Determination of Aflatoxin M1 in Raw Milk from Different Provinces of Ecuador
Abstract
1. Introduction
2. Results
3. Discussion
| Country | AFM1 Maximum Limits in Raw Milk (µg/kg) | Number of Samples | Meanμg/kg | Positive Samples to AFM1 | Samples > 0.5 μg/kg | Samples > 0.05 μg/kg | Type of Analysis | Detection Limit (μg/kg) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| USA | 0.5 | ||||||||
| EU | 0.05 | ||||||||
| Ecuador | 0.5 | 209 | 0.077 | 209 (100%) | 4 (1.91%) | 124 (59.3%) | LFIA | 0.025 | This study |
| 50 | 0.034 | 50 (100%) | 0 (0%) | 2 (4%) | LFIA | 0.02 | [22] | ||
| 88 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ELISA/SNAP | 0.05 | [23] | ||
| Peru | 0.5 | 40 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ELISA/SNAP | 0.05 | [25] |
| Argentina | 0.5 | - | 0.059 | 100% | (0.81%) | (32.65%) | - | - | [26] |
| Mexico | 0.5 | 216 | 0.026 | 117 (54.16%) | 0 (0%) | 31 (27.31%) | ELISA | 0.05 | [27] |
| Chile | 0.05 | 44 | 0.06 | 33 (75%) | 33 (75%) | 16 (36.4%) | HPLC-FL | 0.0091 | [29] |
| Italy | 0.05 | 31702 | 0.00719–0.02253 | - | - | - | LFIA and ELISA | 0.025 | [30] |
| China | 0.5 | 133 | 0.0159 | 100 (75.2%) | 0 (0%) | 0 (0%) | LC-MS/MS | 0.005 | [31] |
| Iran | 0.1 | Meta-analysis 77 studies | 0.056 | Meta-analysis | [33] | ||||
| India | 0.5 | 150 | 0.262 | 77 (51.33%) | 31 (20.67%) | 46 (30.66%) | HPLC | 0.052 | [34] |
| Pakistan | 0.5 | 690 | 0.640 | 690 (100%) | 483 (70%) | 690 (100%) | ELISA | 0.1 | [35] |
| Turkey | 0.5 | 120 | 0.0051 | 107 (89.2%) | 4 (3.33%) | 13 (10.83%) | ELISA | 0.005 | [36] |
| Jordan | 0.5 | 175 | 0.0689 | 175 (100%) | 40 (23%) | 115 (66%) | ELISA | 0.025 | [37] |
| Kenya | 0.5 | 96 | 0.2903 | 96 (100%) | 7 (7.5%) | 64 (66.6%) | ELISA | 0.005 | [38] |
4. Conclusions
5. Materials and Methods
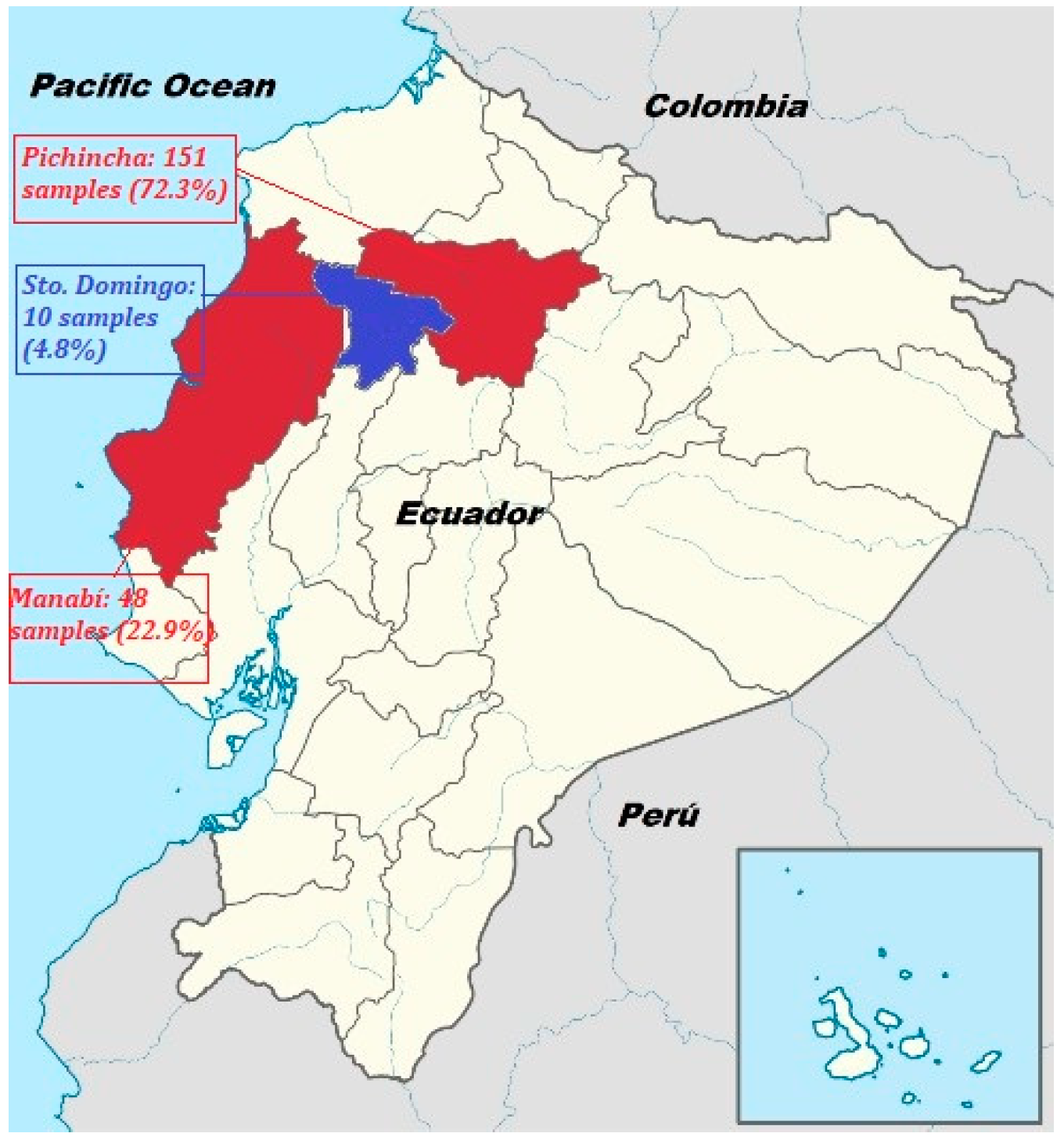
5.1. Sample Collection
5.2. Analysis of AFM1 in Milk by LFIA
5.3. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gimeno, A.; Martins, M.L. Micotoxinas y Micotoxicosis En Animales y Humanos, 3rd ed.; Special Nutrients: Miami, FL, USA, 2011; Available online: http://www.specialnutrients.com/pdf/book/3%20edicion%20MICOTOXINAS%20LR%20Secure.pdf (accessed on 5 April 2020).
- Toso, R.E.; Toribio, M.S.; Diesser, M.; Borello, A.B.; Ardoino, S.M. Affections in Animals and Humans Due to Ingestion or Exposure to Aflatoxins. Preventive Measures to Avoid Toxic Effects. Cienc. Vet. 2018, 20, 51–67. [Google Scholar] [CrossRef]
- Grim, C.J. The Hotspot for (Global) One Health in Primary Food Production: Aflatoxin M1 in Dairy Products. Front. Public Health 2017, 4, 294. [Google Scholar] [CrossRef]
- Rosim, R.E.; De Oliveira, C.A.; Corassin, C.H. Aflatoxina M1 e Aflatoxina B1-Lisina Como Biomarcadores de Avaliação Da Eficiência de Adsorventes Para Aflatoxinas: Artigo de Revisão. Ensaios e Ciência C Biológicas Agrárias e Da Saúde 2018, 22, 171–178. [Google Scholar] [CrossRef]
- Assaf, J.C.; El Khoury, A.; Chokr, A.; Louka, N.; Atoui, A. A Novel Method for Elimnation of Aflatoxin M1 in Milk Using Lactobacillus Rhamnosus GG Biofilm. Int. J. Dairy Technol. 2019, 72, 248–256. [Google Scholar] [CrossRef]
- Akhtar, S.; Shahzad, M.A.; Yoo, S.; Ismail, A.; Hameed, A.; Ismail, T.; Riaz, M. Determination of Aflatoxin M 1 and Heavy Metals in Infant Formula Milk Brands Available in Pakistani Markets. Korean J. Food Sci. Anim. Resour. 2017, 37, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Vaz, A.; Cabral Silva, A.; Rodrigues, A.; Venâncio, A. Detection Methods for Aflatoxin M1 in Dairy Products. Microorganisms 2020, 8, 246. [Google Scholar] [CrossRef]
- Gao, Y.N.; Wang, J.Q.; Li, S.L.; Zhang, Y.D.; Zheng, N. Aflatoxin M1 Cytotoxicity against Human Intestinal Caco-2 Cells Is Enhanced in the Presence of Other Mycotoxins. Food Chem. Toxicol. 2016, 96, 79–89. [Google Scholar] [CrossRef]
- Branch, F.; Darsanaki, R.K.; Mohammadi, M.; Kolavani, M.H.; Issazadeh, K.; Aliabadi, M.A.; Branch, L. Determination of Aflatoxin M1 Levels in Raw Milk Samples in Gilan, Iran. Adv. Stud. Biol. 2013, 5, 151–156. [Google Scholar]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological Properties and Their Involvement in Cancer Development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef]
- Saha Turna, N.; Wu, F. Risk Assessment of Aflatoxin-Related Liver Cancer in Bangladesh. Food Addit. Contam. Part A 2019, 36, 320–326. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Y.; Fei, P.; Liu, J.; Ren, D. A Survey on the Aflatoxin M1 Occurrence in Raw Milk and Dairy Products from Water Buffalo in South China. Food Control 2019, 105, 159–163. [Google Scholar] [CrossRef]
- Udomkun, P.; Nimo, A.; Nagle, M.; Müller, J.; Vanlauwe, B.; Bandyopadhyay, R. Innovative Technologies to Manage a Fl Atoxins in Foods and Feeds and the pro Fi Tability of Application e A Review. Food Control 2017, 76, 127–138. [Google Scholar] [CrossRef]
- Warth, B.; Braun, D.; Ezekiel, C.N.; Turner, P.C.; Degen, G.H.; Marko, D. Biomonitoring of Mycotoxins in Human Breast Milk: Current State and Future Perspectives. Chem. Res. Toxicol. 2016, 29, 1087–1097. [Google Scholar] [CrossRef]
- Awaisheh, S.S.; Rahahleh, R.J.; Algroom, R.M.; Al-Bakheit, A.A.; Al-Khaza’leh, J.M.; Al-Dababseh, B.A. Contamination Level and Exposure Assessment to Aflatoxin M1 in Jordanian Infant Milk Formulas. Ital. J. Food Saf. 2019, 8, 127–130. [Google Scholar] [CrossRef]
- Becker-Algeri, T.A.; Castagnaro, D.; Bortoli, K.; Souza, C.; Drunkler, D.A.; Badiale-Furlong, E. Mycotoxins in Bovine Milk and Dairy Products: A Review. J. Food Sci. 2016, 81, R544–R552. [Google Scholar] [CrossRef]
- Nguyen, T.; Flint, S.; Palmer, J. Control of Aflatoxin M1 in Milk by Novel Methods: A Review. Food Chem. 2020, 311, 125984. [Google Scholar] [CrossRef]
- INEN. Leche Cruda: Requisitos NTE INEN 9. Available online: https://www.gob.ec/sites/default/files/regulations/2018-10/Documento_BL NTE INEN 9 Leche cruda Requisitos.pdf (accessed on 10 January 2020).
- Hassanpour, M.; Rezaie, M.R.; Baghizadeh, A. Practical Analysis of Aflatoxin M1 Reduction in Pasteurized Milk Using Low Dose Gamma Irradiation. J. Environ. Health Sci. Eng. 2019, 17, 863–872. [Google Scholar] [CrossRef]
- Rahmani, J.; Alipour, S.; Miri, A.; Fakhri, Y.; Riahi, S.-M.; Keramati, H.; Moradi, M.; Amanidaz, N.; Pouya, R.H.; Bahmani, Z.; et al. The Prevalence of Aflatoxin M1 in Milk of Middle East Region: A Systematic Review, Meta-Analysis and Probabilistic Health Risk Assessment. Food Chem. Toxicol. 2018, 118, 653–666. [Google Scholar] [CrossRef]
- INEC. Encuesta de Superficie y Producción Agropecuaria Continua. Available online: https://www.ecuadorencifras.gob.ec/documentos/web-inec/Estadisticas_agropecuarias/espac/espac-2018/Presentacion de principales resultados.pdf (accessed on 20 December 2019).
- González, P. Determinación de Aflatoxina M1 En Leche Cruda de Vaca En Centros de Acopio de Pequeños Productores En Las Cinco Provincias de La Sierra Con Mayor Producción En El Ecuador. Master’s Thesis, Universidad De Las Américas-Ecuador, Quito, Ecuador, 2018. Available online: http://dspace.udla.edu.ec/bitstream/33000/9836/1/UDLA-EC-TMACSA-2018-18.pdf (accessed on 5 April 2019).
- Ortiz, M.; Cazar, M. Determinación de La Presencia de Aflatoxina M1 y Antibióticos En Leche Cruda de Las Fincas de Mayor Producción Del Cantón Biblán. Master’s Thesis, Universidad del Azuay-Ecuador, Cuenca, Ecuador, 2014. Available online: http://dspace.uazuay.edu.ec/bitstream/datos/3341/1/10109.pdf (accessed on 6 April 2020).
- Molina, A.; Chavarría, G.; Alfaro-Cascante, M.; Leiva, A.; Granados-Chinchilla, F. Mycotoxins at the Start of the Food Chain in Costa Rica: Analysis of Six Fusarium Toxins and Ochratoxin A between 2013 and 2017 in Animal Feed and Aflatoxin M1 in Dairy Products. Toxins 2019, 11, 312. [Google Scholar] [CrossRef]
- Ortiz, C. Análisis De Aflatoxina M1 En Leche Fresca De Establos. Rev. Investig. Vet. Perú 2009, 20, 139–141. [Google Scholar]
- Signorini, M.L.; Gaggiotti, M.; Molineri, A.; Chiericatti, C.A.; Zapata de Basílico, M.L.; Basílico, J.C.; Pisani, M. Exposure Assessment of Mycotoxins in Cow’s Milk in Argentina. Food Chem. Toxicol. 2012, 50, 250–257. [Google Scholar] [CrossRef]
- Miranda, C. Factores de Riesgo Asociados Con La Ocurrencia de La AFM1 En La Leche Cruda de Vaca En Establos de La Región El Llano, México, Universidad Autónoma de Aguascalientes, 2015. Available online: http://bdigital.dgse.uaa.mx:8080/xmlui/handle/11317/381 (accessed on 11 November 2019).
- Quevedo-Garza, P.A.; Amador-Espejo, G.G.; Salas-García, R.; Ramos-Peña, E.G.; Trujillo, A.J. Aflatoxin M1 Determination in Infant Formulae Distributed in Monterrey, Mexico. Toxins 2020, 12, 100. [Google Scholar] [CrossRef]
- Núñez, C.; Hervé, L. Determinación de La Aflatoxina M1 En Lecherías de La Región Metropolitana y Región de Valparaíso, Universidad de Chile, 2018. Available online: http://repositorio.uchile.cl/bitstream/handle/2250/168623/Determinacion-de-la-aflatoxina.pdf?sequence=4&isAllowed=y (accessed on 5 January 2020).
- Serraino, A.; Bonilauri, P.; Kerekes, K.; Farkas, Z.; Giacometti, F.; Canever, A.; Zambrini, A.V.; Ambrus, Á. Occurrence of Aflatoxin M1 in Raw Milk Marketed in Italy: Exposure Assessment and Risk Characterization. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Xiong, J.; Peng, L.; Zhou, H.; Lin, B.; Yan, P.; Wu, W.; Liu, Y.; Wu, L.; Qiu, Y. Prevalence of Aflatoxin M1 in Raw Milk and Three Types of Liquid Milk Products in Central-South China. Food Control 2020, 108, 106840. [Google Scholar] [CrossRef]
- Li, S.; Min, L.; Wang, G.; Li, D.; Zheng, N.; Wang, J. Occurrence of Aflatoxin M1 in Raw Milk from Manufacturers of Infant Milk Powder in China. Int. J. Environ. Res. Public Health 2018, 15, 879. [Google Scholar] [CrossRef]
- Khaneghahi Abyaneh, H.; Bahonar, A.; Noori, N.; Yazdanpanah, H.; Shojaee AliAbadi, M.H. The Overall and Variations of Aflatoxin M1 Contamination of Milk in Iran: A Systematic Review and Meta-Analysis Study. Food Chem. 2020, 310, 125848. [Google Scholar] [CrossRef]
- Sharma, H.; Jadhav, V.J.; Garg, S.R. Aflatoxin M1 in Milk in Hisar City, Haryana, India and Risk Assessment. Food Addit. Contam. Part B Surveill. 2019, 13, 59–63. [Google Scholar] [CrossRef]
- Akbar, N.; Nasir, M.; Naeem, N.; Ahmad, M.U.D.; Iqbal, S.; Rashid, A.; Imran, M.; Gondal, T.A.; Atif, M.; Salehi, B.; et al. Occurrence and Seasonal Variations of Aflatoxin M1 in Milk from Punjab, Pakistan. Toxins 2019, 11, 574. [Google Scholar] [CrossRef]
- Eker, F.Y.; Muratoglu, K.; Eser, A.G. Detection of Aflatoxin M1 in Milk and Milk Products in Turkey. Environ. Monit. Assess. 2019, 191, 523. [Google Scholar] [CrossRef]
- Sharaf, O. Aflatoxin M1 Levels in Raw Milk, Pasteurized Milk and Infant Formula. Ital. J. Food Saf. 2016, 5, 158–160. [Google Scholar] [CrossRef]
- Kuboka, M.M.; Imungi, J.K.; Njue, L.; Mutua, F.; Grace, D.; Lindahl, J.F. Occurrence of Aflatoxin M1 in Raw Milk Traded in Peri-Urban Nairobi, and the Effect of Boiling and Fermentation. Infect. Ecol. Epidemiol. 2019, 9, 1625703. [Google Scholar] [CrossRef]
- Gutiérrez, R.; Vega, S.; Ruiz, J.L.; Yamazaki, A.; Rivera, J.G.; Escobar, A. Evaluación de Aflatoxina M1 En Leche Orgánica Producida En Tecpatán, Chiapas, México. Rev. Salud Anim. 2013, 35, 33–37. [Google Scholar]
- Peña-Rodas, O.; Martinez-Lopez, R.; Hernandez-Rauda, R. Occurrence of Aflatoxin M1 in Cow Milk in El Salvador: Results from a Two-Year Survey. Toxicol. Rep. 2018, 5, 671–678. [Google Scholar] [CrossRef]
- Yunus, A.; Imtiaz, N.; Khan, H.; Ibrahim, M.; Zafar, Y. Aflatoxin Contamination of Milk Marketed in Pakistan: A Longitudinal Study. Toxins 2019, 11, 110. [Google Scholar] [CrossRef]
- Ismail, A.; Akhtar, S.; Levin, R.E.; Ismail, T.; Riaz, M.; Amir, M. Aflatoxin M1: Prevalence and Decontamination Strategies in Milk and Milk Products. Crit. Rev. Microbiol. 2015, 418–427. [Google Scholar] [CrossRef]
- Dakhili, M.; Shalibeik, S.; Ahmadi, I. Detection of Aflatoxin M1 in Milk from Qom (Aried and Semiaried) Province of Iran. Int. J. Adv. Biotechnol. Res. 2016, 7, 1461–1465. [Google Scholar]
- Hashemi, M. A Survey of Aflatoxin M1 in Cow Milk in Southern Iran. J. Food Drug Anal. 2016, 24, 888–893. [Google Scholar] [CrossRef]
- Horvatović, M.P.; Glamočić, D.; Jajić, I.; Krstović, S.; Guljaš, D.; Gjorgjievski, S. Aflatoxin M 1 in Raw Milk in the Region of Vojvodina. M. Polovinski Horvatović 2016, 66, 239–245. [Google Scholar] [CrossRef]
- Kagera, I.; Kahenya, P.; Mutua, F.; Anyango, G.; Kyallo, F.; Grace, D.; Lindahl, J. Status of Aflatoxin Contamination in Cow Milk Produced in Smallholder Dairy Farms in Urban and Peri-Urban Areas of Nairobi County: A Case Study of Kasarani Sub County, Kenya. Infect. Ecol. Epidemiol. 2019, 9, 1547095. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef]
- INAMHI, I.N. de M. e H. de E. Climas del Ecuador. Available online: http://www.serviciometeorologico.gob.ec/gisweb/TIPO_DE_CLIMAS/PDF/CLIMAS DEL ECUADOR 2016.pdf (accessed on 5 October 2019).
- INEN. Leche y Productos Lácteos. Directrices Para La Toma de Muestras (ISO 707:2008, IDT). Quito-Ecuador 2014. Available online: http://www.normalizacion.gob.ec/wp-content/uploads/downloads/2014/NORMAS_2014/AOC/08092014/nte_inen_iso_6497_extracto.pdf (accessed on 10 January 2020).
- Celis De La Rosa, A.; Labrada, V. Bioestadística, 3rd ed.; Editorial El Manual Moderno S.A. de C. V.: Mexico City, Mexico, 2014; Available online: https://www.academia.edu/21856685/Celis_De_La_Rosa_Alfredo_Y_Labrada_Vanessa_-_Bioestadistica_3ed_ (accessed on 8 February 2020).
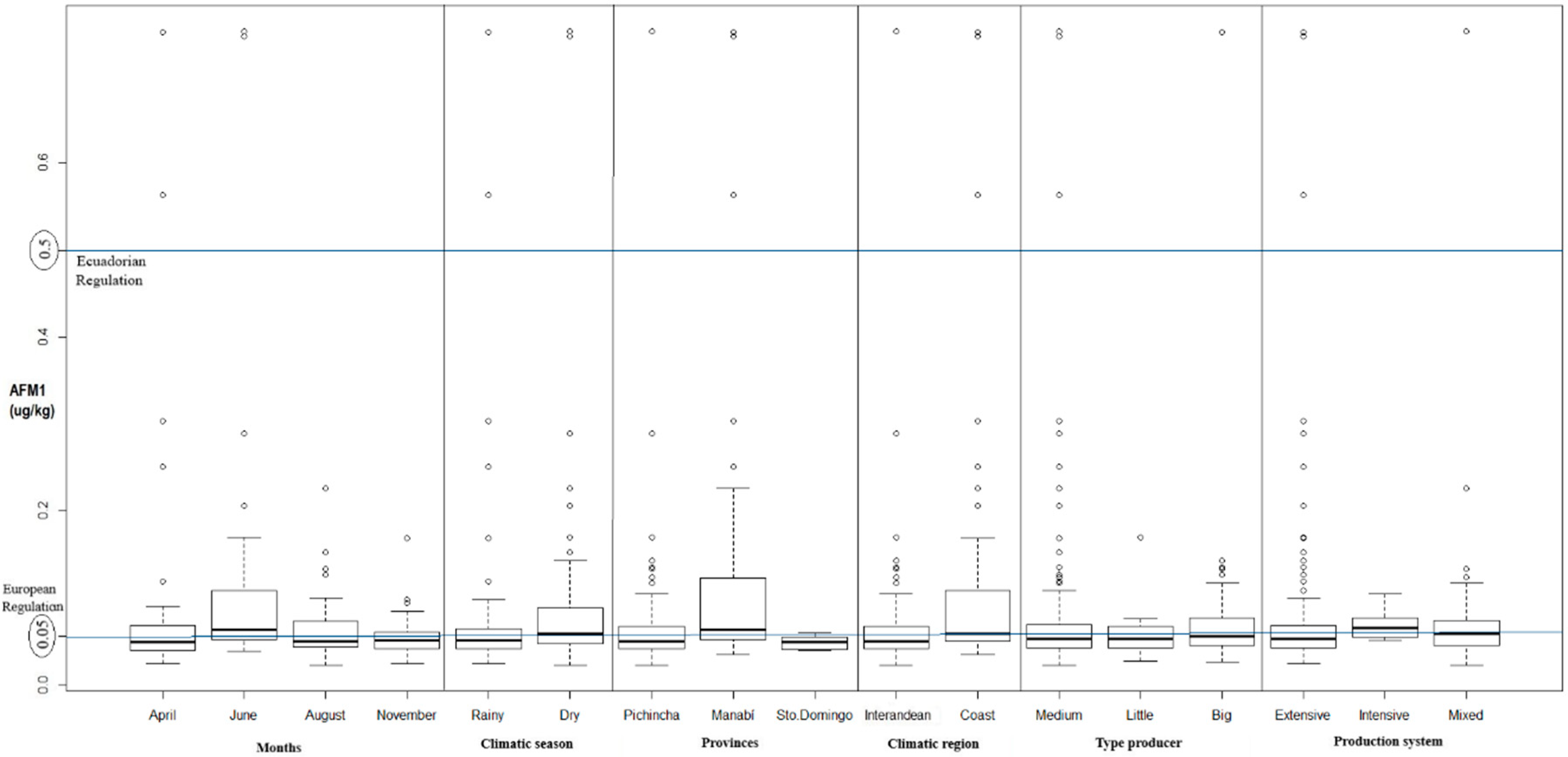
| Variable | Minimum (μg/kg) | Median (μg/kg) | Mean (μg/kg) | Maximum (μg/kg) | p-Value |
|---|---|---|---|---|---|
| Province | |||||
| Manabí | 0.0350 | 0.0640 | 0.1256 | 0.7500 | 5.332 × 10−05 (p ≤ 0.05) |
| Pichincha | 0.0230 | 0.0510 | 0.0639 | 0.7510 | |
| Santo Domingo de los Tsáchilas | 0.0400 | 0.0500 | 0.0485 | 0.0600 | |
| Climatic season | |||||
| Dry | 0.0230 | 0.0580 | 0.0845 | 0.7510 | 0.005509 (p ≤ 0.05) |
| Rainy | 0.0250 | 0.0520 | 0.0701 | 0.7500 | |
| Climatic region | |||||
| Coast | 0.0350 | 0.0590 | 0.1123 | 0.7500 | 0.0008957 (p ≤ 0.05) |
| Inter-Andean | 0.0230 | 0.0510 | 0.0640 | 0.7510 | |
| Variable | Minimum (μg/kg) | Median (μg/kg) | Mean (μg/kg) | Maximum (μg/kg) | p-Value |
|---|---|---|---|---|---|
| Month | |||||
| June | 0.0390 | 0.0640 | 0.1082 | 0.7510 | 0.001406 (p ≤ 0.05) |
| April | 0.0250 | 0.0500 | 0.0862 | 0.7500 | |
| November | 0.0250 | 0.0520 | 0.0554 | 0.1690 | |
| August | 0.0230 | 0.0510 | 0.0656 | 0.2260 | |
| Producer Type | |||||
| Large | 0.0270 | 0.0565 | 0.0768 | 0.7500 | 0.5976 (p ≥ 0.05) |
| Medium | 0.0230 | 0.0540 | 0.0817 | 0.7510 | |
| Small | 0.0280 | 0.0540 | 0.0577 | 0.1700 | |
| Production System | |||||
| Intensive | 0.0520 | 0.0660 | 0.0710 | 0.1050 | 0.3493 (p ≥ 0.05) |
| Mixed | 0.0230 | 0.0590 | 0.0837 | 0.7510 | |
| Extensive | 0.0250 | 0.0540 | 0.0761 | 0.7500 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puga-Torres, B.; Salazar, D.; Cachiguango, M.; Cisneros, G.; Gómez-Bravo, C. Determination of Aflatoxin M1 in Raw Milk from Different Provinces of Ecuador. Toxins 2020, 12, 498. https://doi.org/10.3390/toxins12080498
Puga-Torres B, Salazar D, Cachiguango M, Cisneros G, Gómez-Bravo C. Determination of Aflatoxin M1 in Raw Milk from Different Provinces of Ecuador. Toxins. 2020; 12(8):498. https://doi.org/10.3390/toxins12080498
Chicago/Turabian StylePuga-Torres, Byron, David Salazar, Mayra Cachiguango, Gabriela Cisneros, and Carlos Gómez-Bravo. 2020. "Determination of Aflatoxin M1 in Raw Milk from Different Provinces of Ecuador" Toxins 12, no. 8: 498. https://doi.org/10.3390/toxins12080498
APA StylePuga-Torres, B., Salazar, D., Cachiguango, M., Cisneros, G., & Gómez-Bravo, C. (2020). Determination of Aflatoxin M1 in Raw Milk from Different Provinces of Ecuador. Toxins, 12(8), 498. https://doi.org/10.3390/toxins12080498





