Multi-Mycotoxin Contamination of Maize Silages in Flanders, Belgium: Monitoring Mycotoxin Levels from Seed to Feed
Abstract
1. Introduction
2. Results
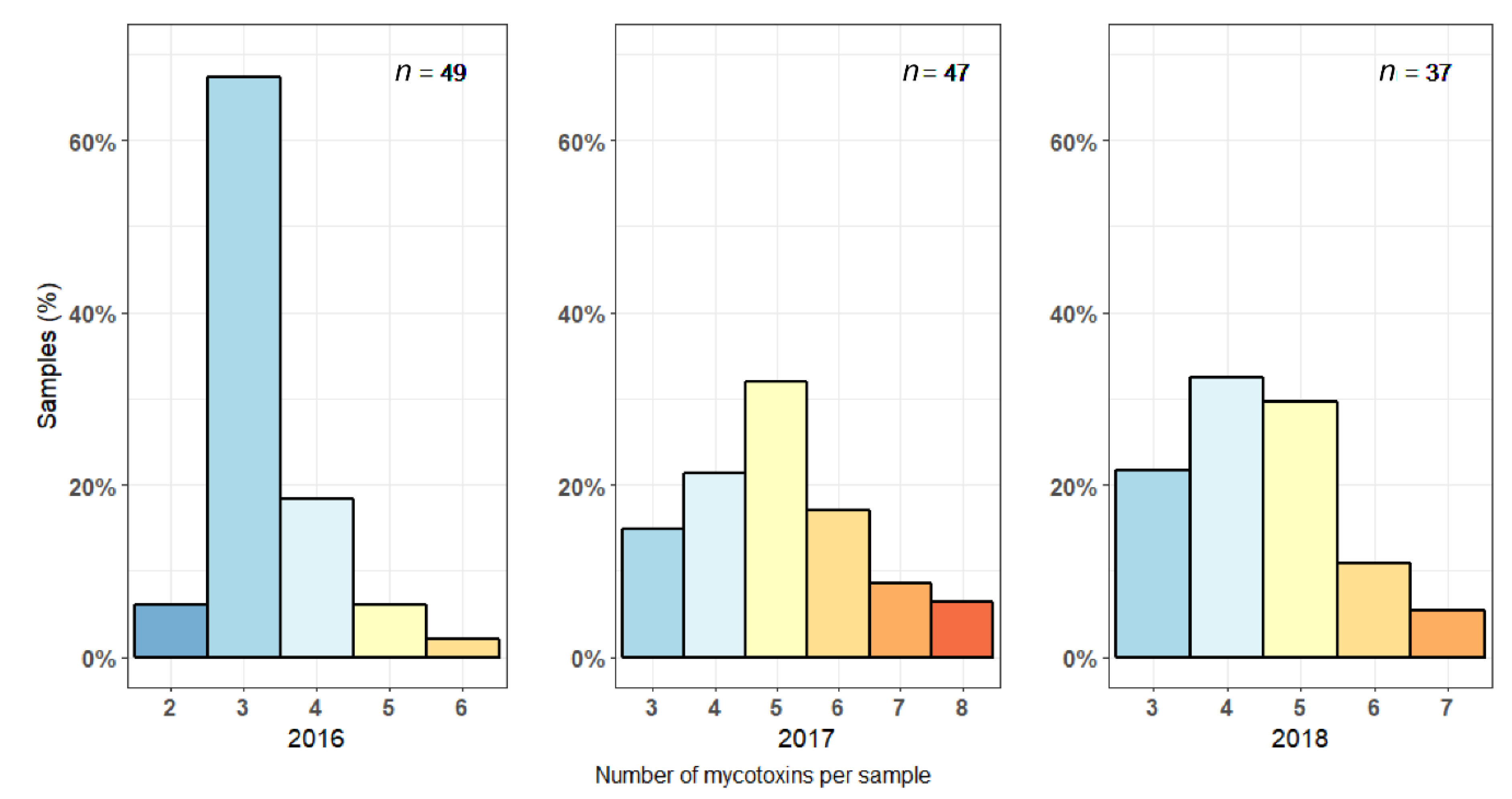
2.1. Mycotoxin Levels in Maize Silages in 2016–2018
| Mycotoxin | Positive Samples (%) | Mean Concentration a (µg/kg DM) | Median Concentration (µg/kg DM) | Max. Concentration (µg/kg DM) | Samples Exceeding EU Recommendation (%) b | |||||||||||||||
| 2016 | 2017 | 2018 | 2016–2018 | 2016 | 2017 | 2018 | 2016–2018 | 2016 | 2017 | 2018 | 2016–2018 | 2016 | 2017 | 2018 | 2016–2018 | 2016 | 2017 | 2018 | 2016–2018 | |
| n samples | 49 | 47 | 37 | 133 | 49 | 47 | 37 | 133 | 49 | 47 | 37 | 133 | 49 | 47 | 37 | 133 | 49 | 47 | 37 | 133 |
| NIV c | 100 | 97.9 | 94.6 | 97.7 | / | / | / | / | / | / | / | / | / | / | / | / | ||||
| DON | 98.0 | 100 | 94.6 | 97.7 | 258 | 1334 | 370 | 670 | 201 | 621 | 222 | 287 | 742 | 8912 | 4466 | 8912 | 0 | 21.3 | 2.7 | 8.3 |
| 3-ADON | n.d. | 29.8 | 2.7 | 11.3 | n.d. | 23 | 29 | 16 | n.d. | 0 | 0 | 0 | n.d. | 183 | 1080 | 1080 | ||||
| 15-ADON | 12.2 | 66.0 | 29.7 | 36.1 | 6.3 | 137 | 49 | 64 | 0 | 80 | 0 | 0 | 108 | 520 | 285 | 520 | ||||
| DON+ d | 98.0 | 100 | 97.3 | 98.5 | 265 | 1495 | 449 | 750 | 226 | 725 | 282 | 318 | 742 | 9583 | 5710 | 9583 | ||||
| ZEN | 4.1 | 74.5 | 32.4 | 36.8 | 11 | 449 | 129 | 199 | 0 | 225 | 0 | 0 | 367 | 3124 | 1428 | 3124 | 0 | 27.7 | 8.1 | 12.0 |
| ENN B | 95.9 | 85.1 | 83.8 | 88.7 | 115 | 78 | 62 | 88 | 63 | 66 | 52 | 57 | 658 | 396 | 353 | 658 | ||||
| AME | 14.3 | 14.9 | 2.7 | 11.3 | 23 | 16 | 5.8 | 16 | 0 | 0 | 0 | 0 | 264 | 154 | 214 | 264 | ||||
| FB1 | n.d. | 23.4 | 64.9 | 26.3 | n.d. | 48 | 184 | 68 | n.d. | 0 | 71 | 0 | n.d. | 715 | 1871 | 1871 | 0 | 0 | 0 | 0 |
| FB2 | n.d. | 4.3 | 27.0 | 9.0 | n.d. | 3.0 | 38 | 12 | n.d. | 0 | 0 | 0 | n.d. | 79 | 449 | 449 | 0 | 0 | 0 | 0 |
| FB3 | n.d. | n.d. | 13.5 | 3.8 | n.d. | n.d. | 9.8 | 2.7 | n.d. | n.d. | 0 | 0 | n.d. | n.d. | 177 | 177 | ||||
| FUM d | n.d. | 23.4 | 64.9 | 26.3 | n.d. | 51 | 232 | 82 | n.d. | 0 | 71 | 0 | n.d. | 795 | 2497 | 2497 | ||||
| ROQ-C | 10.2 | 6.4 | 2.7 | 6.8 | 49 | 18 | 0.4 | 24 | 0 | 0 | 0 | 0 | 1065 | 428 | 14 | 1065 | ||||
| SUM | 100 | 100 | 100 | 100 | 463 | 2107 | 877 | 1159 | 426 | 1303 | 609 | 625 | 1565 | 10,899 | 6329 | 10,899 | ||||
2.2. Correlations between Different Mycotoxins
2.3. Correlations between Mycotoxin Concentrations and Fusarium spp. DNA
2.4. Comparison between Mycotoxin Concentrations at Harvest vs. Net Bag Samples
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Maize Silage Cutting Edge Sampling
5.2. Maize Silage Density Sampling
5.3. Net Bag Sampling
5.4. Silage Quality Analysis
5.5. Mycotoxin Analysis by LC-MS/MS
5.6. qPCR Analysis
5.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A



| Fusarium spp. DNA | Positive Samples (%) | Mean Concentration a (pg/µL) | Median Concentration (pg/µL) | Max. Concentration (pg/µL) |
| F. graminearum | 81.4 | 0.105 | 0.032 | 1.530 |
| F. culmorum | 18.5 | 0.011 | 0 | 0.362 |
| F. verticillioides | 53.7 | 6.778 | 0.008 | 220.924 |
| Silage Quality Parameter | Mean in the Cutting Edge Samples (n = 115) | Mean in the Net Bag Samples (n = 115) |
| pH | 3.79 ± 0.01 | 3.83 ± 0.03 |
| Ammonia content (g/kg DM) | 0.888 ± 0.018 | 0.817 ± 0.076 |
| Lactic acid content (g/kg DM) | 48.73 ± 1.04 | 44.08 ± 2.82 |
| Acetic acid content (g/kg DM) | 15.05 ± 0.46 | 15.02 ± 1.58 |
| Total Fliegscore | 91.3 ± 0.5 | 92.5 ± 1.4 |
| Mycotoxin | Positive Samples (%) | Mean Concentration a (µg/kg) | Median Concentration (µg/kg) | Max. Concentration (µg/kg) | Samples Exceeding EU Recommendation (%) b | |||||||||||||||
| 2016 | 2017 | 2018 | 2016–2018 | 2016 | 2017 | 2018 | 2016–2018 | 2016 | 2017 | 2018 | 2016–2018 | 2016 | 2017 | 2018 | 2016–2018 | 2016 | 2017 | 2018 | 2016–2018 | |
| n samples | 91 | 81 | 85 | 257 | 91 | 81 | 85 | 257 | 91 | 81 | 85 | 257 | 91 | 81 | 95 | 257 | 91 | 81 | 95 | 257 |
| NIV | 98.9 | 100 | 98.8 | 99.2 | 651 | 719 | 882 | 749 | 528 | 461 | 782 | 587 | 2368 | 6776 | 2410 | 6776 | ||||
| DON | 92.3 | 100 | 64.7 | 85.6 | 449 | 558 | 187 | 396 | 263 | 337 | 121 | 215 | 2777 | 5322 | 2111 | 5322 | 2.2 | 3.7 | 1.0 | 2.3 |
| 3-ADON | 78.0 | 29.6 | 15.3 | 42.0 | 54 | 36 | 23 | 38 | 43 | 0 | 0 | 0 | 297 | 380 | 1047 | 1047 | ||||
| 15-ADON | 64.8 | 51.3 | 12.9 | 43.4 | 95 | 81 | 15 | 65 | 71 | 18 | 0 | 0 | 819 | 769 | 249 | 819 | ||||
| DON+ c | 95.6 | 100 | 64.7 | 86.8 | 598 | 675 | 225 | 499 | 377 | 407 | 130 | 262 | 3050 | 6472 | 2111 | 6472 | ||||
| ZEN | 64.8 | 40.7 | 42.4 | 49.8 | 101 | 159 | 176 | 160 | 71 | 0 | 0 | 0 | 1086 | 1412 | 2792 | 2792 | 1.1 | 8.6 | 12.6 | 7.8 |
| ENN B | 42.9 | 18.5 | 45.9 | 36.2 | 133 | 78 | 180 | 150 | 56 | 28 | 71 | 46 | 1375 | 1042 | 1985 | 1985 | ||||
| AOH | n.d. | 3.7 | 9.4 | 4.3 | n.d. | 1.4 | 6.5 | 2.6 | n.d. | 0 | 0 | 0 | n.d. | 49 | 209 | 209 | ||||
| AME | 2.2 | 3.7 | 10.6 | 5.4 | 0.8 | 12 | 20 | 11 | 0 | 0 | 0 | 0 | 49 | 371 | 453 | 453 | ||||
| FB1 d | 2.5 | 19.8 | 61.2 | 28.6 | 1.5 | 61 | 247 | 107 | 0 | 0 | 54 | 0 | 70 | 1363 | 4415 | 4415 | 0 | 0 | 0 | 0 |
| FB2 d | n.d. | 4.9 | 24.7 | 10.2 | n.d. | 9.0 | 62 | 24 | n.d. | 0 | 0 | 0 | n.d. | 413 | 1427 | 1427 | 0 | 0 | 0 | 0 |
| FB3 d | n.d. | 7.4 | 18.8 | 9.0 | n.d. | 3.4 | 18 | 7.4 | n.d. | 0 | 0 | 0 | n.d. | 91 | 451 | 451 | ||||
| FUM c | 2.5 | 19.8 | 61.2 | 28.6 | 1.3 | 74 | 327 | 132 | 0 | 0 | 59 | 0 | 70 | 1783 | 6294 | 6294 | ||||
| DAS | 11.0 | 8.6 | 5.9 | 8.6 | 0.3 | 0.3 | 0.4 | 0.4 | 0 | 0 | 0 | 0 | 6.1 | 10 | 15 | 15 | ||||
| FX | n.d. | 7.4 | 2.4 | 3.1 | n.d. | 14 | 2.7 | 5.4 | n.d. | 0 | 0 | 0 | n.d. | 224 | 162 | 224 | ||||
| T2 | 1.1 | n.d. | 8.2 | 3.1 | 0.2 | n.d. | 6.2 | 2.1 | 0 | n.d. | 0 | 0 | 17 | n.d. | 122 | 122 | 0 | 0 | 0 | 0 |
| STERIG | 1.1 | n.d. | 1.2 | 0.8 | 0.2 | n.d. | 2.6 | 0.9 | 0 | n.d. | 0 | 0 | 15 | n.d. | 73 | 205 | ||||
| ROQ-C d | n.d. | 2.5 | 2.9 | 1.7 | n.d. | 0.6 | 0.6 | 0.4 | n.d. | 0 | 0 | 0 | n.d. | 30 | 25 | 30 | ||||
| TOTAL c | 98.9 | 100 | 100 | 100 | 1485 | 1730 | 1877 | 1692 | 1310 | 1088 | 1596 | 1310 | 4153 | 13,748 | 8309 | 13,748 | ||||
References
- Marin, S.; Sanchis, V.; Magan, N. Water activity, temperature, and pH effects on growth of Fusarium moniliforme and Fusarium proliferatum isolates from maize. Can. J. Microbiol. 1995, 41, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, K.A.; Hurdman, B.F.; Pitt, J.I. Influence of pH on the growth of some toxigenic species of Aspergillus, Penicillium and Fusarium. Int. J. Food Microbiol. 1991, 12, 141–149. [Google Scholar] [CrossRef]
- Kung, L. Silage fermentation & additives. In Direct-Fed Microbial, Enzyme & Forage Additive Compendium; Miller Publishing Co.: Minnetonka, MN, USA, 2001. [Google Scholar]
- Dunière, L.; Sindou, J.; Chaucheyras-Durand, F.; Chevallier, I.; Thévenot-Sergentet, D. Silage processing and strategies to prevent persistence of undesirable microorganisms. Anim. Feed Sci. Technol. 2013, 182, 1–15. [Google Scholar] [CrossRef]
- Mansfield, M.A.; Kuldau, G.A. Microbiological and molecular determination of mycobiota in fresh and ensiled maize silage. Mycologia 2007, 99, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, D.; Bustos-Lopez, M.P.; Gullino, M.L.; Piano, S.; Tabacco, E.; Borreani, G. Evolution of fungal populations in corn silage conserved under polyethylene or biodegradable films. J. Appl. Microbiol. 2015, 119, 510–520. [Google Scholar] [CrossRef]
- Alonso, V.A.; Pereyra, C.M.; Keller, L.A.M.; Dalcero, A.M.; Rosa, C.A.R.; Chiacchiera, S.M.; Cavaglieri, L.R. Fungi and mycotoxins in silage: An overview. J. Appl. Microbiol. 2013, 115, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Cheli, F.; Campagnoli, A.; Dell’Orto, V. Fungal populations and mycotoxins in silages: From occurrence to analysis. Anim. Feed Sci. Technol. 2013, 183, 1–16. [Google Scholar] [CrossRef]
- Garon, D.; Richard, E.; Sage, L.; Bouchart, V.; Pottier, D.; Lebailly, P. Mycoflora and multimycotoxin detection in corn silage: Experimental study. J. Agric. Food Chem. 2006, 54, 3479–3484. [Google Scholar] [CrossRef] [PubMed]
- Storm, I.M.L.D.; Rasmussen, R.R.; Rasmussen, P.H. Occurrence of pre- and post-harvest mycotoxins and other secondary metabolites in Danish maize silage. Toxins 2014, 6, 2256–2269. [Google Scholar] [CrossRef]
- Lepom, P. Occurrence of Fusarium species and their mycotoxins in maize: 1. Method of determining zearalenone in maize and maize silage by means of high performance liquid chomatography (HPLC) using fluorescence detection. Arch. Anim. Nutr. 1988, 38, 799–806. [Google Scholar]
- Boudra, H.; Morgavi, D.P. Reduction in Fusarium toxin levels in corn silage with low dry matter and storage time. J. Agric. Food Chem. 2008, 56, 4523–4528. [Google Scholar] [CrossRef]
- Wambacq, E.; Vanhoutte, I.; Audenaert, K.; De Gelder, L.; Haesaert, G. Occurrence, prevention and remediation of toxigenic fungi and mycotoxins in silage: A review. J. Sci. Food Agric. 2016, 96, 2284–2302. [Google Scholar] [CrossRef]
- Niderkorn, V.; Boudra, H.; Morgavi, D.P. Binding of Fusarium mycotoxins by fermentative bacteria in vitro. J. Appl. Microbiol. 2006, 101, 849–856. [Google Scholar] [CrossRef]
- Latorre, A.; Dagnac, T.; Lorenzo, B.F.; Llompart, M. Occurrence and stability of masked fumonisins in corn silage samples. Food Chem. 2015, 189, 38–44. [Google Scholar] [CrossRef]
- Pelhate, J. Maize silage: Incidence of moulds during conservation. Folia Vet. Lat. 1977, 7, 1–16. [Google Scholar]
- Richard, E.; Heutte, N.; Sage, L.; Pottier, D.; Bouchart, V.; Lebailly, P.; Garon, D. Toxigenic fungi and mycotoxins in mature corn silage. Food Chem. Toxicol. 2007, 45, 2420–2425. [Google Scholar] [CrossRef]
- Taniwaki, M.H.; Hocking, A.D.; Pitt, J.I.; Fleet, G.H. Growth and mycotoxin production by food spoilage fungi under high carbon dioxide and low oxygen atmospheres. Int. J. Food Microbiol. 2009, 132, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Storm, I.M.L.D.; Sørensen, J.L.; Rasmussen, R.R.; Nielsen, K.F.; Thrane, U. Mycotoxins in silage. Stewart Postharvest Rev. 2008, 4, 1–12. [Google Scholar] [CrossRef]
- Wainwright, M.; Ali, T.A.; Killham, K. Anaerobic growth of fungal mycelium from soil particles onto nutrient-free silica gel. Mycol. Res. 1994, 98, 761–762. [Google Scholar] [CrossRef]
- Gibb, E.; Walsh, J.H. Effect of nutritional factors and carbon dioxide on growth of Fusarium moniliforme and other fungi in reduced oxygen concentrations. Trans. Br. Mycol. Soc. 1980, 74, 111–118. [Google Scholar] [CrossRef]
- Marchant, R.; Nigam, P.; Banat, I.M. An unusual facultatively anaerobic filamentous fungus isolated under prolonged enrichment culture conditions. Mycol. Res. 1994, 98, 757–760. [Google Scholar] [CrossRef]
- Cavallarin, L.; Tabacco, E.; Antoniazzi, S.; Borreani, G. Aflatoxin accumulation in whole crop maize silage as a result of aerobic exposure. J. Sci. Food Agric. 2011, 91, 2419–2425. [Google Scholar] [CrossRef]
- Pahlow, G.; Muck, R.E.; Driehuis, F.; Oude Elferink, S.J.W.H.; Spoelstra, S.F. Microbiology of ensiling. In Silage Science and Technology; Buxton, D.R., Muck, R.E., Harrison, J.H., Eds.; American Society of Agronomy: Madison, WI, USA, 2003. [Google Scholar]
- Woolford, M.K. The detrimental effects of air on silage. J. Appl. Bacteriol. 1990, 68, 101–116. [Google Scholar] [CrossRef]
- Vandicke, J.; De Visschere, K.; Croubels, S.; De Saeger, S.; Audenaert, K.; Haesaert, G. Mycotoxins in Flanders’ fields: Occurrence and correlations with Fusarium species in whole-plant harvested maize. Microorganisms 2019, 7, 571. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E.; Schmidt, R.J.; Holmes, B.J.; Muck, R.E. Silage review: Factors affecting dry matter and quality losses in silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef]
- Muck, R.E. Factors influencing silage quality and their implications for management. J. Dairy Sci. 1988, 71, 2992–3002. [Google Scholar] [CrossRef]
- Zachariasova, M.; Dzuman, Z.; Veprikova, Z.; Hajkova, K.; Jiru, M.; Vaclavikova, M.; Zachariasova, A.; Pospichalova, M.; Florian, M.; Hajslova, J. Occurrence of multiple mycotoxins in European feedingstuffs, assessment of dietary intake by farm animals. Anim. Feed Sci. Technol. 2014, 193, 124–140. [Google Scholar] [CrossRef]
- Keller, L.A.M.; González Pereyra, M.L.; Keller, K.M.; Alonso, V.A.; Oliveira, A.A.; Almeida, T.X.; Barbosa, T.S.; Nunes, L.M.T.; Cavaglieri, L.R.; Rosa, C.A.R. Fungal and mycotoxins contamination in corn silage: Monitoring risk before and after fermentation. J. Stored Prod. Res. 2013, 52, 42–47. [Google Scholar] [CrossRef]
- Mansfield, M.A.; Jones, A.D.; Kuldau, G.A. Contamination of fresh and ensiled maize by multiple Penicillium mycotoxins. Phytopathology 2008, 98, 330–336. [Google Scholar] [CrossRef]
- Jard, G.; Liboz, T.; Mathieu, F.; Guyonvarch, A.; Lebrihi, A. Review of mycotoxin reduction in food and feed: From prevention in the field to detoxification by adsorption or transformation. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2011, 28, 1590–1609. [Google Scholar] [CrossRef]
- Van Schooten, H.A.; Philipsen, A.P.; Groten, J.A.M. Handboek Snijmaïs; Wageningen Livestock Research: Wageningen, The Netherlands, 2018. [Google Scholar]
- Tangni, E.K.; Pussemier, L.; Bastiaanse, H.; Haesaert, G.; Foucart, G.; Van Hove, F. Presence of mycophenolic acid, roquefortine C, citrinin and ochratoxin A in maize and grass silages supplied to dairy cattle in Belgium. J. Anim. Sci. Adv. 2013, 3, 598–612. [Google Scholar]
- Driehuis, F.; Spanjer, M.C.; Scholten, J.M.; Te Giffel, M.C. Occurrence of mycotoxins in feedstuffs of dairy cows and estimation of total dietary intakes. J. Dairy Sci. 2008, 91, 4261–4271. [Google Scholar] [CrossRef]
- Auerbach, H.; Oldenburg, E.; Weissbach, F. Incidence of Penicillium roqueforti and roquefortine C in silages. J. Sci. Food Agric. 1998, 76, 565–572. [Google Scholar] [CrossRef]
- Declerck, S.; Van Hove, F.; Héloïse, B.; Pussemier, L.; Tangni, E.; Haesaert, G.; Daemers, E.; Depoorter, J.; Blust, R.; Robbens, J.; et al. Action for the Promotion of and the Co-Operation with the Belgian Coordinated Collections of Micro-organisms, BCCM, Final Report: Characterization of Fungal Species and Mycotoxins Contaminating Silages in Belgium; Belgian Science Policy: Brussels, Belgium, 2009. [Google Scholar]
- Tangni, E.K.; Pussemier, L.; van Hove, F. Mycotoxin contaminating maize and grass silages for dairy cattle feeding: Current state and challenges. J. Anim. Sci. Adv. 2013, 3, 492–511. [Google Scholar]
- Schmidt, P.; Novinski, C.O.; Junges, D.; Almeida, R.; de Souza, C.M. Concentration of mycotoxins and chemical composition of corn silage: A farm survey using infrared thermography. J. Dairy Sci. 2015, 98, 6609–6619. [Google Scholar] [CrossRef]
- Dagnac, T.; Latorre, A.; Fernández Lorenzo, B.; Llompart, M. Validation and application of a liquid chromatography-tandem mass spectrometry based method for the assessment of the co-occurrence of mycotoxins in maize silages from dairy farms in NW Spain. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2016, 33, 1850–1863. [Google Scholar] [CrossRef]
- Kosicki, R.; Błajet-Kosicka, A.; Grajewski, J.; Twaruzek, M. Multiannual mycotoxin survey in feed materials and feedingstuffs. Anim. Feed Sci. Technol. 2016, 215, 165–180. [Google Scholar] [CrossRef]
- Cogan, T.; Hawkey, R.; Higgie, E.; Lee, M.R.F.; Mee, E.; Parfitt, D.; Raj, J.; Roderick, S.; Walker, N.; Ward, P.; et al. Silage and total mixed ration hygienic quality on commercial farms: Implications for animal production. Grass Forage Sci. 2017, 72, 601–613. [Google Scholar] [CrossRef]
- Kovalsky, P.; Kos, G.; Nährer, K.; Schwab, C.; Jenkins, T.; Schatzmayr, G.; Sulyok, M.; Krska, R. Co-occurrence of regulated, masked and emerging mycotoxins and secondary metabolites in finished feed and maize–An extensive survey. Toxins 2016, 8, 363. [Google Scholar] [CrossRef]
- Pleadin, J.; Vulić, A.; Perši, N.; Škrivanko, M.; Capek, B.; Cvetnić, Ž. Annual and regional variations of aflatoxin B1 levels seen in grains and feed coming from Croatian dairy farms over a 5-year period. Food Control 2015, 47, 221–225. [Google Scholar] [CrossRef]
- Glamočić, D.; Polovinski Horvatović, M.; Jajić, I.; Krstović, S.; Guljaš, D. Occurrence of aflatoxin B1, ochratoxin A and zearalenone in maize silage in the region of Vojvodina, Serbia. Acta Vet. Brno. 2019, 69, 106–115. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef]
- Panasiuk, L.; Jedziniak, P.; Pietruszka, K.; Piatkowska, M.; Bocian, L. Frequency and levels of regulated and emerging mycotoxins in silage in Poland. Mycotoxin Res. 2019, 35, 17–25. [Google Scholar] [CrossRef]
- Reisinger, N.; Schürer-Waldheim, S.; Mayer, E.; Debevere, S.; Antonissen, G.; Sulyok, M.; Nagl, V. Mycotoxin occurrence in maize silage-A neglected risk for bovine gut health? Toxins 2019, 11, 577. [Google Scholar] [CrossRef]
- Driehuis, F.; Spanjer, M.C.; Scholten, J.M.; Te Giffel, M.C. Occurrence of mycotoxins in maize, grass and wheat silage for dairy cattle in the Netherlands. Food Addit. Contam. Part B 2008, 1, 41–50. [Google Scholar] [CrossRef]
- Rasmussen, R.R.; Storm, I.M.L.D.; Rasmussen, P.H.; Smedsgaard, J.; Nielsen, K.F. Multi-mycotoxin analysis of maize silage by LC-MS/MS. Anal. Bioanal. Chem. 2010, 397, 765–776. [Google Scholar] [CrossRef]
- Baliukoniene, V.; Bakutis, B.; Vaivadaite, T.; Bartkiene, E.; Jovaišiene, J. Prevalence of fungi and mycotoxins in silage and milk in Lithuania. Vet. ir Zootech. 2012, 59, 3–9. [Google Scholar]
- Grajewski, J.; Blajet-Kosicka, A.; Twaruzek, M.; Kosicki, R. Occurrence of mycotoxins in Polish animal feed in years 2006–2009. J. Anim. Physiol. Anim. Nutr. 2012, 96, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Shimshoni, J.A.; Cuneah, O.; Sulyok, M.; Krska, R.; Galon, N.; Sharir, B.; Shlosberg, A. Mycotoxins in corn and wheat silage in Israel. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2013, 30, 1614–1625. [Google Scholar] [CrossRef]
- González Pereyra, M.L.; Alonso, V.A.; Sager, R.; Morlaco, M.B.; Magnoli, C.E.; Astoreca, A.L.; Rosa, C.A.R.; Chiacchiera, S.M.; Dalcero, A.M.; Cavaglieri, L.R. Fungi and selected mycotoxins from pre- and postfermented corn silage. J. Appl. Microbiol. 2008, 104, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Commission, E. Commission Recommendation 2006/576/EC of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, L229, 7–9. [Google Scholar]
- Commission, E. Commission recommendation of 27 March 2013 on the presence of T-2 and HT-2 in cereals and cereal products (2013/165/EU). Off. J. Eur. Union 2013, 91, 12–15. [Google Scholar] [CrossRef]
- Gallo, A.; Bertuzzi, T.; Giuberti, G.; Moschini, M.; Bruschi, S.; Cerioli, C.; Masoero, F. New assessment based on the use of principal factor analysis to investigate corn silage quality from nutritional traits, fermentation end products and mycotoxins. J. Sci. Food Agric. 2016, 96, 437–448. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E. The relationship of silage temperature with the microbiological status of the face of corn silage bunkers. J. Dairy Sci. 2010, 93, 2620–2629. [Google Scholar] [CrossRef]
- Pleadin, J.; Vulić, A.; Zadravec, M.; Lešić, T.; Benić, M.; Tkalec, V.J.; Vahčić, N. Presence of Fusarium mycotoxins in feedstuffs and cow milk sampled from Croatian farms during 2015. Mljekarstvo 2017, 67, 102–111. [Google Scholar] [CrossRef]
- Tsiplakou, E.; Anagnostopoulos, C.; Liapis, K.; Haroutounian, S.A.; Zervas, G. Determination of mycotoxins in feedstuffs and ruminant’s milk using an easy and simple LC-MS/MS multiresidue method. Talanta 2014, 130, 8–19. [Google Scholar] [CrossRef]
- Bebber, D.P.; Ramotowski, M.A.T.; Gurr, S.J. Crop pests and pathogens move polewards in a warming world. Nat. Clim. Chang. 2013, 3, 985–988. [Google Scholar] [CrossRef]
- Battilani, P.; Toscano, P.; Van Der Fels-Klerx, H.J.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A.; Pascale, M.; Logrieco, A. Mycotoxin risks under a climate change scenario in Europe. Trends Food Sci. Technol. 2019, 84, 38–40. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Lima, N. How will climate change affect mycotoxins in food? Food Res. Int. 2010, 43, 1902–1914. [Google Scholar] [CrossRef]
- Ogunade, I.M.; Martinez-Tuppia, C.; Queiroz, O.C.M.; Jiang, Y.; Drouin, P.; Wu, F.; Vyas, D.; Adesogan, A.T. Silage review: Mycotoxins in silage: Occurrence, effects, prevention, and mitigation. J. Dairy Sci. 2018, 101, 4034–4059. [Google Scholar] [CrossRef]
- De Boevre, M.; Landschoot, S.; Audenaert, K.; Maene, P.; Di Mavungu, J.D.; Eeckhout, M.; Haesaert, G.; De Saeger, S. Occurrence and within field variability of Fusarium mycotoxins and their masked forms in maize crops in Belgium. World Mycotoxin J. 2014, 7, 91–102. [Google Scholar] [CrossRef]
- Commission, E. Commission regulation (EU) No 574/2011 of 16 June 2011 amending Annex I to directive 2002/32/EC of the European Parliament and of the Council as regards maximum levels for nitrite, melamine, Ambrosia spp. and carry-over of certain coccidiostats and. Off. J. Eur. Union 2011, 159, 7–24. [Google Scholar] [CrossRef]
- Tangni, E.K.; Wambacq, E.; Bastiaanse, H.; Haesaert, G.; Pussemier, L.; De Poorter, J.; Foucart, G.; Van Hove, F. Survey of fungal diversity in silages supplied to dairy cattle in Belgium over a two-year period. J. Anim. Sci. Adv. 2017, 7, 1861–1873. [Google Scholar] [CrossRef]
- Harris, L.J.; Desjardins, A.E.; Plattner, R.D.; Nicholson, P.; Butler, G.; Young, J.C.; Weston, G.; Proctor, R.H.; Hohn, T.M. Possible role of trichothecene mycotoxins in virulence of Fusarium graminearum on maize. Plant Dis. 1999, 83, 954–960. [Google Scholar] [CrossRef]
- Proctor, R.H.; Desjardins, A.E.; McCormick, S.P.; Plattner, R.D.; Alexander, N.J.; Brown, D.W. Genetic analysis of the role of trichothecene and fumonisin mycotoxins in the virulence of Fusarium. Eur. J. Plant Pathol. 2002, 108, 691–698. [Google Scholar] [CrossRef]
- Venkatesh, N.; Keller, N.P. Mycotoxins in conversation with bacteria and fungi. Front. Microbiol. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Audenaert, K.; Vanheule, A.; Höfte, M.; Haesaert, G. Deoxynivalenol: A major player in the multifaceted response of Fusarium to its environment. Toxins 2013, 6, 1–19. [Google Scholar] [CrossRef]
- Reverberi, M.; Ricelli, A.; Zjalic, S.; Fabbri, A.A.; Fanelli, C. Natural functions of mycotoxins and control of their biosynthesis in fungi. Appl. Microbiol. Biotechnol. 2010, 87, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.; Reape, T.J.; Rocha, O.; Doyle, S.M.; Kacprzyk, J.; Doohan, F.M.; McCabe, P.F. The Fusarium mycotoxin deoxynivalenol can inhibit plant apoptosis-like programmed cell death. PLoS ONE 2013, 8, e69542. [Google Scholar] [CrossRef]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; De Saeger, S.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef]
- Birr, T.; Jensen, T.; Preußke, N.; Sönnichsen, F.D.; De Boevre, M.; De Saeger, S.; Hasler, M.; Verreet, J.-A.; Klink, H. Occurrence of Fusarium Mycotoxins and Their Modified Forms in Forage Maize Cultivars. Toxins 2021, 13, 110. [Google Scholar] [CrossRef]
- Uegaki, R.; Tsukiboshi, T.; Tohno, M. Changes in the concentrations of fumonisin, deoxynivalenol and zearalenone in corn silage during ensilage. Anim. Sci. J. 2013, 84, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.; De Boevre, M.; De Saeger, S.; Preußke, N.; Sönnichsen, F.D.; Kramer, E.; Klink, H.; Verreet, J.A.; Birr, T. Effect of ensiling duration on the fate of deoxynivalenol, zearalenone and their derivatives in maize silage. Mycotoxin Res. 2019, 36, 127–136. [Google Scholar] [CrossRef]
- Mansfield, M.A.; De Wolf, E.D.; Kuldau, G.A. Relationships between weather conditions, agronomic practices, and fermentation characteristics with deoxynivalenol content in fresh and ensiled maize. Plant Dis. 2005, 89, 1151–1157. [Google Scholar] [CrossRef]
- Lepom, P.; Knabe, O.; Baath, H. Occurrence of Fusarium strains and their mycotoxins in corn silage. 7. Formation of deoxynivalenol (DON) in a silage corn plot artificially inoculated with Fusarium culmorum and the effect of silaging on the stability of the DON formed. Arch. Tierernahr. 1990, 40, 1005–1012. [Google Scholar] [CrossRef]
- Kjeldahl, J. Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern. Zeitschrift für Anal. Chemie 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Ohmomo, S.; Tanaka, O.; Kitamoto, H. Analysis of organic acids in silage by high-performance liquid chromatography. Bull. Natl. Grassl. Res. Inst. 1993, 48, 51–56. [Google Scholar]
- Flieg, O. A key for the evaluation of silage samples. Futterbau und Giirfutterbereitung 1938, 1, 112–128. [Google Scholar]
- Monbaliu, S.; Van Poucke, C.; Detavernier, C.T.L.; Dumoultn, F.; Van Velde, M.D.E.; Schoeters, E.; Van Dyck, S.; Averkieva, O.; Van Peteghem, C.; De Saeger, S. Occurrence of mycotoxins in feed as analyzed by a multi-mycotoxin LC-MS/MS method. J. Agric. Food Chem. 2010, 58, 66–71. [Google Scholar] [CrossRef]
- Monbaliu, S.; Wu, A.; Zhang, D.; Van Peteghem, C.; De Saeger, S. Multimycotoxin UPLC−MS/MS for Tea, Herbal Infusions and the Derived Drinkable Products. J. Agric. Food Chem. 2010, 58, 12664–12671. [Google Scholar] [CrossRef]
- Nicolaisen, M.; Suproniene, S.; Nielsen, L.K.; Lazzaro, I.; Spliid, N.H.; Justesen, A.F. Real-time PCR for quantification of eleven individual Fusarium species in cereals. J. Microbiol. Methods 2009, 76, 234–240. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 10 March 2021).





| Mycotoxin | Mean Concentration before Ensiling (µg/kg DM) | Mean Concentration after Ensiling (µg/kg DM) | Difference before−after Ensiling (%) |
| DON | 664 ± 241 | 532 ± 199 | −19.9 |
| 3-ADON | 49 ± 19 | 14 ± 9 | −70.9 *** |
| 15-ADON | 90 ± 40 | 63 ± 22 | −30.5 |
| DON+ a | 803 ± 297 | 609 ± 225 | −24.2 |
| ZEN | 326 ± 77 | 147 ± 45 | −54.7 * |
| ENN B | 67 ± 24 | 55 ± 16 | −17.4 |
| AME | 16 ± 12 | 8.9 ± 6.2 | −44.7 |
| FB1 | 315 ± 204 | 155 ± 86 | −50.9 |
| FB2 | 94 ± 66 | 46 ± 29 | −51.0 |
| FB3 | 29 ± 21 | 12 ± 11 | −59.4 |
| FUM a | 437 ± 291 | 212 ± 125 | −51.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vandicke, J.; De Visschere, K.; Ameye, M.; Croubels, S.; De Saeger, S.; Audenaert, K.; Haesaert, G. Multi-Mycotoxin Contamination of Maize Silages in Flanders, Belgium: Monitoring Mycotoxin Levels from Seed to Feed. Toxins 2021, 13, 202. https://doi.org/10.3390/toxins13030202
Vandicke J, De Visschere K, Ameye M, Croubels S, De Saeger S, Audenaert K, Haesaert G. Multi-Mycotoxin Contamination of Maize Silages in Flanders, Belgium: Monitoring Mycotoxin Levels from Seed to Feed. Toxins. 2021; 13(3):202. https://doi.org/10.3390/toxins13030202
Chicago/Turabian StyleVandicke, Jonas, Katrien De Visschere, Maarten Ameye, Siska Croubels, Sarah De Saeger, Kris Audenaert, and Geert Haesaert. 2021. "Multi-Mycotoxin Contamination of Maize Silages in Flanders, Belgium: Monitoring Mycotoxin Levels from Seed to Feed" Toxins 13, no. 3: 202. https://doi.org/10.3390/toxins13030202
APA StyleVandicke, J., De Visschere, K., Ameye, M., Croubels, S., De Saeger, S., Audenaert, K., & Haesaert, G. (2021). Multi-Mycotoxin Contamination of Maize Silages in Flanders, Belgium: Monitoring Mycotoxin Levels from Seed to Feed. Toxins, 13(3), 202. https://doi.org/10.3390/toxins13030202








