tailed shrew, Blarina brevicauda. Genome Biol. Evol. 2020, evaa115. [Google Scholar] [CrossRef]
- Delpietro, H.A.; Russo, R.G. Acquired resistance to saliva anticoagulants by prey previously fed upon by vampire bats (Desmodus rotundus): Evidence for immune response. J. Mammal. 2009, 90, 1132–1138. [Google Scholar] [CrossRef]
- Tellgren-Roth, A.; Dittmar, K.; Massey, S.E.; Kemi, C.; Tellgren-Roth, C.; Savolainen, P.; Lyons, L.A.; Liberles, D.A. Keeping the blood flowing–plasminogen activator genes and feeding behavior in vampire bats. Naturwissenschaften 2009, 96, 39–47. [Google Scholar] [CrossRef]
- Low, D.H.W.; Sunagar, K.; Undheim, E.A.B.; Ali, S.A.; Alagon, A.C.; Ruder, T.; Jackson, T.N.W.; Pineda Gonzales, S.; King, G.F.; Jones, A.; et al. Dracula’s children: Molecular evolution of vampire bat venom. J. Proteom. 2013, 89, 95–111. [Google Scholar] [CrossRef]
- Kakumanu, R.; Hodgson, W.C.; Ravi, R.; Alagon, A.; Harris, R.J.; Brust, A.; Alewood, P.F.; Kemp-Harper, B.K.; Fry, B.G. Vampire venom: Vasodilatory mechanisms of vampire bat (Desmodus rotundus) blood feeding. Toxins 2019, 11, 26. [Google Scholar] [CrossRef]
- Nekaris, K.A.I.; Moore, R.S.; Rode, E.J.; Fry, B.G. Mad, bad and dangerous to know: The biochemistry, ecology and evolution of slow loris venom. J. Venom Anim. Toxins Incl. Trop. Dis. 2013, 19, 21. [Google Scholar] [CrossRef]
- Nekaris, K.A.I.; Weldon, A.; Imron, M.A.; Maynard, K.Q.; Nijman, V.; Poindexter, S.A.; Queiroz Morcatty, T. Venom in furs: Facial masks as aposematic signals in a venomous mammal. Toxins 2019, 11, 93. [Google Scholar] [CrossRef]
- Scheib, H.; Nekaris, K.A.I.; Rode-Margono, J.; Ragnarsson, L.; Baumann, K.; Dobson, J.S.; Wirdateti, W.; Nouwens, A.; Nijman, V.; Martelli, P.; et al. The toxicological intersection between allergen and toxin: A structural comparison of the cat dander allergenic protein Fel d1 and the slow loris brachial gland secretion protein. Toxins 2020, 12, 86. [Google Scholar] [CrossRef]
- Nekaris, K.A.I.; Campera, M.; Nijman, V.; Birot, H.; Rode-Margono, E.J.; Fry, B.G.; Weldon, A.; Wirdateti, W.; Imron, M.A. Slow lorises use venom as weapon in intraspecific competition. Curr. Biol. 2020, 30, R1252–R1253. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Salamin, N.; Ohdachi, S.D.; Barrière, P.; Vogel, P. Molecular phylogenetics of shrews (Mammalia: Soricidae) reveal timing of transcontinental colonizations. Mol. Phylogenet. Evol. 2007, 44, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Mebs, D. Studies on biological and enzymatic activities of salivary glands from the European hedgehog (Erinaceus europaeus). Toxicon 1999, 37, 1635–1638. [Google Scholar] [CrossRef]
- Uemura, D.; Kita, M.; Arimoto, H.; Kitamura, M. Recent aspects of chemical ecology: Natural toxins, coral communities, and symbiotic relationships. Pure Appl. Chem. 2009, 81, 1093–1111. [Google Scholar] [CrossRef]
- Kita, M. Bioorganic studies on the key natural products from venomous mammals and marine invertebrates. Bull. Chem. Soc. Jpn. 2012, 85, 1175–1185. [Google Scholar] [CrossRef]
- Folinsbee, K.E. Evolution of venom across extant and extinct eulipotyphlans. Compt. Rend. Palevol. 2013, 12, 531–542. [Google Scholar] [CrossRef]
- Pearson, O.P. The submaxillary glands of shrews. Anat. Record 1950, 107, 161–165. [Google Scholar] [CrossRef]
- Pearson, O.P. A toxic substance from the salivary glands of a mammal (short-tailed shrew). In Venoms; Buckley, E.E., Porges, N., Eds.; American Association for the Advancement of Science: Washington, DC, USA, 1956; pp. 55–58. [Google Scholar]
- Turvey, S.T. Evolution of non-homologous venom delivery systems in West Indian Insectivores? J. Vertebr. Paleontol. 2010, 30, 1294–1299. [Google Scholar] [CrossRef]
- Rabb, G.B. Toxic salivary glands in the primitive insectivore Solenodon. Nat. Hist. Miscellanea 1959, 190, 1–3. [Google Scholar]
- Pucek, M. The effect of the venom of the European water shrew (Neomys fodiens fodiens Pennant) on certain experimental animals. Acta Theriol. 1959, 3, 93–108. [Google Scholar] [CrossRef]
- Pucek, M. Neomys anomalus Cabrera, 1907–a venomous mammal. Bull. De L’Académie Pol. Des Sci. 1969, 17, 569–573. [Google Scholar]
- Tomasi, E.T. Function of venom in the short-tailed shrew, Blarina brevicauda. J. Mammal. 1978, 59, 852–854. [Google Scholar] [CrossRef]
- Martin, I.G. Venom of the short-tailed shrew (Blarina brevicauda) as an insect immobilizing agent. J. Mammal. 1981, 62, 189–192. [Google Scholar] [CrossRef]
- Tapisso, J.T. How Historical and Present Climate Conditions Affected the Distribution of the Mediterranean Water Shrew? A Phylogeographical and Ecological Approach. Ph.D. Thesis, University of Lisbon, Lisbon, Portugal, 2014; p. 207. [Google Scholar]
- Igea, J.; Aymerich, P.; Bannikova, A.A.; Gosálbez, J.; Castresana, J. Multilocus species trees and species delimitation in a temporal context: Application to the water shrews of the genus Neomys. BMC Evol. Biol. 2015, 15, 209. [Google Scholar] [CrossRef]
- Querejeta, M.; Castresana, J. Evolutionary history of the endemic water shrew Neomys anomalus: Recurrent phylogeographic patterns in semi-aquatic mammals of the Iberian Peninsula. Ecol. Evol. 2018, 8, 10138–10146. [Google Scholar] [CrossRef]
- Punzo, F. Behavioral interactions between the shrew Blarina carolinensis and four species of Florida mice in the presence and absence of mouse nestlings. Fla. Sci. 2003, 66, 63–67. [Google Scholar]
- Laerm, J.; Ford, W.M.; Chapman, B.R. Southern short-tailed shrew Blarina carolinensis (Bachman, 1837). In The Land Manager’s Guide to Mammals of the South; Trani, M.K., Ford, W.M., Chapman, B.R., Eds.; USDA Forest Service & The Nature Conservancy, Southeastern Region: Durham, NC, USA, 2007; pp. 70–74. [Google Scholar]
- Hofmann, J.E. Field Manual of Illinois Mammals; Illinois Natural History Survey: Champaign, IL, USA, 2008. [Google Scholar]
- Thompson, C.W.; Choate, J.R.; Genoways, H.H.; Finck, E.I. Blarina hylophaga (Soricomorpha: Soricidae). Mammal. Spec. 2011, 43, 94–103. [Google Scholar] [CrossRef]
- Lopez-Jurado, L.F.; Mateo, J.A. Evidence of venom in the Canarian shrew (Crocidura canariensis): Immobilizing effects on the Atlantic lizard (Gallotia atlantica). J. Zool. 1996, 239, 394–395. [Google Scholar] [CrossRef]
- Nussbaum, R.A.; Maser, C. Observations of Sorex palustris preying on Dicamptodon emsatus. Murrelet 1969, 50, 23–24. [Google Scholar]
- Kingdon, J. The Kingdon Field Guide to African Mammals, 2nd ed.; Bloomsbury Wildlife: London, UK, 2015. [Google Scholar]
- Camargo, I.; Álvarez-Castañeda, S.T. Analyses of predation behavior of the desert shrew Notiosorex crawfordi. Mammalia 2019, 83, 276–280. [Google Scholar] [CrossRef]
- Rychlik, L. Rząd: Owadożery–Eulipotyphla [Order: Eulipotyphla]. In Zoologia, tom 3, część 3. Ssaki; Błaszak, C., Ed.; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2020; pp. 192–237. (In Polish) [Google Scholar]
- Evans, A.C. The identity of earthworms stored by moles. Proc. Zool. Soc. 1948, 118, 356–359. [Google Scholar] [CrossRef]
- Macdonald, D.W. Predation on earthworms by terrestrial vertebrates. In Earthworm Ecology; Satchell, J.E., Ed.; Springer: Dordrecht, The Netherlands, 1983; pp. 393–414. [Google Scholar]
- Kowalski, K.; Duk, K. Kopalne ssaki jadowite z nadrzędu Eulipotyphla [The venomous extinct eulipotyphlans]. Kosmos 2016, 65, 93–102, (In Polish with English summary). [Google Scholar]
- Jin, C.-Z.; Sun, C.-K.; Zhang, Y.-Q. Lunanosorex (Insectivora, Mammalia) from the Pliocene of north China. Vert. PalAs 2007, 45, 74–88. [Google Scholar]
- McDowell, S.B., Jr. The Greater Antillean insectivores. Bull. Am. Mus. Nat. Hist. 1958, 115, 117–214. [Google Scholar]
- Folinsbee, K.E.; Müller, J.; Reisz, R.R. Canine grooves: Morphology, function, and relevance to venom. J. Vertebr. Paleontol. 2007, 27, 547–551. [Google Scholar] [CrossRef]
- Bennàsar, M.; Cáceres, I.; Cuenca-Bescós, G.; Huguet, R.; Blain, H.-A.; Rofes, J. Exceptional biting capacities of the Early Pleistocene fossil shrew Beremendia fissidens (Soricidae, Eulipotyphla, Mammalia): New taphonomic evidence. Hist. Biol. 2015, 27, 978–986. [Google Scholar] [CrossRef]
- Hurum, J.H.; Luo, Z.X.; Kielan-Jaworowska, Z. Were mammals originally venomous? Acta Palaeontol. Pol. 2006, 51, 1–11. [Google Scholar]
- Kielan-Jaworowska, Z. W Poszukiwaniu Wczesnych SSAKÓW. Ssaki ery Dinozaurów [In Search of Early Mammals. Mammals of the Dinosaur Era]; Wydawnictwa Uniwersytetu Warszawskiego: Warszawa, Poland, 2013. (In Polish) [Google Scholar]
- Orr, C.M.; Delezene, L.K.; Scott, J.E.; Tocheri, M.W.; Schwartz, G.T. The comparative method and the inference of venom-delivery systems in fossil mammals. J. Vertebr. Paleontol. 2007, 27, 541–546. [Google Scholar] [CrossRef]
- Pearson, O.P. On the cause and nature of a poisonous action produced by the bite of a shrew (Blarina brevicauda). J. Mammal. 1942, 23, 159–166. [Google Scholar] [CrossRef]
- Ellis, S.; Krayer, O. Properties of a toxin from the salivary gland of the shrew Blarina brevicauda. J. Pharmac. Exp. Ther. 1955, 114, 127–137. [Google Scholar]
- Pucek, M. Die toxische Wirkung der Glandulae submaxillares bei Neomys fodiens fodiens Schreb. Bull. De L’Académie Pol. Des Sci. 1957, 5, 301–306. [Google Scholar]
- Pucek, M. Chemistry and pharmacology of insectivore venoms. In Venomous Animals and Their Venoms; Bücherl, W., Buckley, E.E., Deulofeu, V., Eds.; Academic Press: New York, NY, USA, 1968; pp. 43–50. [Google Scholar]
- DeMeules, D.H. Possible anti-adrenalin action of shrew venom. J. Mammal. 1954, 35, 425. [Google Scholar] [CrossRef]
- Kowalski, K.; Marciniak, P.; Rosiński, G.; Rychlik, L. Toxic activity and protein identification from the parotoid gland secretion of the common toad Bufo bufo. Comp. Biochem. Physiol. C 2018, 205, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Adamski, Z.; Bufo, S.A.; Chowański, S.; Falabella, P.; Lubawy, J.; Marciniak, P.; Pacholska- Bogalska, J.; Salvia, R.; Scrano, L.; Słocińska, M.; et al. Beetles as model organisms in physiological, biomedical and environmental studies–a review. Front. Physiol. 2019, 10, 319. [Google Scholar] [CrossRef] [PubMed]
- The Earl of Cranbrook. The feeding habits of the water shrew, Neomys fodiens bicolor Shaw, in captivity and the effect of its attack upon its prey. Proc. Zool. Soc. Lond. 1959, 133, 245–249. [Google Scholar] [CrossRef]
- Buchalczyk, T.; Pucek, Z. Food storage of the European water shrew, Neomys fodiens (Pennant, 1771). Acta Theriol. 1963, 19, 376–379. [Google Scholar] [CrossRef]
- Wołk, K. The winter food of the European water shrew. Acta Theriol. 1976, 21, 117–129. [Google Scholar] [CrossRef]
- Churchfield, S. The feeding ecology of the European water shrew. Mammal. Rev. 1985, 15, 13–21. [Google Scholar] [CrossRef]
- Castién, E. The diet of Neomys fodiens in the Spanish western Pyrenees. Folia Zool. 1995, 44, 297–303. [Google Scholar]
- Haberl, W. Food storage, prey remains and notes on occasional vertebrates in the diet of the Eurasian water shrew, Neomys fodiens. Folia Zool. 2002, 51, 93–102. [Google Scholar]
- Churchfield, S.; Rychlik, L. Diets and coexistence in Neomys and Sorex shrews in Białowieża forest, eastern Poland. J. Zool. 2006, 269, 381–390. [Google Scholar] [CrossRef]
- Varona, L.S. Remarks on the biology and zoogeography of Solenodon (Atopogale) cubanus Peters, 1861 (Mammalia, Insectivora). Bijdr. Dierkd. 1983, 53, 93–98. [Google Scholar] [CrossRef]
- Michalak, I. Reproduction and behaviour of the Mediterranean water shrew under laboratory conditions. Säugetierkd. Mitt. 1982, 30, 307–310. [Google Scholar]
- Michalak, I. Keeping and breeding the Eurasian water shrew Neomys fodiens under laboratory conditions. Int. Zoo Yearb. 1987, 26, 223–228. [Google Scholar] [CrossRef]
- Bajkowska, U.; Chętnicki, W.; Fedyk, S. Breeding of the common shrew, Sorex araneus, under laboratory conditions. Folia Zool. 2009, 58, 1–8. [Google Scholar]
- Kita, M.; Okumura, Y.; Ohdachi, S.D.; Oba, Y.; Yoshikuni, M.; Nakamura, Y.; Kido, H.; Uemura, D. Purification and characterization of blarinasin, a new tissue kallikrein-like protease from the short-tailed shrew Blarina brevicauda: Comparative studies with blarina toxin. Biol. Chem. 2005, 386, 177–182. [Google Scholar] [CrossRef]
- Stewart, J.M. Peptide composition for cancer treatment by inhibiting TRPV6 calcium channel activity. US-8211857-B2, 3 July 2012. [Google Scholar]
- Bowen, C.V.; DeBay, D.; Ewart, H.S.; Gallant, P.; Gormley, S.; Ilenchuk, T.T.; Iqbal, U.; Lutes, T.; Martina, M.; Mealing, G.; et al. In vivo detection of human TRPV6-rich tumors with anti-cancer peptides derived from soricidin. PLoS ONE 2013, 8, e58866. [Google Scholar] [CrossRef] [PubMed]
- Tu, A.T.; Hendon, R.R. Characterization of lizard venom hyaluronidase and evidence for its action as a spreading factor. Comp. Biochem. Physiol. B 1983, 76, 377–383. [Google Scholar] [CrossRef]
- Wood, J.P.; Ellery, P.E.; Maroney, S.A.; Mast, A.E. Biology of tissue factor pathway inhibitor. Blood 2014, 123, 2934–2943. [Google Scholar] [CrossRef]
- Van Hoek, M.L. Antimicrobial peptides in reptiles. Pharmaceuticals 2014, 7, 723–753. [Google Scholar] [CrossRef]
- Doley, R.; Zhou, X.; Kini, R.M. Snake venom phospholipase A2 enzymes. In Handbook of Venoms and Toxins of Reptiles; Mackessy, S.P., Ed.; CRC Press: New York, NY, USA, 2010; pp. 173–198. [Google Scholar]
- Harris, J.B.; Scott-Davey, T. Secreted phospholipases A2 of snake venoms: Effects on the peripheral neuromuscular system with comments on the role of phospholipases A2 in disorders of the CNS and their uses in industry. Toxins 2013, 5, 2533–2571. [Google Scholar] [CrossRef]
- Lenting, P.J.; van Mourik, J.A.; Mertens, K. The life cycle of coagulation factor VIII in view of its structure and function. Blood 1998, 92, 3983–3996. [Google Scholar] [CrossRef]
- Kalapos, M.P. Methylglyoxal in living organisms: Chemistry, biochemistry, toxicology and biological implications. Toxicol. Lett. 1999, 110, 145–175. [Google Scholar] [CrossRef]
- De Plater, G.; Martin, R.L.; Milburn, P.J. A pharmacological and biochemical investigation of the venom from the platypus (Ornithorhynchus anatinus). Toxicon 1995, 33, 157–169. [Google Scholar] [CrossRef]
- Sannaningaiah, D.; Subbaiah, G.K.; Kempaiah, K. Pharmacology of spider venom toxins. Toxin Rev. 2014, 33, 206–220. [Google Scholar] [CrossRef]
- Fry, B.G.; Casewell, N.R.; Wüster, W.; Vidal, N.; Young, B.; Jackson, T.N.W. The structural and functional diversification of the Toxicofera reptile venom system. Toxicon 2012, 60, 434–448. [Google Scholar] [CrossRef]
- Harris, R.J.; Arbuckle, K. Tempo and mode of the evolution of venom and poison in tetrapods. Toxins 2016, 8, 193. [Google Scholar] [CrossRef]
- Ligabue-Braun, R. Venom use in mammals: Evolutionary aspects. In Evolution of Venomous Animals and Their Toxins; Toxinology; Gopalakrishnakone, P., Malhotra, A., Eds.; Springer Science+Business Media: Dordrecht, The Netherlands, 2016. [Google Scholar]
- Arbuckle, K. Evolutionary context of venom in animals. In Evolution of Venomous Animals and Their Toxins; Gopalakrishnakone, P., Malhotra, A., Eds.; Toxinology; Springer: Dordrecht, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Schendel, V.; Rash, L.D.; Jenner, R.A.; Undheim, E.A.B. The diversity of venom: The importance of behavior and venom system morphology in understanding its ecology and evolution. Toxins 2019, 11, 666. [Google Scholar] [CrossRef]
- Arbuckle, K. Ecological function of venom in Varanus, with a compilation of dietary records from the literature. Biawak 2009, 3, 46–56. [Google Scholar]
- Tucker, A.S. Salivary gland adaptations: Modification of the glands for novel uses. In Salivary Glands. Development, Adaptations and Disease; Tucker, A.S., Miletich, I., Eds.; Frontiers of Oral Biology: Basel, Karger, 2010; Volume 14, pp. 21–31. [Google Scholar]
- Pahari, S.; Bickford, D.; Fry, B.G.; Kini, R.M. Expression pattern of three-finger toxin and phospholipase A2 genes in the venom glands of two sea snakes, Lapemis curtus and Acalyptophis peronii: Comparison of evolution of these toxins in land snakes, sea kraits and sea snakes. BMC Evol. Biol. 2007, 7, 175. [Google Scholar] [CrossRef]
- Phuong, M.A.; Mahardika, G.N.; Alfaro, M.E. Dietary breadth is positively correlated with venom complexity in cone snails. BMC Genom. 2016, 17, 401. [Google Scholar] [CrossRef]
- Davies, E.-L.; Arbuckle, K. Coevolution of snake venom toxic activities and diet: Evidence that ecological generalism favours toxicological diversity. Toxins 2019, 11, 711. [Google Scholar] [CrossRef]
- Weese, D.A.; Duda, T.F., Jr. Effects of predator-prey interactions on predator traits: Differentiation of diets and venoms of a marine snail. Toxins 2019, 11, 299. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.H. Predation on shrews by frogs. J. Mammal. 1951, 32, 219. [Google Scholar] [CrossRef]
- Pucek, Z. Keys to Vertebrates of Poland. Mammals; PWN-Polish Scientific Publishers: Warszawa, Poland, 1981; pp. 1–367. [Google Scholar]
- George, S.B.; Choate, J.R.; Genoways, H.H. Blarina brevicauda. Mamm. Species 1986, 261, 1–9. [Google Scholar]
- Korpimäki, E.; Norrdahl, K. Low proportion of shrews in the diet of small mustelids in western Finland. Zeitschr. Säugetierk. 1987, 52, 257–260. [Google Scholar]
- Saarikko, J. Risk of predation and foraging activity in shrews. Ann. Zool. Fenn. 1992, 29, 291–299. [Google Scholar]
- Gliwicz, J.; Taylor, J.R.E. Comparing life histories of shrews and rodents. Acta Theriol. 2002, 47 (Suppl. 1), 185–208. [Google Scholar] [CrossRef]
- Korpimäki, E.; Norrdahl, K. Avian and mammalian predators of shrews in Europe: Regional differences, between-year and seasonal variation, and mortality due to predation. Ann. Zool. Fennici 1989, 26, 389–400. [Google Scholar]
- Kauhala, K.; Laukkanen, P.; Rége von, I. Summer food composition and food niche overlap of the raccoon dog, red fox and badger in Finland. Ecography 1998, 21, 457–463. [Google Scholar] [CrossRef]
- Osten-Sacken, N.; Rychlik, L. Diät vom Marderhund (Nyctereutes procyonoides) in Westpolen–erste Ergebnisse. In Populationsökologie von Raubsäugerarten; Beiträge zur Jagd- und Wildforschung; Stubbe, M., Ed.; Gesellschaft für Wildtier- und Jagdforschung e.V.: Berlin, Germany, 2011; Volume 36, pp. 171–180. [Google Scholar]
- Drygala, F.; Werner, U.; Zoller, H. Diet composition of the invasive raccoon dog (Nyctereutes procyonoides) and the native red fox (Vulpes vulpes) in north-east Germany. Hystrix Ital. J. Mamm. 2013, 24, 190–194. [Google Scholar]
- Rychlik, L. Changes in prey size preferences during successive stages of foraging in the Mediterranean water shrew Neomys anomalus. Behaviour 1999, 136, 345–365. [Google Scholar] [CrossRef]
- Eadie, W.R. The short-tailed shrew and field mouse predation. J. Mammal. 1944, 25, 359–364. [Google Scholar] [CrossRef]
- Pernetta, J.C. A note on the predation of Smooth newt, Triturus vulgaris by European water shrew, Neomys fodiens bicolor. J. Zool. 1976, 179, 215–216. [Google Scholar]
- Kraft von, R.; Pleyer, G. Zur Ernährungsbiologie der Europäischen Wasserspitzmaus Neomys fodiens (Pennant, 1771), an Fischteichen. Zeitschr. Säugetierk. 1978, 43, 321–330. [Google Scholar]
- O’Reilly, R.A. Shrew preying on ribbon snake. J. Mammal. 1949, 30, 309. [Google Scholar] [CrossRef]
- Eadie, W.R. Shrew-mouse predation during low mouse abundance. J. Mammal. 1948, 29, 35–37. [Google Scholar] [CrossRef]
- Martinsen, D.L. Energetics and activity patterns of short-tailed shrews (Blarina) on restricted diets. Ecology 1969, 50, 505–510. [Google Scholar] [CrossRef]
- Getz, L.L.; Larson, C.M.; Lindstrom, K.A. Blarina brevicauda as a predator on nestling voles. J. Mammal. 1992, 73, 591–596. [Google Scholar] [CrossRef]
- Rood, J.P. Habits of the short-tailed shrew in captivity. J. Mammal. 1958, 39, 499–507. [Google Scholar] [CrossRef]
- Eadie, W.R. Shrew predation and vole populations on a localized area. J. Mammal. 1952, 33, 185–189. [Google Scholar] [CrossRef]
- Fulk, G.W. The effect of shrews on the space utilization of voles. J. Mammal. 1972, 53, 461–478. [Google Scholar] [CrossRef]
- Tupikova, N.V. [The diet and nature of daily cycle of activity of shrews from central region of U.S.S.R.]. Zool. Zhurnal 1949, 28, 561–572. (In Russian) [Google Scholar]
- Skoczeń, S. Gromadzenie zapasów pokarmowych przez niektóre ssaki owadożerne (Insectivora) [Food hoarding in selected insectivorous mammals (Insectivora)]. Prz. Zool. 1970, 14, 243–248. (In Polish) [Google Scholar]
- Köhler, D. Zum Verhaltensinventar der Wasserspitzmaus (Neomys fodiens). Säugetierkd. Inf. 1984, 2, 175–199. [Google Scholar]
- Lorenz, K.Z. The taming of the shrew. In King Salomon’s Ring. New Light on Animal Ways; Lorenz, K.Z., Ed.; Methuen & Co. Ltd.: London, UK, 1952; pp. 92–113. [Google Scholar]
- Ottenwalder, J.A. Observations on the habitat and ecology of the Hispaniolan solenodon (Solenodon paradoxus) in the Dominican Republic. In Ecologia de les Illes. Monografies de la Societat d’Historia Natural de les Balears No. 6, and Monografia de l’Institut d’Estudis Balearics No. 66; Alcover, J.A., Ed.; Institut d’Estudis Baleŕrics: Palma de Mallorca, Spain, 1999; pp. 123–168. [Google Scholar]
- Derbridge, J.J.; Posthumus, E.E.; Chen, H.L.; Koprowski, J.L. Solenodon paradoxus (Soricomorpha: Solenodontidae). Mamm. Species 2015, 47, 100–106. [Google Scholar] [CrossRef]
- Robinson, D.E.; Brodie, D., Jr. Food hoarding behavior in the short-tailed shrew Blarina brevicauda. Am. Midl. Nat. 1982, 108, 369–375. [Google Scholar] [CrossRef]
- Ingram, W.M. Snail associates of Blarina brevicauda talpoides (Say). J. Mammal. 1942, 23, 255–258. [Google Scholar] [CrossRef]
- Lawrence, B. Brief comparison of short-tailed shrew and reptile poisons. J. Mammal. 1945, 26, 393–396. [Google Scholar] [CrossRef]
- Brodie, E.D., Jr.; Formanowicz, D.R., Jr. Palatability and antipredator behavior of the tree frog Hyla versicolor to the shrew Blarina brevicauda. J. Herpetol. 1981, 15, 235–236. [Google Scholar] [CrossRef]
- Maier, T.J. Predatory behaviors of Blarina brevicauda toward a fossorial Eastern spadefoot toad (Scaphiopus holbrookii). In Advances in the Biology of the Soricidae II; Merritt, J.F., Churchfield, S., Hutterer, R., Sheftel, B.I., Eds.; Special Publication of the International Society of Shrew Biologists no. 01; The International Society of Shrew Biologists: New York, NY, USA, 2005; pp. 361–366. [Google Scholar]
- Haberl, W. Prey handling times and partial prey consumption in five species of European shrews (Soricidae, Insectivora). Pak. J. Biol. Sci. 1998, 1, 53–54. [Google Scholar] [CrossRef]
- Taylor, J.R.E. Evolution of energetic strategies in shrews. In Evolution of Shrews; Wójcik, J.M., Wolsan, M., Eds.; Mammal Research Institute of the Polish Academy of Sciences: Białowieża, Poland, 1998; pp. 309–346. [Google Scholar]
- Yang, D.C.; Deuis, J.R.; Dashevsky, D.; Dobson, J.; Jackson, T.N.W.; Brust, A.; Xie, B.; Koludarov, I.; Debono, J.; Hendrikx, I.; et al. The snake with the scorpion’s sting: Novel three-finger toxin sodium channel activators from the venom of the long-glanded blue coral snake (Calliophis bivirgatus). Toxins 2016, 8, 303. [Google Scholar] [CrossRef]
- Rychlik, L.; Jancewicz, E. Prey size, prey nutrition, and food handling by shrews of different body sizes. Behav. Ecol. 2002, 13, 216–223. [Google Scholar] [CrossRef]
- Martin, I.G. Factors affecting food hoarding in the short-tailed shrew Blarina brevicauda. Mammalia 1984, 48, 65–71. [Google Scholar] [CrossRef]
- Rychlik, L. Food handling by the gregarious Mediterranean water shrew Neomys anomalus. Folia Zool. 1999, 48, 161–172. [Google Scholar]
- Merritt, J.F. The Biology of Small Mammals; The Johns Hopkins University Press: Baltimore, MA, USA, 2010. [Google Scholar]
- Hamilton, W.J., Jr. The food of the Soricidae. J. Mammal. 1930, 11, 26–39. [Google Scholar] [CrossRef]
- Formanowicz, D.R.J.; Bradley, P.J.; Brodie, E.D.J. Food hoarding by the least shrew (Cryptotis parva): Intersexual and prey type effects. Am. Midl. Nat. 1989, 122, 26–33. [Google Scholar] [CrossRef]
- Pattie, D.L. Behavior of captive marsh shrews (Sorex bendirii). Murrelet 1969, 50, 27–32. [Google Scholar] [CrossRef]
- Maser, C.; Hooven, E.F. Notes on the behavior and food habits of captive Pacific shrews, Sorex pacificus pacificus. Northwest Sci. 1974, 48, 81–95. [Google Scholar]
- Merritt, J.F. Winter survival adaptations of the short-tailed shrew (Blarina brevicauda) in an Appalachian montane forest. J. Mammal. 1986, 67, 450–464. [Google Scholar] [CrossRef]
- Kardynia, P.; Bogdziewicz, M.; Rychlik, L. Interspecific differences influencing seasonal changes in food hoarding and consumption in shrews. In Programme & Abstract Book of the International Colloquium Biology of the Soricidae IV, Poznań, Poland, 11–14 September 2016; Rychlik, L., von Merten, S., Kardynia, P., Eds.; Adam Mickiewicz University, Kontekst Publishing House: Poznań, Poland, 2016; p. 19. [Google Scholar]
- Platt, W.J. The social organisation and territoriality of short-tailed shrew (Blarina brevicauda) population in old-field habitats. Anim. Behav. 1976, 24, 305–318. [Google Scholar] [CrossRef]
- Hawkins, A.E.; Jewell, P.A. Food consumption and energy requirements of captive British shrews and the mole. Proc. Zool. Soc. Lond. 1962, 138, 137–155. [Google Scholar] [CrossRef]
- Hanski, I. Food consumption, assimilation and metabolic rate in six species of shrew (Sorex and Neomys). Ann. Zool. Fenn. 1984, 21, 157–165. [Google Scholar]
- Hanski, I. Population biological consequences of body size in Sorex. In Advances in the Biology of Shrews; Merritt, J.F., Kirkland, G.L., Jr., Rose, R.K., Eds.; Special Publication of Carnegie Museum of Natural History no. 18; Carnegie Museum of Natural History: Pittsburgh, PA, USA, 1994; pp. 15–26. [Google Scholar]
- Hanski, I. What does a shrew do in an energy crisis? In Behavioural Ecology. Ecological Consequences of Adaptive Behaviour. The 25th Symposium of the British Ecological Society, Reading, 1984; Sibly, R.M., Smith, R.H., Eds.; Blackwell: Oxford, UK, 1985; pp. 247–252. [Google Scholar]
- Saarikko, J. Foraging behaviour of shrews. Ann. Zool. Fenn. 1989, 26, 411–423. [Google Scholar]
- McNamara, J.M.; Houston, A.I.; Krebs, J.R. Why hoard? The economics of food storing in tits, Parus spp. Behav. Ecol. 1990, 1, 12–23. [Google Scholar] [CrossRef]
- Dickman, C.R. Body size, prey size, and community structure in insectivorous mammals. Ecology 1988, 69, 569–580. [Google Scholar] [CrossRef]
- Rychlik, L. Competition and coexistence of shrews. In Essays on Mammals of Białowieża Forest; Jędrzejewska, B., Wójcik, J.M., Eds.; Mammal Research Institute, Polish Academy of Sciences: Białowieża, Poland, 2004; pp. 161–170. [Google Scholar]
- Churchfield, S. Dietary separation in three species of shrew inhabiting water-cress beds. J. Zool. (Lond.) 1984, 204, 211–228. [Google Scholar] [CrossRef]
- Churchfield, S. Niche dynamics, food resources, and feeding strategies in multispecies communities of shrews. In The Biology of the Soricidae; Findley, J.S., Yates, T.L., Eds.; The Museum of Southwestern Biology, University of New Mexico: Albuquerque, NM, USA, 1991; pp. 23–34. [Google Scholar]
- Churchfield, S.; Sheftel, B.I. Food niche overlap and ecological separation in a multi-species community of shrews in the Siberian taiga. J. Zool. 1994, 234, 105–124. [Google Scholar] [CrossRef]
- Vander Wall, S.B. Food Hoarding in Animals; University of Chicago Press: Chicago, IL, USA, 1990. [Google Scholar]
- Rychlik, L. Overlap of temporal niches among four sympatric species of shrews. Acta Theriol. 2005, 50, 175–188. [Google Scholar] [CrossRef]
- Croin Michielsen, N. Intraspecific and interspecific competition in the shrews Sorex araneus L. and S. minutus L. Arch. Neerl. Zool. 1966, 17, 73–174. [Google Scholar] [CrossRef]
- Hawes, M.L. Home range, territoriality, and ecological separation in sympatric shrews, Sorex vagrans and Sorex obscurus. J. Mammal. 1977, 58, 354–367. [Google Scholar] [CrossRef]
- Neet, C.R. Prey size and body size in syntopic populations of the shrews Sorex araneus, S. coronatus, S. minutus and Neomys fodiens. In Proceedings of the 5th International Theriological Congress, Rome, Italy, 22–29 August 1989; p. 428. [Google Scholar]
- Dickman, C.R. Mechanisms of competition among insectivorous mammals. Oecologia 1991, 85, 464–471. [Google Scholar] [CrossRef]
- Castién, E.; Gosalbez, J. Habitat and food preferences in a guild of insectivorous mammals in the Western Pyrenees. Acta Theriol. 1999, 44, 1–13. [Google Scholar] [CrossRef]
- Rychlik, L. Habitat preferences of four sympatric species of shrews. Acta Theriol. 2000, 45 (Suppl. 1), 173–190. [Google Scholar] [CrossRef]
- Bunn, D.S. Fighting and moult in shrews. J. Zool. (Lond.) 1966, 148, 580–582. [Google Scholar] [CrossRef]
- Köhler, D. Zum interspecifischen Verhalten von Neomys fodiens und Sorex araneus. Säugetierkd. Inf. 1985, 2, 299–300. [Google Scholar]
- Krushinska, N.L.; Rychlik, L. Intra- and interspecific antagonistic behaviour in two sympatric species of water shrews: Neomys fodiens and N. anomalus. J. Ethol. 1993, 11, 11–21. [Google Scholar] [CrossRef]
- Krushinska, N.L.; Rychlik, L.; Pucek, Z. Agonistic interactions between resident and immigrant sympatric water shrews: Neomys fodiens and N. anomalus. Acta Theriol. 1994, 39, 227–247. [Google Scholar] [CrossRef]
- Rychlik, L. Evolution of social systems in shrews. In Evolution of Shrews; Wójcik, J.M., Wolsan, M., Eds.; Mammal Research Institute of the Polish Academy of Sciences: Białowieża, Poland, 1998; pp. 347–406. [Google Scholar]
- Rychlik, L.; Zwolak, R. Interspecific aggression and behavioural domination among four sympatric species of shrews. Can. J. Zool. 2006, 84, 434–448. [Google Scholar] [CrossRef]
- Hamilton, W.J., Jr. The food of small forest mammals in eastern United States. J. Mammal. 1941, 22, 250–263. [Google Scholar] [CrossRef]
- Eadie, W.R. Predation on Sorex by Blarina. J. Mammal. 1949, 30, 308–309. [Google Scholar] [CrossRef][Green Version]
- Persson, L. Asymmetrical competition: Are larger animals competitively superior? Am. Natural. 1985, 126, 261–266. [Google Scholar] [CrossRef]
- McCue, M. Cost of producing venom in three North American pitviper species. Copeia 2006, 2006, 818–825. [Google Scholar] [CrossRef]
- Pintor, A.F.V.; Winter, K.L.; Krockenberger, A.K.; Seymour, J.E. Venom physiology and composition in a litter of Common Death Adders (Acanthopis antarcticus) and their parents. Toxicon 2011, 57, 68–75. [Google Scholar] [CrossRef]
- Li, M.; Fry, B.G.; Manjunatha Kini, R. Eggs-only diet: Its implications for the toxin profile changes and ecology of the marbled sea snake (Aipysurus eydouxii). J. Mol. Evol. 2005, 60, 81–89. [Google Scholar] [CrossRef]
- Vaughan, T.A.; Ryan, J.M.; Czaplewski, N.J. Mammalogy; Jones and Bartlett Learning: Burlington, MA, USA, 2015. [Google Scholar]
- McCue, M.D. Snakes survive starvation by employing supply- and demand-side economic strategies. Zoology 2007, 110, 318–327. [Google Scholar] [CrossRef]
- McCue, M.D.; Lillywhite, H.B.; Beaupre, S.J. Physiological responses to starvation in snakes: Low energy specialists. In Comparative Physiology of Fasting, Starvation, and Food Limitation; McCue, M.D., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 103–131. [Google Scholar]
- MacArthur, R.H.; Pianka, E.R. On optimal use of a patchy environment. Am. Nat. 1966, 100, 603–609. [Google Scholar] [CrossRef]
- Schoener, T.W. Theory of feeding strategies. Annu. Rev. Ecol. Evol. Syst. 1971, 2, 369–404. [Google Scholar] [CrossRef]
- Marten, G.G. An optimization equation for predation. Ecology 1973, 54, 92–101. [Google Scholar] [CrossRef]

| Taxon | Grooved Teeth | Number, Form and Position of Grooves | Lower Inci-sors Forming a Trough | Enlarged Fossa in Mandibles | Venom-Secreting Salivary Gland |
|---|---|---|---|---|---|
| Extant eulipotyphlans | |||||
| Shrews (Blarina, Neomys, Chimarrogale?, Crocidura?, Notiosorex?, Scutisorex?, Sorex?) | I1 | one shallow groove, open, lingual side | yes | yes | submandibular |
| Solenodons (Solenodon) | I2 | one deep groove forming a channel, anterolingual side | no | yes | submandibular |
| Moles (Talpa?) | no | none | no | ? | submandibular? |
| Extinct eulipotyphlans | |||||
| Shrews Beremendia fissidens, B. minor and B. pohaiensis | I1 | one shallow groove, open, lingual side | yes | yes | submandibular |
| Neomys newtoni and N. browni | I1 | one shallow groove, open, lingual side | yes | yes | submandibular |
| Dolinasorex glyphodon | I1 | one narrow but conspicuous groove, open, lingual side | yes | yes? | submandibular |
| Lunanosorex lii | I1 | two grooves, open, lingual and buccal sides | yes | yes | submandibular |
| Siamosorex debonisi | I2 | one deep but open, mesiolingual side | no | no | submandibular |
| Solenodons Solenodon arredondoi and S. marcanoi | I2 | one deep groove forming a channel, anterolingual side | no | yes | submandibular |
| Nesophontids Nesophontes (~9 species) | C1 | two open grooves: deep and wide on anterior side, deeper and narrow on anterolingual side | no | no | parotid? |
| Species | Venom Components | Venom Activity | References |
|---|---|---|---|
| American short-tailed shrew Blarina brevicauda | blarina toxin (BLTX) | proteolytic and hypotensive activity | [30] |
| blarinasin | nontoxic | [107] | |
| soricidin | inhibition of the movement of Ca across the cellular membrane | [108,109] | |
| kallikrein 1 (KLK1-BL2) serine protease | hypotensive effects in vivo | [45] | |
| phospholipase A2 (PLA2) | cardio-, myo- and neurotoxicity, pro- and anticoagulant effects | [45] | |
| antileukoproteinase (SLPI) | inhibition of serine-proteases, antimicrobial activity | [45] | |
| hyaluronidase PH-20 | facilitation of toxin spreading | [45] | |
| tissue factor pathway inhibitor 2 protein | inhibition of blood coagulation | [45] | |
| Eurasian water shrew Neomys fodiens | phospholipase A2 (PLA2) | paralytic effects cardiotoxic activity in vitro | [17,43] |
| hyaluronidase | facilitation of toxin spreading | [17,43] | |
| lysozyme C | antimicrobial defense | [17,43] | |
| Hispaniolan solenodon Solenodon paradoxus | kallikrein 1 (KLK1) serine protease | hypotensive effects in vivo | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalski, K.; Rychlik, L. Venom Use in Eulipotyphlans: An Evolutionary and Ecological Approach. Toxins 2021, 13, 231. https://doi.org/10.3390/toxins13030231
Kowalski K, Rychlik L. Venom Use in Eulipotyphlans: An Evolutionary and Ecological Approach. Toxins. 2021; 13(3):231. https://doi.org/10.3390/toxins13030231
Chicago/Turabian StyleKowalski, Krzysztof, and Leszek Rychlik. 2021. "Venom Use in Eulipotyphlans: An Evolutionary and Ecological Approach" Toxins 13, no. 3: 231. https://doi.org/10.3390/toxins13030231
APA StyleKowalski, K., & Rychlik, L. (2021). Venom Use in Eulipotyphlans: An Evolutionary and Ecological Approach. Toxins, 13(3), 231. https://doi.org/10.3390/toxins13030231






