The Efficacy of Composite Essential Oils against Aflatoxigenic Fungus Aspergillus flavus in Maize
Abstract
1. Introduction
2. Results
2.1. Antifungal Effect of Essential Oils on Aspergillus flavus
2.2. Determination of Minimum Inhibitory Concentration (MIC) of Cinnamon, Oregano and Lemongrass EOs
2.3. Combinations of EOs with Synergistic Inhibitory Activity against A. flavus
2.4. Inhibitory Effect of Cinnamon, Oregano and Lemongrass (COL)-CEO against AFB1 Biosynthesis at the Transcriptional Level
2.5. Chemical Characterization of EOs
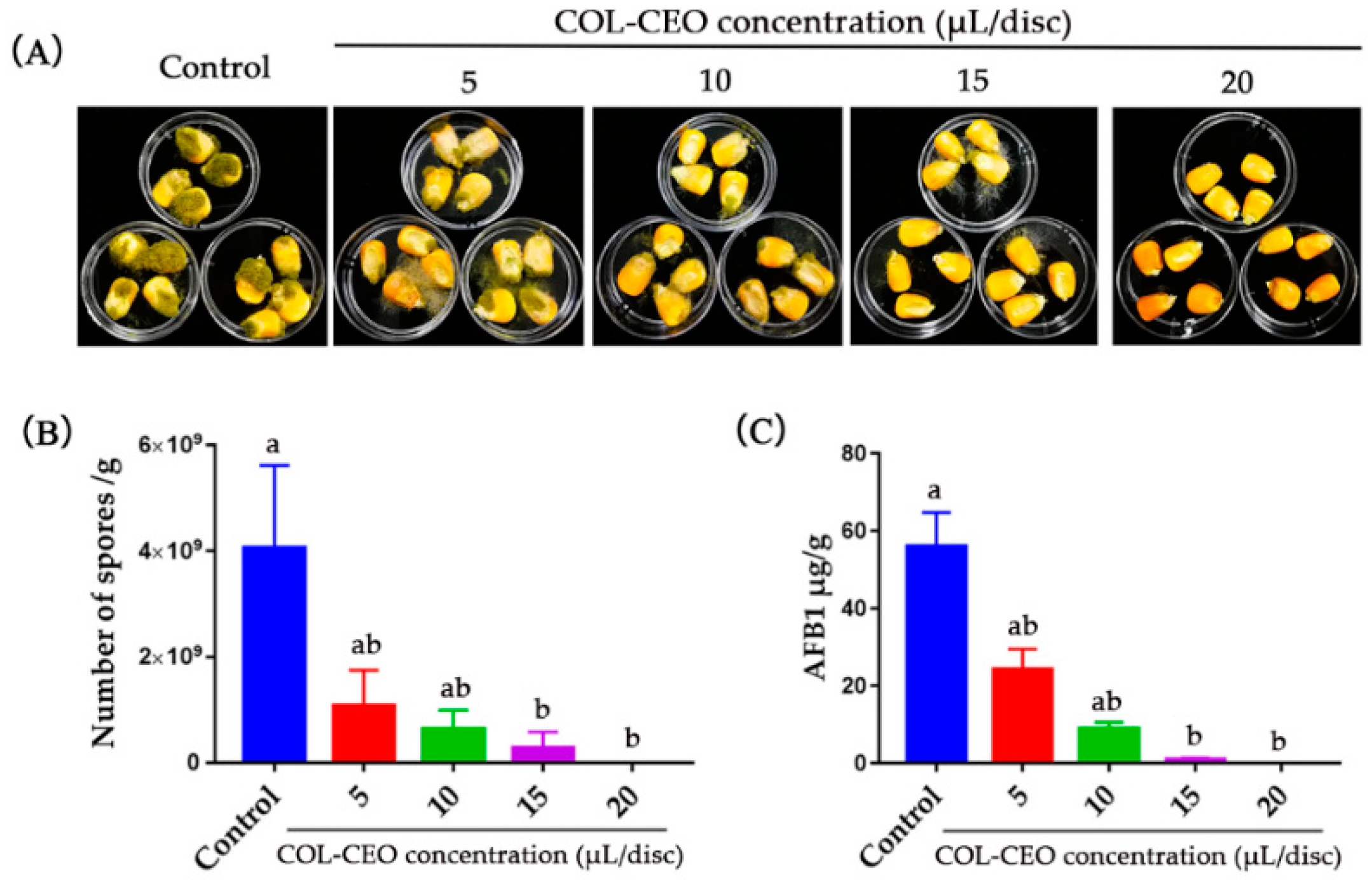
2.6. Antifungal Activity of COL-CEO against A. flavus on Maize Grains
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Essential Oils and Fungal Strain
5.2. Antifungal Activity of EOs on Growth of A. flavus
5.3. Determination of Minimum Inhibitory Concentration (MIC)
5.4. Combinatorial Assays
5.5. RNA Extraction and RT-qPCR Analysis
5.6. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of the EOs
5.7. Fourier Transform Infrared Spectroscopy (FTIR) Analysis of EOs
5.8. CEO Vapors against A. flavus on Maize Grains
5.9. Aflatoxin Analysis
5.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Magan, N.; Aldred, D.J. Post-harvest control strategies: Minimizing mycotoxins in the food chain. Int. J. Food Microbiol. 2007, 119, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Jian, F.J.; Al Mamun, M.A.; White, N.D.G.; Jayas, D.S.; Fields, P.G.; McCombe, J. Safe storage times of FINOLA (R) hemp (Cannabis sativa) seeds with dockage. J. Stored Prod. Res. 2019, 83, 34–43. [Google Scholar] [CrossRef]
- Shafiekhani, S.; Wilson, S.A.; Atungulu, G.G. Impacts of storage temperature and rice moisture content on color characteristics of rice from fields with different disease management practices. J. Stored Prod. Res. 2018, 78, 89–97. [Google Scholar] [CrossRef]
- Suleiman, R.; Bern, C.J.; Brumm, T.J.; Rosentrater, K.A. Impact of moisture content and maize weevils on maize quality during hermetic and non-hermetic storage. J. Stored Prod. Res. 2018, 78, 1–10. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajslova, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2019. [Google Scholar] [CrossRef]
- Robens, J.; Cardwell, K. The costs of mycotoxin management to the USA: Management of aflatoxins in the United States. J. Toxicol. Toxin Rev. 2003, 22, 139–152. [Google Scholar] [CrossRef]
- Dadzie, M.A.; Oppong, A.; Ofori, K.; Eleblu, J.S.; Ifie, E.B.; Blay, E.; Obeng-Bio, E.; Appiah-Kubi, Z.; Warburton, M.L. Distribution of Aspergillus flavus and aflatoxin accumulation in stored maize grains across three agro-ecologies in Ghana. Food Control 2019, 104, 91–98. [Google Scholar] [CrossRef]
- Kovač, M.; Šubaric, D.; Bulaic, M.; Kovač, T.; Šarkanj, B. Yesterday masked, today modified; what do mycotoxins bring next? Arch. Ind. Hyg. Toxicol. 2018, 69, 196–214. [Google Scholar] [CrossRef]
- James, A.; Zikankuba, V.L. Mycotoxins contamination in maize alarms food safety in sub-Sahara Africa. Food Control 2018, 90, 372–381. [Google Scholar] [CrossRef]
- Klich, M.A. Aspergillus flavus: The major producer of aflatoxin. Mol. Plant Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef]
- Singh, P.; Callicott, K.A.; Orbach, M.J.; Cotty, P.J. Molecular analysis of S-morphology aflatoxin producers from the United States reveals previously unknown diversity and two new taxa. Front. Microbiol. 2020, 11, 1236. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer. Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene (No.82); World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- CDC. Outbreak of aflatoxin poisoning-eastern and central provinces, Kenya, January–July 2004. Morb. Mortal. Wkly Rep. 2004, 53, 790–793. [Google Scholar]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar] [CrossRef] [PubMed]
- Bankole, S.A.; Ogunsanwo, B.M.; Eseigbe, D.A. Aflatoxins in Nigerian dry-roasted groundnuts. Food Chem. 2005, 89, 503–506. [Google Scholar] [CrossRef]
- Lewis, L.; Onsongo, M.; Njapau, H.; Schurz-Rogers, H.; Luber, G.; Kieszak, S.; Nyamongo, J.; Backer, L.; Dahiye, A.M.; Misore, A.; et al. Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in eastern and central Kenya. Environ. Health Perspect. 2005, 113, 1763–1767. [Google Scholar] [CrossRef]
- Singh, P.; Cotty, P.J. Aflatoxin contamination of dried red chilies: Contrasts between the United States and Nigeria, two markets differing in regulation enforcement. Food Control 2017, 80, 374–379. [Google Scholar] [CrossRef]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- United States Food and Drug Administration. Action Levels for Poisonous or Deleterious Substances in Human Food and Animal Feed; USFDA: Washington, DC, USA, 2000. Available online: http://www.cfsan.fda.gov/~lrd/fdaact.html#afla (accessed on 20 August 2000).
- Gonzalez-Fandos, E.; Herrera, B. Efficacy of propionic acid against Listeria monocytogenes attached to poultry skin during refrigerated storage. Food Control 2013, 34, 601–606. [Google Scholar] [CrossRef]
- Rutenberg, R.; Bernstein, S.; Fallik, E.; Paster, N.; Poverenov, E. The improvement of propionic acid safety and use during the preservation of stored grains. Crop Protect. 2018, 110, 191–197. [Google Scholar] [CrossRef]
- Agrafioti, P.; Athanassiou, C.G.; Subramanyam, B. Efficacy of heat treatment on phosphine resistant and susceptible populations of stored product insects. J. Stored Prod. Res. 2019, 81, 100–106. [Google Scholar] [CrossRef]
- Šarkanj, B.; Molnar, M.; Čačić, M.; Gille, L. 4-Methyl-7-hydroxycoumarin antifungal and antioxidant activity enhancement by substitution with thiosemicarbazide and thiazolidinone moieties. Food Chem. 2013, 139, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Čačić, M.; Pavić, V.; Molnar, M.; Šarkanj, B.; Has-Schön, E. Design and synthesis of some new 1,3,4-thiadiazines with coumarin moieties and their antioxidative and antifungal activity. Molecules 2014, 19, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Kovač, T.; Borišev, I.; Crevar, B.; Čačić Kenjerić, F.; Kovač, M.; Strelec, I.; Ezekiel, C.N.; Sulyok, M.; Krska, R.; Šarkanj, B. Fullerol C60(OH)24 nanoparticles modulate aflatoxin B1 biosynthesis in Aspergillus flavus. Sci. Rep. 2018, 8, 12855. [Google Scholar] [CrossRef] [PubMed]
- Kovač, T.; Šarkanj, B.; Klapec, T.; Borišev, I.; Kovač, M.; Nevistic, A.; Strelec, I. Fullerol C60(OH)24 nanoparticles and mycotoxigenic fungi: A preliminary investigation into modulation of mycotoxin production. Environ. Sci. Pollut. Res. 2017, 24, 16673–16681. [Google Scholar] [CrossRef]
- Kovač, T.; Šarkanj, B.; Klapec, T.; Borišev, I.; Kovač, M.; Nevistić, A.; Strelec, I. Antiaflatoxigenic effect of fullerene C60 nanoparticles at environmentally plausible concentrations. AMB Express 2018, 8, 14. [Google Scholar] [CrossRef]
- Brandhorst, T.T.; Klein, B.S. Uncertainty surrounding the mechanism and safety of the post-harvest fungicide fludioxonil. Food Chem. Toxicol. 2019, 123, 561–565. [Google Scholar] [CrossRef]
- Jerković, I.; Kranjac, M.; Marijanović, Z.; Šarkanj, B.; Cikoš, A.M.; Aladić, K.; Pedisić, S.; Jokić, S. Chemical diversity of Codium bursa (Olivi) C. Agardh headspace compounds, volatiles, fatty acids and insight into its antifungal activity. Molecules 2019, 24, 842. [Google Scholar] [CrossRef]
- Kapetanakou, A.E.; Nestora, S.; Evageliou, V.; Skandamis, P.N. Sodium alginate-cinnamon essential oil coated apples and pears: Variability of Aspergillus carbonarius growth and ochratoxin A production. Food Res. Int. 2019, 119, 876–885. [Google Scholar] [CrossRef]
- Reyes-Jurado, F.; Cervantes-Rincon, T.; Bach, H.; Lopez-Malo, A.; Palou, E. Antimicrobial activity of Mexican oregano (Lippia berlandieri), thyme (Thymus vulgaris), and mustard (Brassica nigra) essential oils in gaseous phase. Ind. Crop Prod. 2019, 131, 90–95. [Google Scholar] [CrossRef]
- Thanaboripat, D.; Suvathi, Y.; Kurdhom, M.; Rakphung, S. Effect of lemongrass oil and powder on growth and aflatoxin production by Aspergillus flavus IMI242648 in maize. New Biotechnol. 2014, 31, S146. [Google Scholar] [CrossRef]
- Hasheminejad, N.; Khodaiyan, F.; Safari, M. Improving the antifungal activity of clove essential oil encapsulated by chitosan nanoparticles. Food Chem. 2019, 275, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Hossain, F.; Follett, P.; Vu, K.D.; Salmieri, S.; Senoussi, C.; Lacroix, M. Radiosensitization of Aspergillus niger and Penicillium chrysogenum using basil essential oil and ionizing radiation for food decontamination. Food Control 2014, 45, 156–162. [Google Scholar] [CrossRef]
- Rasooli, I.; Owlia, P. Chemoprevention by thyme oils of Aspergillus parasiticus growth and aflatoxin production. Phytochemistry 2005, 66, 2851–2856. [Google Scholar] [CrossRef] [PubMed]
- Gundewadi, G.; Sarkar, D.J.; Rudra, S.G.; Singh, D. Preparation of basil oil nanoemulsion using Sapindus mukorossi pericarp extract: Physico-chemical properties and antifungal activity against food spoilage pathogens. Ind. Crop Prod. 2018, 125, 95–104. [Google Scholar] [CrossRef]
- Boukaew, S.; Prasertsan, P.; Sattayasamitsathit, S. Evaluation of antifungal activity of essential oils against aflatoxigenic Aspergillus flavus and their allelopathic activity from fumigation to protect maize seeds during storage. Ind. Crop Prod. 2017, 97, 558–566. [Google Scholar] [CrossRef]
- Crespo, Y.A.; Bravo Sanchez, L.R.; Quintana, Y.G.; Cabrera, A.S.T.; Bermudez Del Sol, A.; Mayancha, D.M.G. Evaluation of the synergistic effects of antioxidant activity on mixtures of the essential oil from Apium graveolens L., Thymus vulgaris L. and Coriandrum sativum L. using simplex-lattice design. Heliyon 2019, 5, e01942. [Google Scholar] [CrossRef]
- Elhidar, N.; Nafis, A.; Kasrati, A.; Goehler, A.; Bohnert, J.A.; Abbad, A.; Hassani, L.; Mezrioui, N.E. Chemical composition, antimicrobial activities and synergistic effects of essential oil from Senecio anteuphorbium, a Moroccan endemic plant. Ind. Crop Prod. 2019, 130, 310–315. [Google Scholar] [CrossRef]
- Soulaimani, B.; Nafis, A.; Kasrati, A.; Rochdi, A.; Mezrioui, N.E.; Abbad, A.; Hassani, L. Chemical composition, antimicrobial activity and synergistic potential of essential oil from endemic Lavandula maroccana (Mill.). S. Afr. J. Bot. 2019, 125, 202–206. [Google Scholar] [CrossRef]
- Marin, S.; Velluti, A.; Munoz, A.; Ramos, A.J.; Sanchis, V. Control of fumonisin B1 accumulation in naturally contaminated maize inoculated with Fusarium verticillioides and Fusarium proliferatum, by cinnamon, clove, lemongrass, oregano and palmarosa essential oils. Eur. Food Res. Technol. 2003, 217, 332–337. [Google Scholar] [CrossRef]
- Li, Y.Q.; Kong, D.X.; Wu, H. Analysis and evaluation of essential oil components of cinnamon barks using GC-MS and FTIR spectroscopy. Ind. Crop Prod. 2013, 41, 269–278. [Google Scholar] [CrossRef]
- Wan, J.; Zhong, S.B.; Schwarz, P.; Chen, B.C.; Rao, J.J. Physical properties, antifungal and mycotoxin inhibitory activities of five essential oil nanoemulsions: Impact of oil compositions and processing parameters. Food Chem. 2019, 291, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Rajinder Pal, M.; Abhilash, R.; Vikas, J. Essential oils: An impending substitute of synthetic antimicrobial agents to avercome antimicrobial resistance. Curr. Drug Targets 2019, 20, 605–624. [Google Scholar]
- Tantaoui-Elaraki, A.; Beraoud, L. Inhibition of growth and aflatoxin production in Aspergillus parasiticus by essential oils of selected plant materials. J. Environ. Pathol. Toxicol. Oncol. 1994, 13, 67–72. [Google Scholar] [PubMed]
- Bluma, R.; Amaiden, M.R.; Daghero, J.; Etcheverry, M. Control of Aspergillus section Flavi growth and aflatoxin accumulation by plant essential oils. J. Appl. Microbiol. 2008, 105, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Koc, F.; Kara, S. Environmental factors affecting efficacy of some essential oils and potassium sorbate to control growth of Aspergillus flavus, Aspergillus parasiticus on wheat and maize grains. J. Agric. Sci. Technol. 2014, 16, 1325–1334. [Google Scholar]
- Ozcakmak, S.; Dervisoglu, M.; Pembeci-Kodolbas, C.; Sagdic, O. Effects of thyme and rosemary essential oils on the growth of two aflatoxigenic Aspergillus flavus strains. J. Appl. Bot. Food Qual. 2010, 83, 170–174. [Google Scholar]
- Chaemsanit, S.; Matan, N.; Matan, N. Effect of peppermint oil on the shelf-life of dragon fruit during storage. Food Control 2018, 90, 172–179. [Google Scholar] [CrossRef]
- Garcia-Sotelo, D.; Silva-Espinoza, B.; Perez-Tello, M.; Olivas, I.; Alvarez-Parrilla, E.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Antimicrobial activity and thermal stability of rosemary essential oil:beta-cyclodextrin capsules applied in tomato juice. LWT-Food Sci. Technol. 2019, 111, 837–845. [Google Scholar] [CrossRef]
- Sukcharoen, O.; Sirirote, P.; Thanaboripat, D. Control of aflatoxigenic strains by Cinnamomum porrectum essential oil. J. Food Sci. Technol. Mysore 2017, 54, 2929–2935. [Google Scholar] [CrossRef]
- Manso, S.; Cacho-Nerin, F.; Becerril, R.; Nerin, C. Combined analytical and microbiological tools to study the effect on Aspergillus flavus of cinnamon essential oil contained in food packaging. Food Control 2013, 30, 370–378. [Google Scholar] [CrossRef]
- Hossain, F.; Follett, P.; Vu, K.D.; Harich, M.; Salmieri, S.; Lacroix, M. Evidence for synergistic activity of plant-derived essential oils against fungal pathogens of food. Food Microbiol. 2016, 53, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A.M.; Scheffer, J.J.; Baerheim Svendsen, A. Antimicrobial activities of essential oils. A 1976-1986 literature review on possible applications. Pharm. Weekbl. Sci. 1987, 9, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, R.J.; Yang, B. Extraction of essential oils from five cinnamon leaves and identification of their volatile compound compositions. Innov. Food Sci. Emerg. Technol. 2009, 10, 289–292. [Google Scholar] [CrossRef]
- Cai, R.; Hu, M.M.; Zhang, Y.J.; Niu, C.; Yue, T.L.; Yuan, Y.H.; Wang, Z.L. Antifungal activity and mechanism of citral, limonene and eugenol against Zygosaccharomyces rouxii. LWT Food Sci. Technol. 2019, 106, 50–56. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; Contreras, M.D.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- Ehrlich, K.C.; Yu, J.; Cotty, P.J. Aflatoxin biosynthesis gene clusters and flanking regions. J. Appl. Microbiol. 2005, 99, 518–527. [Google Scholar] [CrossRef]
- Yu, J.J.; Chang, P.K.; Ehrlich, K.C.; Cary, J.W.; Bhatnagar, D.; Cleveland, T.E.; Payne, G.A.; Linz, J.E.; Woloshuk, C.P.; Bennett, J.W. Clustered pathway genes in aflatoxin biosynthesis. Appl. Environ. Microbiol. 2004, 70, 1253–1262. [Google Scholar] [CrossRef]
- Alkhayyat, F.; Yu, J.H. Chapter Five—Upstream Regulation of Mycotoxin Biosynthesis. Adv. Appl. Microbiol. 2014, 86, 251–278. [Google Scholar] [CrossRef]
- Grintzalis, K.; Vernardis, S.I.; Klapa, M.I.; Georgiou, C.D. Role of oxidative stress in sclerotial differentiation and aflatoxin B1 biosynthesis in Aspergillus flavus. Appl. Environ. Microbiol. 2014, 80, 5561–5571. [Google Scholar] [CrossRef]
- Kenne, G.J.; Gummadidala, P.M.; Omebeyinje, M.H.; Mondal, A.M.; Bett, D.K.; McFadden, S.; Bromfield, S.; Banaszek, N.; Velez-Martinez, M.; Mitra, C.; et al. Activation of aflatoxin biosynthesis alleviates total ROS in Aspergillus parasiticus. Toxins 2018, 10, 57. [Google Scholar] [CrossRef]
- Reverberi, M.; Zjalic, S.; Ricelli, A.; Punelli, F.; Camera, E.; Fabbri, C.; Picardo, M.; Fanelli, C.; Fabbri, A.A. Modulation of antioxidant defense in Aspergillus parasiticus is involved in aflatoxin biosynthesis: A role for the apyapA gene. Eukaryot. Cell 2008, 7, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Fountain, J.C.; Bajaj, P.; Pandey, M.; Nayak, S.N.; Yang, L.; Kumar, V.; Jayale, A.S.; Chitikineni, A.; Zhuang, W.; Scully, B.T.; et al. Oxidative stress and carbon metabolism influence Aspergillus flavus transcriptome composition and secondary metabolite production. Sci. Rep. 2016, 6, 38747. [Google Scholar] [CrossRef]
- Kim, J.H.; Yu, J.J.; Mahoney, N.; Chan, K.L.; Molyneux, R.J.; Varga, J.; Bhatnagar, D.; Cleveland, T.E.; Nierman, W.C.; Campbell, B.C. Elucidation of the functional genomics of antioxidant-based inhibition of aflatoxin biosynthesis. Int. J. Food Microbiol. 2008, 122, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Li, J.L.; Lu, Z.S.; Liu, Y.; Li, C.M. Transient transmembrane secretion of H2O2: A mechanism for the citral-caused inhibition of aflatoxin production from Aspergillus flavus. Chem. Commun. 2015, 51, 17424–17427. [Google Scholar] [CrossRef] [PubMed]
- Narasaiah, K.V.; Sashidhar, R.B.; Subramanyam, C. Biochemical analysis of oxidative stress in the production of aflatoxin and its precursor intermediates. Mycopathologia 2006, 162, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Shang, B.; Wang, L.; Lu, Z.; Liu, Y. Cinnamaldehyde inhibits fungal growth and aflatoxin B1 biosynthesis by modulating the oxidative stress response of Aspergillus flavus. Appl. Microbiol. Biotechnol. 2016, 100, 1355–1364. [Google Scholar] [CrossRef]
- Božik, M.; Císarová, M.; Tančinová, D.; Kouřimská, L.; Hleba, L.; Klouček, P. Selected essential oil vapours inhibit growth of Aspergillus spp. in oats with improved consumer acceptability. Ind. Crop Prod. 2017, 98, 146–152. [Google Scholar] [CrossRef]
- Matusiak, K.; Machnowski, W.; Wrzosek, H.; Polak, J.; Rajkowska, K.; Smigielski, K.; Kunicka-Styczynska, A.; Gutarowska, B. Application of Cinnamomum zeylanicum essential oil in vapour phase for heritage textiles disinfection. Int. Biodeterior. Biodegrad. 2018, 131, 88–96. [Google Scholar] [CrossRef]
- El Ouadi, Y.; Manssouri, M.; Bouyanzer, A.; Majidi, L.; Bendaif, H.; Elmsellem, H.; Shariati, M.A.; Melhaoui, A.; Hammouti, B. Essential oil composition and antifungal activity of Melissa officinalis originating from north-Est Morocco, against postharvest phytopathogenic fungi in apples. Microb. Pathog. 2017, 107, 321–326. [Google Scholar] [CrossRef]
- Nikkhah, M.; Hashemi, M.; Najafi, M.B.H.; Farhoosh, R. Synergistic effects of some essential oils against fungal spoilage on pear fruit. Int. J. Food Microbiol. 2017, 257, 285–294. [Google Scholar] [CrossRef]
- Tullio, V.; Nostro, A.; Mandras, N.; Dugo, P.; Banche, G.; Cannatelli, M.A.; Cuffini, A.M.; Alonzo, V.; Carlone, N.A. Antifungal activity of essential oils against filamentous fungi determined by broth microdilution and vapour contact methods. J. Appl. Microbiol. 2007, 102, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Susc, E.C.A. Terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin. Microbiol. Infect. 2000, 6, 503–508. [Google Scholar]
- Fratini, F.; Mancini, S.; Turchi, B.; Friscia, E.; Pistelli, L.; Giusti, G.; Cerri, D. A novel interpretation of the Fractional Inhibitory Concentration Index: The case Origanum vulgare L. and Leptospermum scoparium JR et G. Forst essential oils against Staphylococcus aureus strains. Microbiol. Res. 2017, 195, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Shao, Q.; Li, C.; Zhao, K.; Jiang, L.; Fan, J.; Jiang, H.; Tao, F. An efficient Agrobacterium-mediated transformation method for aflatoxin generation fungus Aspergillus flavus. J. Microbiol. 2018, 56, 356–364. [Google Scholar] [CrossRef]
- Han, G.M.; Li, C.P.; Xiang, F.Z.; Zhao, Q.Q.; Zhao, Y.; Cai, R.H.; Cheng, B.J.; Wang, X.W.; Tao, F. Genome-wide association study leads to novel genetic insights into resistance to Aspergillus flavus in maize kernels. BMC Plant Biol. 2020, 20. [Google Scholar] [CrossRef] [PubMed]
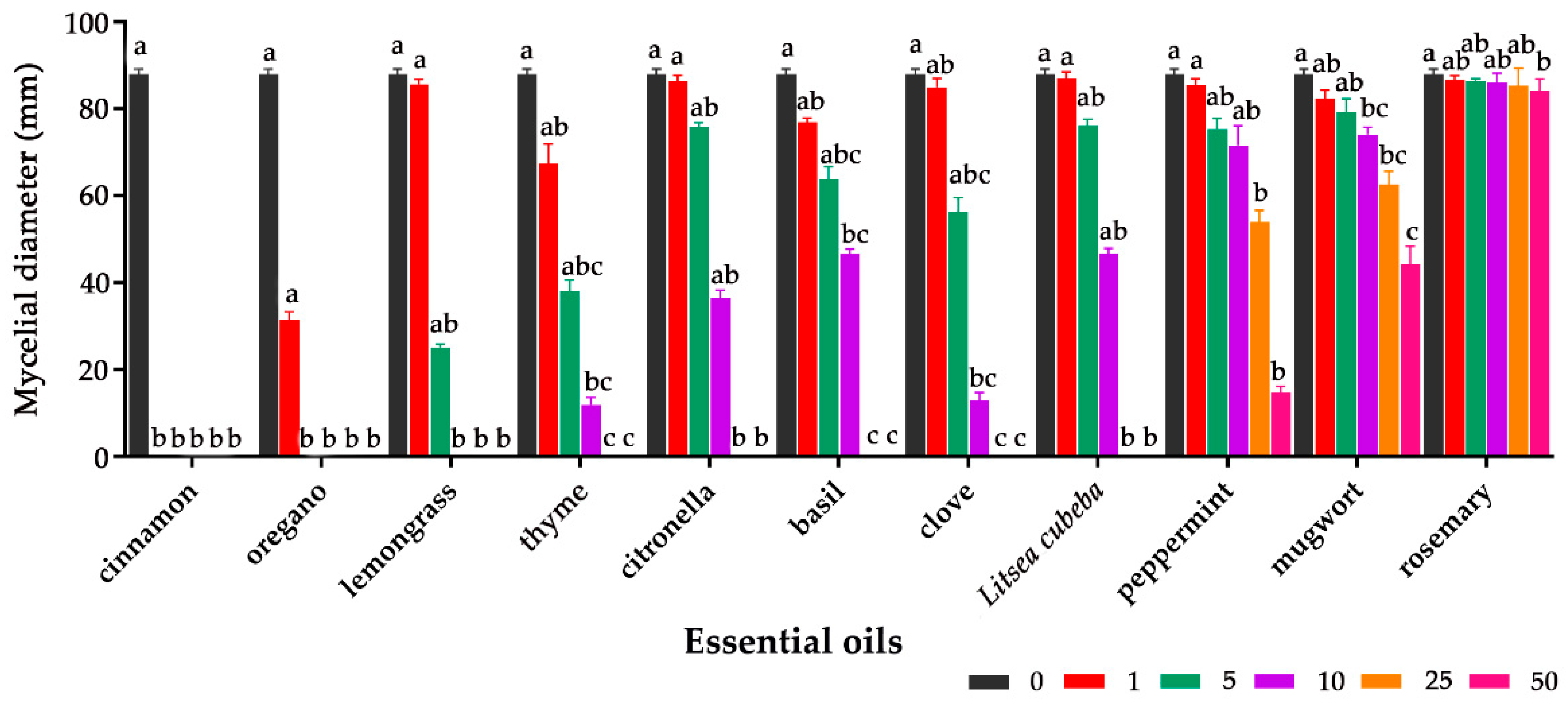
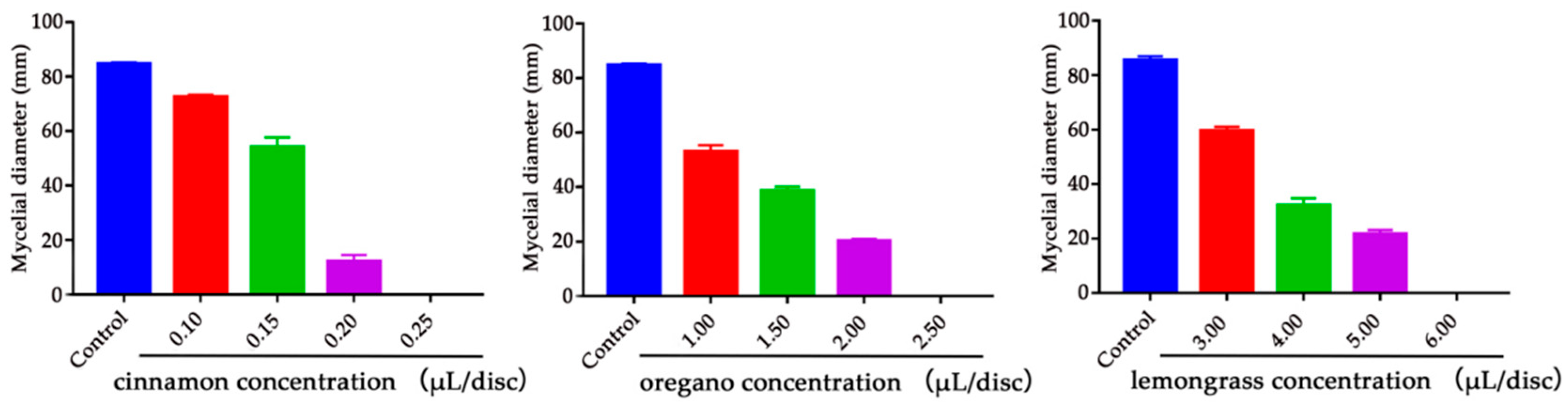
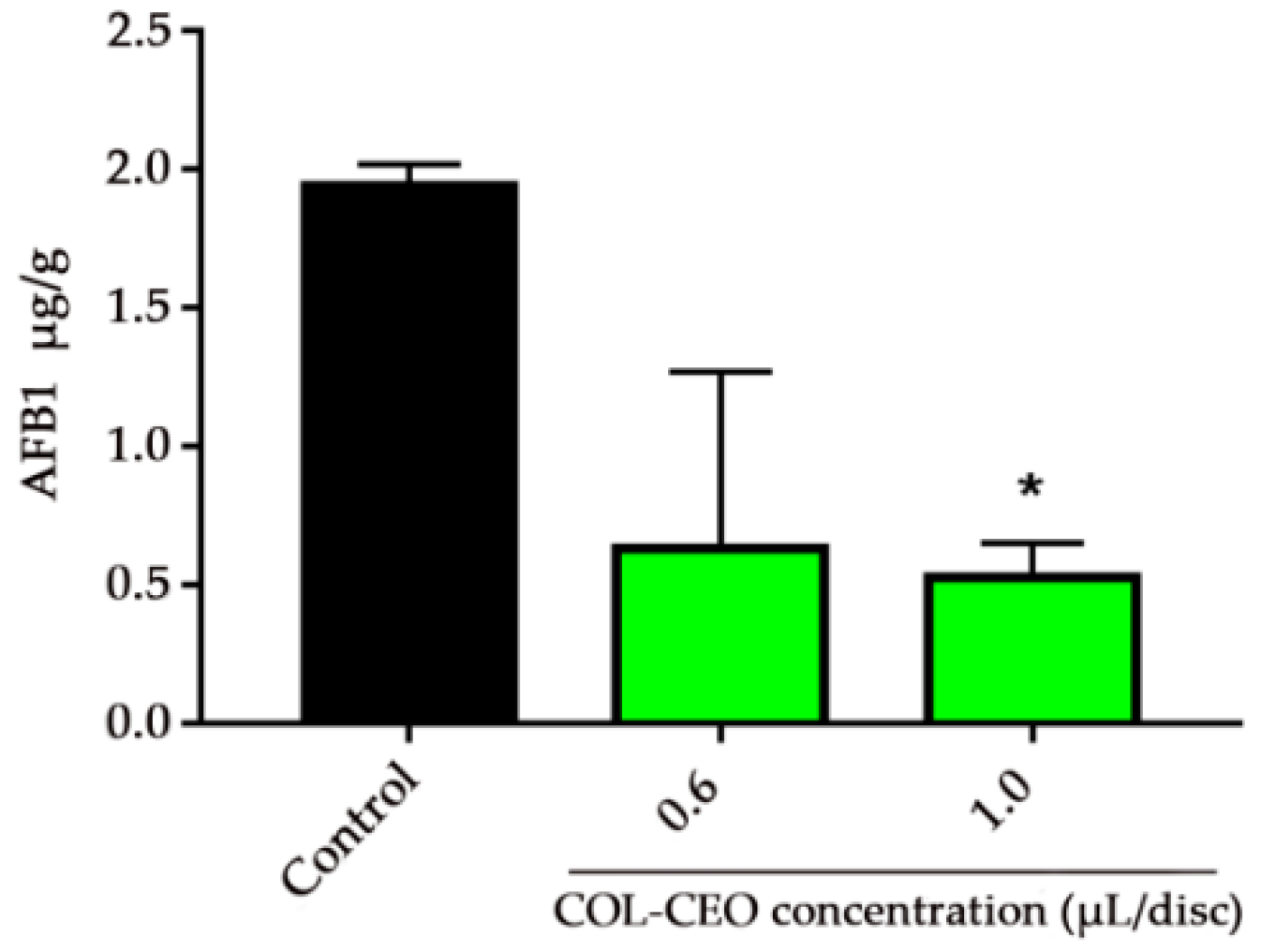
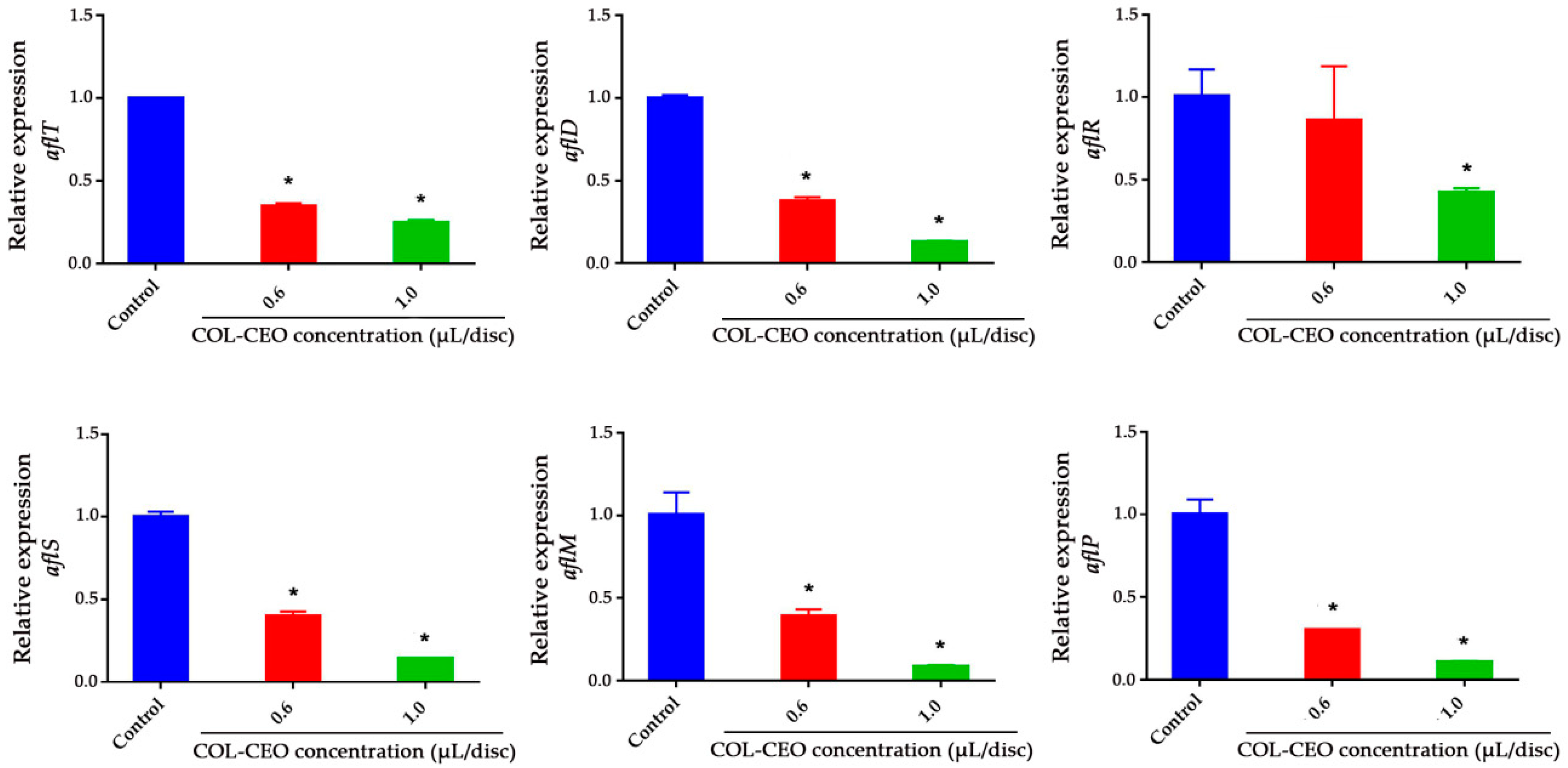


| Concentration of EO (μL/disc) | Total | FIC | FICI | Antifungal Activity | ||||
|---|---|---|---|---|---|---|---|---|
| Cinnamon | Oregano | Lemongrass | Cinnamon | Oregano | Lemongrass | |||
| 0.1250 | 0.6250 | - | 0.750 | 0.5000 | 0.2500 | - | 0.7500 | Additive |
| 0.1250 | - | 1.5000 | 1.625 | 0.5000 | - | 0.2500 | 0.7500 | Additive |
| - | 1.2500 | 1.5000 | 2.750 | - | 0.5000 | 0.2500 | 0.7500 | Additive |
| 0.0313 | 0.1563 | 1.5000 | 1.6876 | 0.1250 | 0.0625 | 0.2500 | 0.4375 | Synergistic |
| Peak No. | Retention Time, Minutes | Name | Cinnamon% | Oregano% | Lemongrass% | COL-CEO% |
|---|---|---|---|---|---|---|
| 1 | 15.607 | 6,6-Trimethyl-(1 theta)-bicyclo [3.1.1]hept-2-en | 0.08 | 0.03 | ND | ND |
| 2 | 16.637 | Benzaldehyde | 1.04 | ND | ND | ND |
| 3 | 18.883 | Limonene | ND | 0.02 | 5.83 | 4.29 |
| 4 | 22.785 | (S)-Cis-verbenol | 0.09 | ND | 0.89 | 0.63 |
| 5 | 23.026 | Phenylpropyl aldehyde | 0.19 | ND | ND | ND |
| 6 | 24.583 | Carveol | 2.20 | ND | 1.70 | 1.18 |
| 7 | 25.062 | (Z)-Citral | 0.43 | ND | 43.66 | 33.44 |
| 8 | 25.45 | Phenethyl acetate | 0.09 | ND | ND | ND |
| 9 | 25.835 | (E)-Citral | ND | ND | 43.55 | 32.88 |
| 10 | 26.271 | Cinnamaldehyde | 89.33 | 0.03 | 0.1 | 3.76 |
| 11 | 26.349 | Thymol | ND | 13.26 | ND | 1.78 |
| 12 | 26.717 | Carvacrol | ND | 84.96 | ND | 19.84 |
| 13 | 27.428 | 2,3-Epoxygeranial | 0.19 | 0.02 | 1.30 | 0.76 |
| 14 | 27.822 | Hexa-1,3-dien-1-ylbenzene | 0.15 | ND | ND | ND |
| 15 | 28.479 | (2,2,6-Trimethylbicyclo [4.1.0]hept-1-yl)methanol | 0.17 | 0.08 | 1.49 | 0.94 |
| 16 | 28.711 | 2-Pentadec-12-ynoxyoxane | ND | ND | 0.06 | 0.07 |
| 17 | 28.979 | 2-Vinylnaphthalene | 0.17 | ND | ND | ND |
| 18 | 32.786 | (E)-2-Methoxycinnamaldehyde | 4.66 | ND | ND | ND |
| 19 | 35.951 | 4-Phenylbenzaldehyde | 0.30 | ND | ND | ND |
| 20 | 46.199 | 3,3,5,5-Tetramethyl-8-(3-methylbutyl)-6,7-dihydro-2H-s-indacen-1-one | ND | 0.59 | ND | ND |
| total | 99.09 | 98.99 | 98.58 | 99.57 |
| Gene | Primer Sequence (5’–3’) | Target Fragment Length (bp) |
|---|---|---|
| β-tubulin | F: GACACCGTTGTTGAGCCCTA | 135 |
| R: GTCACCGTAAGAGGGGTTGG | ||
| aflT | F: CCATTCGCAACCAATCACC | 105 |
| R: GCCAATCGACATGATCACGC | ||
| aflD | F: GCCATCACGGTCAAGAGAAG | 106 |
| R: GCCTTCAGCGACGGTTAGTC | ||
| aflR | F: CCACTACCACCGTTTCAGGC | 88 |
| R: ATGCCAGCACCTTGAGAACG | ||
| aflS | F: CGCTCACTCGGATGAACTGG | 109 |
| R: CCAGACTCGGCCTTAGCTTC | ||
| aflM | F: ATCCTGAGGCAACTGCGAAG | 100 |
| R: TCACGCAAGCAGTGTTAGAGC | ||
| aflP | F: AACTCCCTCCTCCACCGAAC | 109 |
| R: CGATTGTGTCGAGTGATGTGG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, F.; Zhao, Q.; Zhao, K.; Pei, H.; Tao, F. The Efficacy of Composite Essential Oils against Aflatoxigenic Fungus Aspergillus flavus in Maize. Toxins 2020, 12, 562. https://doi.org/10.3390/toxins12090562
Xiang F, Zhao Q, Zhao K, Pei H, Tao F. The Efficacy of Composite Essential Oils against Aflatoxigenic Fungus Aspergillus flavus in Maize. Toxins. 2020; 12(9):562. https://doi.org/10.3390/toxins12090562
Chicago/Turabian StyleXiang, Fangzhi, Qianqian Zhao, Kai Zhao, Hao Pei, and Fang Tao. 2020. "The Efficacy of Composite Essential Oils against Aflatoxigenic Fungus Aspergillus flavus in Maize" Toxins 12, no. 9: 562. https://doi.org/10.3390/toxins12090562
APA StyleXiang, F., Zhao, Q., Zhao, K., Pei, H., & Tao, F. (2020). The Efficacy of Composite Essential Oils against Aflatoxigenic Fungus Aspergillus flavus in Maize. Toxins, 12(9), 562. https://doi.org/10.3390/toxins12090562





