Multiple Mycotoxins in Kenyan Rice
Abstract
1. Introduction
| Mycotoxin | Major Producing Fungi [12,14,16,23,36,37,38,39,40,41] | Human Health Impact [18,42,43,44,45,46,47,48] | Regulatory Limit (ppb) in Human Food [24,49,50,51,52,53,54] |
|---|---|---|---|
| Aflatoxin | Aspergillus flavus, A. parasiticus, and A. nomius | Liver cancer | 10 |
| Citrinin | Many species of Aspergillus (e.g., A. niger), Penicillium (e.g., P. citrinum), and Monascus (e.g., M. pallens) | Potential carcinogen and nephrotoxicity | 100 |
| Diacetoxyscirpenol | Fusarium spp. (mainly F. langsethiae, F. poae, F. sporotrichioides, and F. sambucinum) | Vomiting alimentary toxic aleukia | 100 |
| Deoxynivalenol | Fusarium spp. (e.g., Gibberella zeae) | Nausea and vomiting | 1000 |
| Fumonisin | Fusarium spp. (e.g., F. verticillioides and F. proliferatum) | Esophageal cancer | 2000 |
| HT2 | Fusarium spp. (e.g., F. langsethiae, F. poae, and F. sporotrichioides) | Alimentary toxic aleukia | 50 |
| T2 | Fusarium spp. (e.g., F. langsethiae, F. poae, and F. sporotrichioides) | Alimentary toxic aleukia | 50 |
| Ochratoxin A | Aspergillus ochraceus, A. carbonarius, A. niger, and Penicillium verrucosum | Potential human carcinogen and kidney damage | 5 |
| Sterigmatocystin | Aspergillus versicolor | Esophageal and lung cancer | NR |
2. Results
2.1. Description of Datasets and Its Distribution
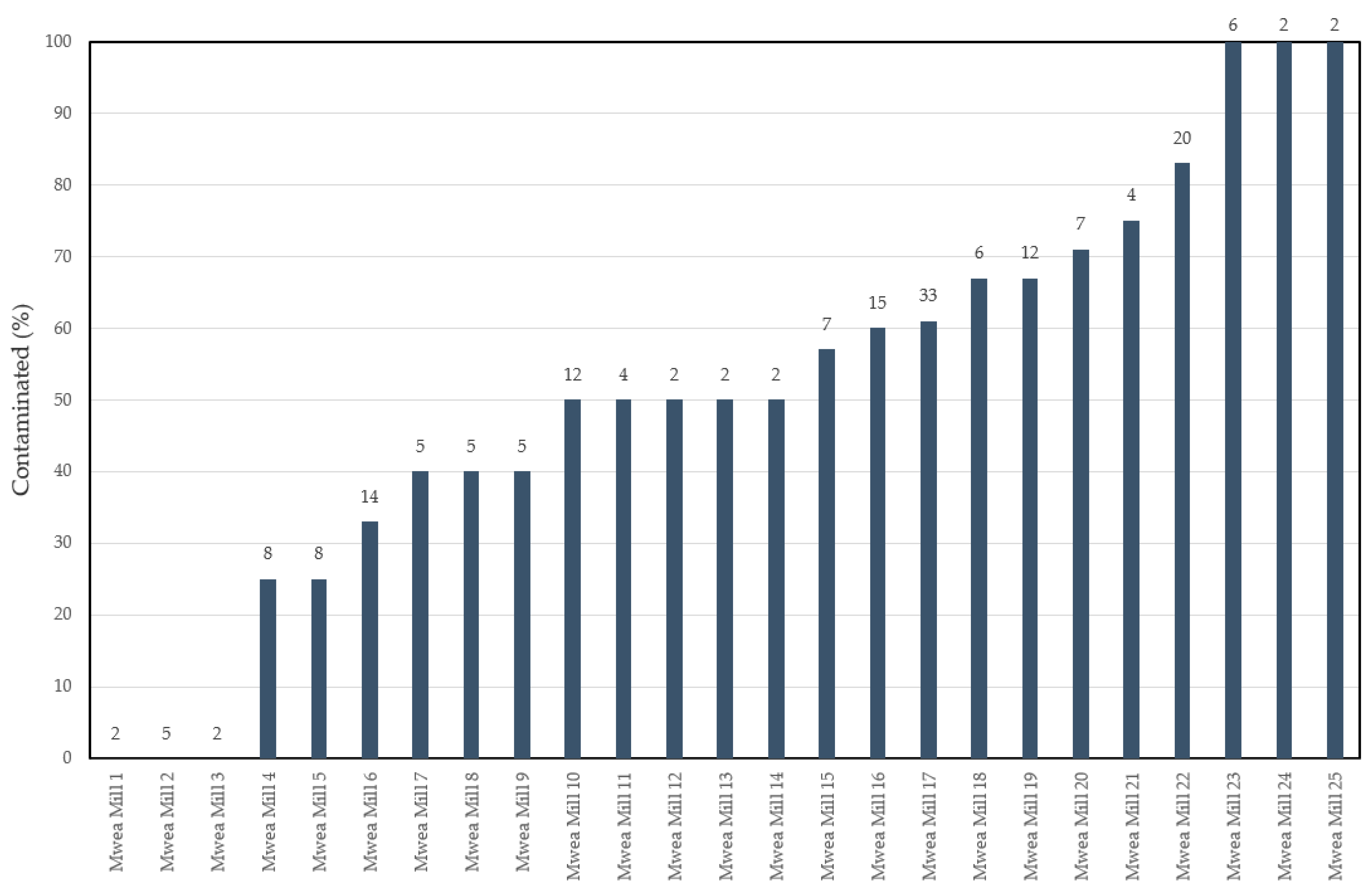
2.2. Mycotoxin Profiles in Rice Samples from the Mwea Irrigation Scheme
2.3. Mycotoxin Profiles in NIBAM 11 and NIBAM 109 Rice Cultivars
2.4. Effects of Paddy Harvesting Date on the Occurrence of Mycotoxins
2.5. Effects of Pre- and Postharvest Management and Handling on the Occurrence of Mycotoxins
3. Discussion
4. Materials and Methods
4.1. Study Site
4.2. Sampling of Rice
4.3. Milling of Rice Grain in the Laboratory
4.4. Mycotoxin Analysis
4.4.1. Mycotoxin Analysis Using ELISA
4.4.2. Mycotoxin Analysis of Samples Using LC–MS/MS
Chemicals and Reagents
Preparation of Mycotoxin Standards for Use in LC–MS/MS
Sample Preparation and Extraction
Sample Analysis in LC–MS/MS
4.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Awika, J. Major Cereal Grains Production and Use around the World. In Advances in Cereal Science: Implications to Food Processing and Health Promotion; American Chemical Society: Washington, DC, USA, 2011; Volume 1089, pp. 1–13. [Google Scholar]
- Arouna, A.; Lokossou, J.C.; Wopereis, M.C.S.; Bruce-Oliver, S.; Roy-Macauley, H. Contribution of improved rice varieties to poverty reduction and food security in sub-Saharan Africa. Glob. Food Sec. 2017, 14, 54–60. [Google Scholar] [CrossRef]
- Kega, V.M.; Gikonyo, E.W.; Muriithi, C.W.; Macharia, J.M.K.; Muthoni, L. Rice Cultivation Manual; Kenya Agricultural and Livestock Research Organization: Nairobi, Kenya, 2015; p. 63. Available online: https://www.kalro.org/files/Rice-Cultivation-Manual.pdf (accessed on 16 November 2020).
- Ntiritu, G.M. Estimating Rice Production Efficiency in Kenya: A Case Study of Mwea Irrigation Scheme. Master′s Thesis, University of Nairobi, Nairobi, Kenya, 2014. [Google Scholar]
- Williams, R.J.; McDonald, D. Grain molds in the tropics: Problems and importance. Ann. Rev. Phytopathol. 2003, 21, 153–178. [Google Scholar] [CrossRef]
- Kumar, D.; Kalita, P. Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Perincherry, L.; Lalak-Kańczugowska, J.; Stępień, Ł. Fusarium-produced mycotoxins in plant-pathogen interactions. Toxins 2019, 11, 664. [Google Scholar] [CrossRef]
- Soni, P.; Gangurde, S.S.; Ortega-Beltran, A.; Kumar, R.; Parmar, S.; Sudini, H.K.; Lei, Y.; Ni, X.; Huai, D.; Fountain, J.C.; et al. Functional biology and molecular mechanisms of host-pathogen interactions for aflatoxin contamination in groundnut (Arachis hypogaea L.) and maize (Zea mays L.). Front. Microbiol. 2020, 11, 227. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Tesha, B.J.; Mghase, J.; Rodenburg, J. Increasing paddy yields and improving farm management: Results from participatory experiments with good agricultural practices (GAP) in Tanzania. Paddy Water Environ. 2018, 16, 749–766. [Google Scholar] [CrossRef]
- Mousa, W.; Ghazali, F.M.; Jinap, S.; Ghazali, H.M.; Radu, S. Modelling the effect of water activity and temperature on growth rate and aflatoxin production by two isolates of Aspergillus flavus on paddy. J. Appl. Microbiol. 2011, 111, 1262–1274. [Google Scholar] [CrossRef]
- Magan, N.; Hope, R.; Cairns, V.; Aldred, D. Post-harvest fungal ecology: Impact of fungal growth and mycotoxin accumulation in stored grain. Eur. J. Plant Pathol. 2003, 109, 723–730. [Google Scholar] [CrossRef]
- Abbas, H.K.; Cartwright, R.D.; Shier, W.T.; Abouzied, M.M.; Bird, C.B.; Rice, L.G.; Ross, P.F.; Sciumbato, G.L.; Meredith, F.I. Natural occurrence of fumonisins in rice with Fusarium sheath rot disease. Plant Dis. 1998, 82, 22–25. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497. [Google Scholar] [CrossRef]
- Payne, G.A. Process of contamination by aflatoxin producing fungi and their impacts on crops. In Mycotoxins in Agriculture and Food Safey; Sinha, K.K., Bhatnagar, D., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1998. [Google Scholar]
- Beattie, S.; Schwarz, P.B.; Horsley, R.; Barr, J.; Casper, H.H. The effect of grain storage conditions on the viability of Fusarium and deoxynivalenol production in infested malting barley. J. Food Prot. 1998, 61, 103–106. [Google Scholar] [CrossRef]
- Lattanzio, V.M.; Ciasca, B.; Haidukowski, M.; Infantino, A.; Visconti, A.; Pascale, M. Mycotoxin profile of Fusarium langsethiae isolated from wheat in Italy: Production of type-A trichothecenes and relevant glucosyl derivatives. J. Mass Spectrom. 2013, 48, 1291–1298. [Google Scholar] [CrossRef]
- Čulig, B.; Bevardi, M.; Bošnir, J.; Serdar, S.; Lasić, D.; Racz, A.; Galić, A.; Kuharić, Ž. Presence of citrinin in grains and its possible health effects. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 22–30. [Google Scholar] [CrossRef]
- Hamid, A.S.; Tesfamariam, I.G.; Zhang, Y.; Zhang, Z.G. Aflatoxin B1-induced hepatocellular carcinoma in developing countries: Geographical distribution, mechanism of action and prevention. Oncol. Lett. 2013, 5, 1087–1092. [Google Scholar] [CrossRef]
- Saito, M.; Enomoto, M.; Tatsuno, T. Yellowed rice toxins: Luteroskyrin and related compounds, chlorine-containing compounds and citrinin. In Microbial Toxins; Ciegler, A., Kadis, S., Ajl, S.J., Eds.; Academic Press: New York, NY, USA, 1971; Volume VI, pp. 299–380. [Google Scholar]
- Marasas, W.F. Fumonisins: History, world-wide occurrence and impact. Adv. Exp. Med. Biol. 1996, 392, 1–17. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: From simple to complex mycotoxins. Toxins 2011, 3, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Mbundi, L.; Gallar-Ayala, H.; Khan, M.R.; Barber, J.L.; Losada, S.; Busquets, R. Advances in the analysis of challenging food contaminants: Nanoparticles, bisphenols, mycotoxins, and brominated flame retardants. In Advances in Molecular Toxicology; Fishbein, J.C., Heilman, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 8, pp. 35–105. [Google Scholar]
- Bui-Klimke, T.R.; Wu, F. Ochratoxin A and human health risk: A review of the evidence. Crit. Rev. Food Sci. Nutr. 2015, 55, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- Tabata, S. Yeasts and Molds | Mycotoxins: Aflatoxins and Related Compounds. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 801–811. [Google Scholar]
- Sommer, N.F.; Buchanan, J.R.; Fortlage, R.J. Aflatoxin and sterigmatocystin contamination of pistachio nuts in orchards. Appl. Environ. Microbiol. 1976, 32, 64. [Google Scholar] [CrossRef]
- EFSA. Scientific opinion on the risk for public and animal health related to the presence of sterigmatocystin in food and feed. EFSA J. 2013, 11, 3254. [Google Scholar] [CrossRef]
- Hell, K.; Mutegi, C.K. Aflatoxin control and prevention strategies in key crops of Sub-Saharan Africa. Afr. J. Microbiol. Res. 2011, 5, 459–466. [Google Scholar]
- Mutiga, S.K.; Hoffmann, V.; Harvey, J.W.; Milgroom, M.G.; Nelson, R.J. Assessment of Aflatoxin and Fumonisin Contamination of Maize in Western Kenya. Phytopathology 2015, 105, 1250–1261. [Google Scholar] [CrossRef]
- Mutegi, C.K.; Ngugi, H.K.; Hendriks, S.L.; Jones, R.B. Prevalence and factors associated with aflatoxin contamination of peanuts from Western Kenya. Int. J. Food Microbiol. 2009, 130, 27–34. [Google Scholar] [CrossRef]
- Daniel, J.H.; Lewis, L.W.; Redwood, Y.A.; Kieszak, S.; Breiman, R.F.; Flanders, W.D.; Bell, C.; Mwihia, J.; Ogana, G.; Likimani, S.; et al. Comprehensive assessment of maize aflatoxin levels in Eastern Kenya, 2005-2007. Environ. Health Persp. 2011, 119, 1794–1799. [Google Scholar] [CrossRef]
- Makun, H.A.; Gbodi, T.A.; Akanya, O.H.; Salako, E.A.; Ogbadu, G.H. Fungi and some mycotoxins contaminating rice (Oryza Sativa) in Niger State, Nigeria. Afr. J. Biotechnol. 2007, 6, 099–108. [Google Scholar]
- Tang, E.N.; Ndindeng, S.A.; Bigoga, J.; Traore, K.; Silue, D.; Futakuchi, K. Mycotoxin concentrations in rice from three climatic locations in Africa as affected by grain quality, production site, and storage duration. Food Sci. Nutr. 2019, 7, 1274–1287. [Google Scholar] [CrossRef]
- Youmma, D.; Masanga, J.; Nyerere, A.; Runo, S.; Ambang, Z. Towards managing and controlling aflatoxin producers within Aspergillus species in infested rice grains collected from local markets in Kenya. Toxins 2019, 11, 544. [Google Scholar] [CrossRef]
- Mapiemfu-Lamare, D.; Ndindeng, S.A.; Ambang, Z.; Tang, E.; Ngome, A.; Johnson, J.-M.; Tanaka, A.; Saito, K. Physical rice grain quality as affected by biophysical factors and pre-harvest practices. Int. J. Plant Prod. 2017, 11. [Google Scholar] [CrossRef]
- Kato, G.; Tu, L. Evaluation of Irrigation Water Resources Availability and Climate Change Impacts—A Case Study of Mwea Irrigation Scheme, Kenya. Water 2020, 12, 2330. [Google Scholar] [CrossRef]
- Rheeder, J.P.; Marasas, W.F.O.; Vismer, H.F. Production of fumonisin analogs by Fusarium species. Appl. Environ. Microbiol. 2002, 68, 2101–2105. [Google Scholar] [CrossRef] [PubMed]
- Hamuel, J.D. The occurrence, properties and significance of citrinin mycotoxin. J. Plant Pathol. Microbiol. 2015, 6, 2. [Google Scholar]
- Agrios, G.N. Plant diseases caused by fungi. In Plant Pathology, 5th ed.; Agrios, G.N., Ed.; Academic Press: San Diego, CA, USA, 2005; pp. 385–614. [Google Scholar]
- Kokkonen, M.; Jestoi, M.; Laitila, A. Mycotoxin production of Fusarium langsethiae and Fusarium sporotrichioides on cereal-based substrates. Mycotoxin Res. 2012, 28, 25–35. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Aspergillus and related teleomorphs. In Food Spoilage Microorganisms; Blackburn, C.d.W., Ed.; Woodhead Publishing: Cambridge, UK, 2006; pp. 451–487. [Google Scholar]
- Knutsen, H.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Risk to human and animal health related to the presence of 4,15-diacetoxyscirpenol in food and feed. EFSA J. 2018, 16, e05367. [Google Scholar] [CrossRef] [PubMed]
- Flajs, D.; Peraica, M. Toxicological properties of citrinin. Arh. Hig. Rada. Toksikol. 2009, 60, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Haschek, W.M.; Voss, K.A. Mycotoxins. In Haschek and Rousseaux′s Handbook of Toxicologic Pathology, 3rd ed.; Haschek, W.M., Rousseaux, C.G., Wallig, M.A., Eds.; Academic Press: Boston, MA, USA, 2013; pp. 1187–1258. [Google Scholar]
- Arcella, D.; Gergelova, P.; Innocenti, M.; Steinkellner, H. Human and animal dietary exposure to T-2 and HT-2 toxin. EFSA J. 2017, 15, e04972. [Google Scholar] [PubMed]
- Perkowski, J.; Chełkowski, J.; Wakuliński, W. Mycotoxins in cereal grain. Part 13. Deoxynivalenol and 3-acetyl-deoxynivalenol in wheat kernels and chaff with head fusariosis symptoms. Nahrung 1990, 34, 325–328. [Google Scholar] [CrossRef]
- Myburg, R.B.; Dutton, M.F.; Chuturgoon, A.A. Cytotoxicity of fumonisin B1, diethylnitrosamine, and catechol on the SNO esophageal cancer cell line. Environ. Health Perspect. 2002, 110, 813–815. [Google Scholar] [CrossRef]
- Wang, J.; Huang, S.; Xing, L.; Shen, H.; Yan, X.; Wang, J.; Zhang, X. Role of hMLH1 in sterigmatocystin-induced G₂ phase arrest in human esophageal epithelial Het-1A cells in vitro. Toxicol. Lett. 2013, 217, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Hope, J.H.; Hope, B.E. A review of the diagnosis and treatment of ochratoxin a inhalational exposure associated with human illness and kidney disease including focal segmental glomerulosclerosis. J. Environ. Public Health 2012, 2012, 835059. [Google Scholar] [CrossRef]
- Petersen, A.B. Expert Advice on Appropriate Criteria and Limits for Contaminants in Ready to Use Therapeutic Foods; World Food Programme and United Nations Children’s Fund: New York, NY, USA, 2018. [Google Scholar]
- Duarte, S.C.; Lino, C.M.; Pena, A. Mycotoxin food and feed regulation and the specific case of ochratoxin A: A review of the worldwide status. Food Addit. Contam. Part A 2010, 27, 1440–1450. [Google Scholar] [CrossRef]
- Pan, T.M.; Hsu, W.H. Monascus-Fermented Products. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 815–825. [Google Scholar]
- European Comission. Opinion of the Scientific Committee on Food on Fusarium Toxins: Part 6: Group Evaluation of T-2 Toxin, HT-2 Toxin, Nivalenol and Deoxynivalenol; EC: Brussel, Belgium, 2002. [Google Scholar]
- European-Commission-(EC). Commission Recommendation of 27 March 2013 on the presence of T-2 and HT-2 toxin in cereals and cereal products. Off. J. Eur. Union 2013, L91, 12–15. [Google Scholar]
- Gong, Y.Y.; Routledge, M.; Kimanya, M.E.; Musoke, G.; Nelson, F.; Sonoiya, S.; Manyong, V. Aflatoxin Standards for Food Program; International Institute of Tropical Agriculture (IITA): Dar es Salaam, Tanzania, 2015; p. 30. [Google Scholar]
- Tanaka, K.; Sago, Y.; Zheng, Y.; Nakagawa, H.; Kushiro, M. Mycotoxins in rice. Int. J. Food Microbiol. 2007, 119, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Azziz-Baumgartner, E.; Lindblade, K.; Gieseker, K.; Rogers, H.S.; Kieszak, S.; Njapau, H.; Schleicher, R.; McCoy, L.F.; Misore, A.; DeCock, K.; et al. Case-control study of an acute aflatoxicosis outbreak, Kenya, 2004. Environ. Health Perspect. 2005, 113, 1779–1783. [Google Scholar] [CrossRef] [PubMed]
- Wakhisi, J.; Patel, K.; Buziba, N.; Rotich, J. Esophageal cancer in north Rift Valley of Western Kenya. Afr. Health Sci. 2005, 5, 157–163. [Google Scholar]
- Sun, Z.T.; Lu, P.X.; Gail, M.H.; Pee, D.; Zhang, Q.N.; Ming, L.H.; Wang, J.B.; Wu, Y.; Liu, G.T.; Wu, Y.Y.; et al. Increased risk of hepatocellular carcinoma in male hepatitis B surface antigen carriers with chronic hepatitis who have detectable urinary aflatoxin metabolite M1. Hepatology 1999, 30, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Righetti, L.; Korber, T.; Rolli, E.; Galaverna, G.; Suman, M.; Bruni, R.; Dall’Asta, C. Plant biotransformation of T2 and HT2 toxin in cultured organs of Triticum durum Desf. Sci. Rep. 2019, 9, 14320. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in occurrence, importance, and mycotoxin control strategies: Prevention and detoxification in foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef]
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 1995; Volume 63, pp. 443–465. [Google Scholar]
- Richard-Molard, D.; Lesage, L.; Cahagnier, B. Effect of water activity on mold growth and mycotoxin production. In Properties of Water in Foods; Simatos, D., Multon, J.L., Eds.; Springer: Dordrecht, The Netherlands, 1985; pp. 273–292. [Google Scholar]
- Mannaa, M.; Kim, K.D. Influence of temperature and water activity on deleterious fungi and mycotoxin production during grain storage. Mycobiology 2017, 45, 240–254. [Google Scholar] [CrossRef]
- Carlson, D.B.; Williams, D.E.; Spitsbergen, J.M.; Ross, P.F.; Bacon, C.W.; Meredith, F.I.; Riley, R.T. Fumonisin B1 promotes aflatoxin B1 and N-methyl-N’-nitro-nitrosoguanidine-initiated liver tumors in rainbow trout. Toxicol. Appl. Pharmacol. 2001, 172, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Perez-Nadales, E.; Nogueira, M.F.; Baldin, C.; Castanheira, S.; El Ghalid, M.; Grund, E.; Lengeler, K.; Marchegiani, E.; Mehrotra, P.V.; Moretti, M.; et al. Fungal model systems and the elucidation of pathogenicity determinants. Fungal Genet. Biol. 2014, 70, 42–67. [Google Scholar] [CrossRef] [PubMed]
- Samejima, H.; Katsura, K.; Kikuta, M.; Njinju, S.M.; Kimani, J.M.; Yamauchi, A.; Makihara, D. Analysis of rice yield response to various cropping seasons to develop optimal cropping calendars in Mwea, Kenya. Plant Prod. Sci. 2020, 23, 297–305. [Google Scholar] [CrossRef]
- Xavier, R.; Eychenne, N.; Delos, M.; Folcher, L. Withdrawal of maize protection by herbicides and insecticides increases mycotoxin contamination near maximum thresholds. Agron. Sustain. Dev. 2016, 36, 1–10. [Google Scholar] [CrossRef]
- Geraldo, M.; Tessmann, D.; Kemmelmeier, C. Production of mycotoxins by Fusarium graminearum isolated from small cereals (wheat, triticale and barley) affected with scab disease in Southern Brazil. Braz J. Microbiol. 2006, 37. [Google Scholar] [CrossRef]
- Vogelgsang, S.; Beyer, M.; Pasquali, M.; Jenny, E.; Musa, T.; Bucheli, T.D.; Wettstein, F.E.; Forrer, H.-R. An eight-year survey of wheat shows distinctive effects of cropping factors on different Fusarium species and associated mycotoxins. Eur. J. Agron. 2019, 105, 62–77. [Google Scholar] [CrossRef]
- Mutiga, S.K.; Chepkwony, N.; Hoekenga, O.A.; Flint-Garcia, S.A.; Nelson, R.J. The role of ear environment in postharvest susceptibility of maize to toxigenic Aspergillus flavus. Plant Breed. 2019, 138, 38–50. [Google Scholar] [CrossRef]
- Swamy, Y.; Bhattacharya, K. Breakage of rice during milling—Effect of kernel defects and grain dimension. J. Food Process Eng. 2007, 3, 29–42. [Google Scholar] [CrossRef]
- Tong, C.; Gao, H.; Luo, S.; Liu, L.; Bao, J. Impact of postharvest operations on rice grain quality: A review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 626–640. [Google Scholar] [CrossRef]
- Mutiga, S.K.; Mushongi, A.A.; Kangéthe, E.K. Enhancing food safety through adoption of long-term technical advisory, financial, and storage support services in maize growing areas of East Africa. Sustainability 2019, 11, 2827. [Google Scholar] [CrossRef]
- Kundu, C.; Ishii, M.; Sato, K.; Wanjogu, R.; Makihara, D.; Yamauchi, A.; Masunaga, T. An assessement of paddy production system in central kenya with special reference to micronutrients. J. Agric. Sci. 2017, 9, 49. [Google Scholar] [CrossRef][Green Version]




| Toxin | Sample Contamination (ppb) | |||||
|---|---|---|---|---|---|---|
| LOD | Samples with Detectable Toxin (n; %) | Range | Median | 75th Percentile | >ML (n; %) | |
| Aflatoxin (AF) † | 0.26 | 110; 55.5 | 0–993 | 0.2 | 1.7 | 27; 13.5 |
| Aflatoxin G2 | 0.1 | 3; 1.5 | 0–0.6 | 0 | 0 | NA |
| Aflatoxin G1 | 0.05 | 6; 3 | 0–1 | 0 | 0 | NA |
| Aflatoxin B2 | 0.07 | 22; 11 | 0–72 | 0 | 0 | NA |
| Aflatoxin B1 | 0.04 | 108; 54 | 0–921 | 0.2 | 1.7 | NA |
| Citrinin | 0.1 | 111; 55.5 | 0–9 | 0.2 | 0.4 | 0 |
| Diacetoxyscirpenol | 0.05 | 41; 20.5 | 0–24 | 0 | 0 | 0 |
| Deoxynivalenol ¥ | 500 | 1; 0.5 | 0–510 | 81 | 207 | 0 |
| Fumonisin (FB) † | 2.35 | 52; 26 | 0–76 | 0 | 7.3 | 0 |
| Fumonisin B1 | 1.53 | 0 | 0 | 0 | 0 | NA |
| Fumonisin B2 | 0.15 | 1; 0.5 | 0–8 | 0 | 0 | NA |
| Fumonisin B3 | 0.67 | 52; 26 | 0–76 | 0 | 6.3 | NA |
| HT2 + T2 † | 0.04 | 29; 14.5 | 0–62 | 0 | 0 | 1; 0.5 |
| HT2 | 0.03 | 6; 3 | 0–49 | 0 | 0 | NA |
| T2 | 0.01 | 37; 18.5 | 0– 45 | 0 | 0 | NA |
| Ochratoxin A | 0.19 | 60; 30 | 0–111 | 0 | 0.5 | 12; 6 |
| Sterigmatocystin | 0.03 | 149; 74.5 | 0–7 | 0.2 | 0.3 | NA |
| Sterigmatocystin | Citrinin | Aflatoxin | Ochratoxin A | Fumonisin | Diacetoxyscirpenol | HT2 + T2 | Deoxynivalenol | |
|---|---|---|---|---|---|---|---|---|
| Sterigmatocystin | 48 | 46 | 25 | 20 | 15 | 16 | 0.5 | |
| Citrinin | 0.26; 0.0002 | 35 | 15 | 16.5 | 13.5 | 13 | 0.5 | |
| Aflatoxin | 0.19; 0.0065 | NS | 20 | 15 | 12.5 | 11.5 | 0.5 | |
| Ochratoxin A | NS | 0.17; 0.0149 | 0.15; 0.0377 | 6.5 | 9 | 10 | 0.5 | |
| Fumonisin | NS | NS | NS | NS | 7 | 9.5 | 0 | |
| Diacetoxyscirpenol | NS | NS | NS | 0.15; 0.0294 | NS | 7.5 | 0.5 | |
| HT2 + T2 | NS | NS | NS | 0.22; 0.0019 | 0.23; 0.001 | 0.21; 0.0028 | 0 | |
| Deoxynivalenol | NS | NS | NS | NS | NS | NS | NS |
| Model | Factor (p-Value) | ||||||
|---|---|---|---|---|---|---|---|
| Toxin | r2 | p-Value | Paddy Harvest Date | Locality of Miller | Source of Paddy | Miller | Cultivar |
| Aflatoxin | 0.4 | 0.0255 | 0.993 | NI | 0.4106 | 0.0382 | <0.0001 |
| Citrinin | 0.16 | 0.4961 | 0.1575 | 0.6212 | 0.3398 | 0.4875 | NI |
| Diacetoxyscirpenol | 0.37 | 0.2341 | 0.3344 | NI | 0.0387 | 0.5012 | 0.1873 |
| Deoxynivalenol | NA | NA | NA | NA | NA | NA | NA |
| Fumonisin | 0.26 | 0.0369 | 0.0007 | 0.8425 | 0.3508 | 0.1313 | 0.0005 |
| Ochratoxin A | 0.21 | 0.1956 | 0.6977 | 0.0622 | 0.2952 | 0.1541 | 0.4661 |
| Sterigmatocystin | 0.33 | 0.6138 | 0.077 | NI | 0.4774 | 0.983 | NI |
| HT2 + T2 | 0.16 | 0.3433 | NI | NI | 0.5955 | NI | 0.0188 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutiga, S.K.; Mutuku, J.M.; Koskei, V.; Gitau, J.K.; Ng’ang’a, F.; Musyoka, J.; Chemining’wa, G.N.; Murori, R. Multiple Mycotoxins in Kenyan Rice. Toxins 2021, 13, 203. https://doi.org/10.3390/toxins13030203
Mutiga SK, Mutuku JM, Koskei V, Gitau JK, Ng’ang’a F, Musyoka J, Chemining’wa GN, Murori R. Multiple Mycotoxins in Kenyan Rice. Toxins. 2021; 13(3):203. https://doi.org/10.3390/toxins13030203
Chicago/Turabian StyleMutiga, Samuel K., J. Musembi Mutuku, Vincent Koskei, James Kamau Gitau, Fredrick Ng’ang’a, Joyce Musyoka, George N. Chemining’wa, and Rosemary Murori. 2021. "Multiple Mycotoxins in Kenyan Rice" Toxins 13, no. 3: 203. https://doi.org/10.3390/toxins13030203
APA StyleMutiga, S. K., Mutuku, J. M., Koskei, V., Gitau, J. K., Ng’ang’a, F., Musyoka, J., Chemining’wa, G. N., & Murori, R. (2021). Multiple Mycotoxins in Kenyan Rice. Toxins, 13(3), 203. https://doi.org/10.3390/toxins13030203






