Low Doses of Mycotoxin Mixtures below EU Regulatory Limits Can Negatively Affect the Performance of Broiler Chickens: A Longitudinal Study
Abstract
1. Introduction
2. Results
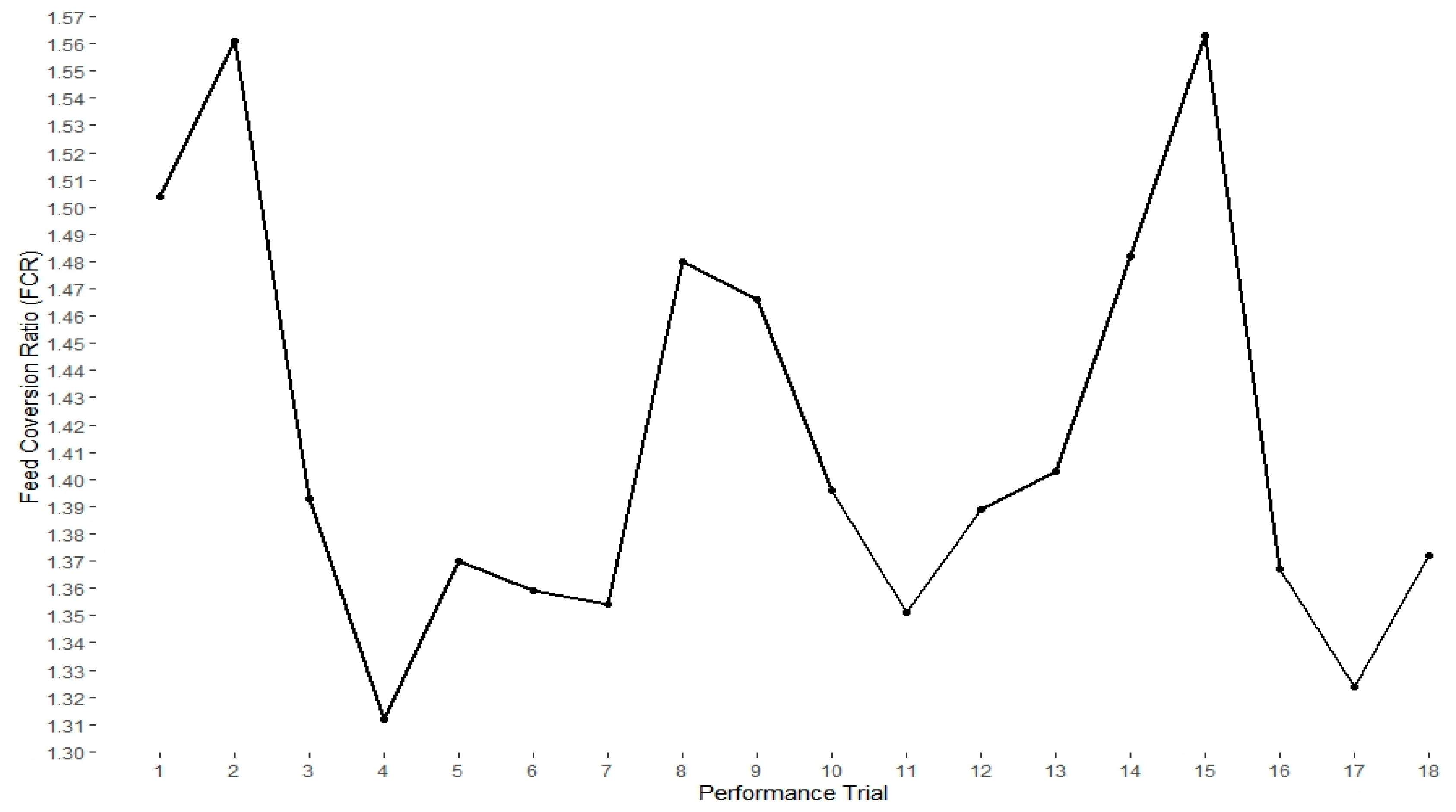
2.1. Animal Performance
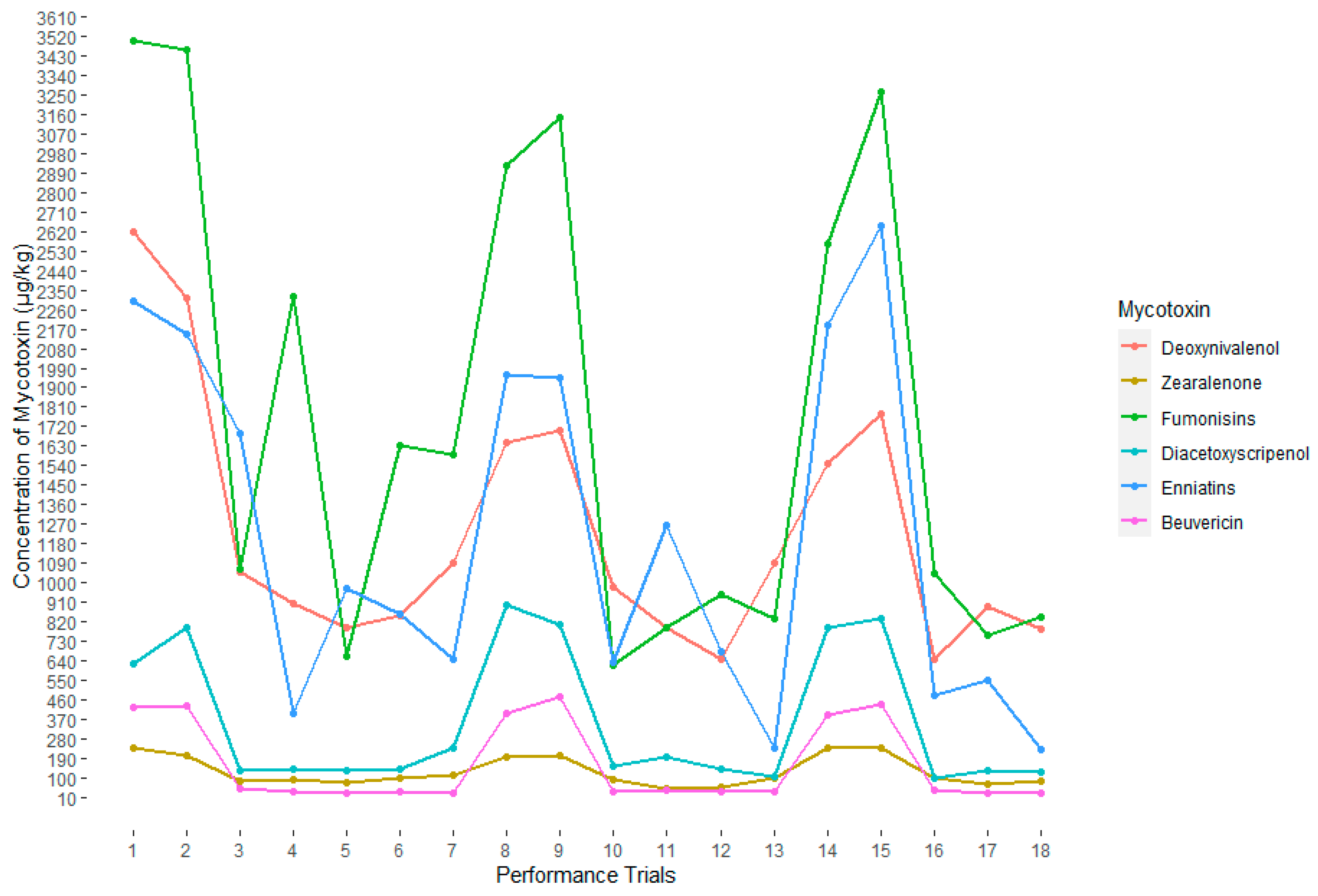
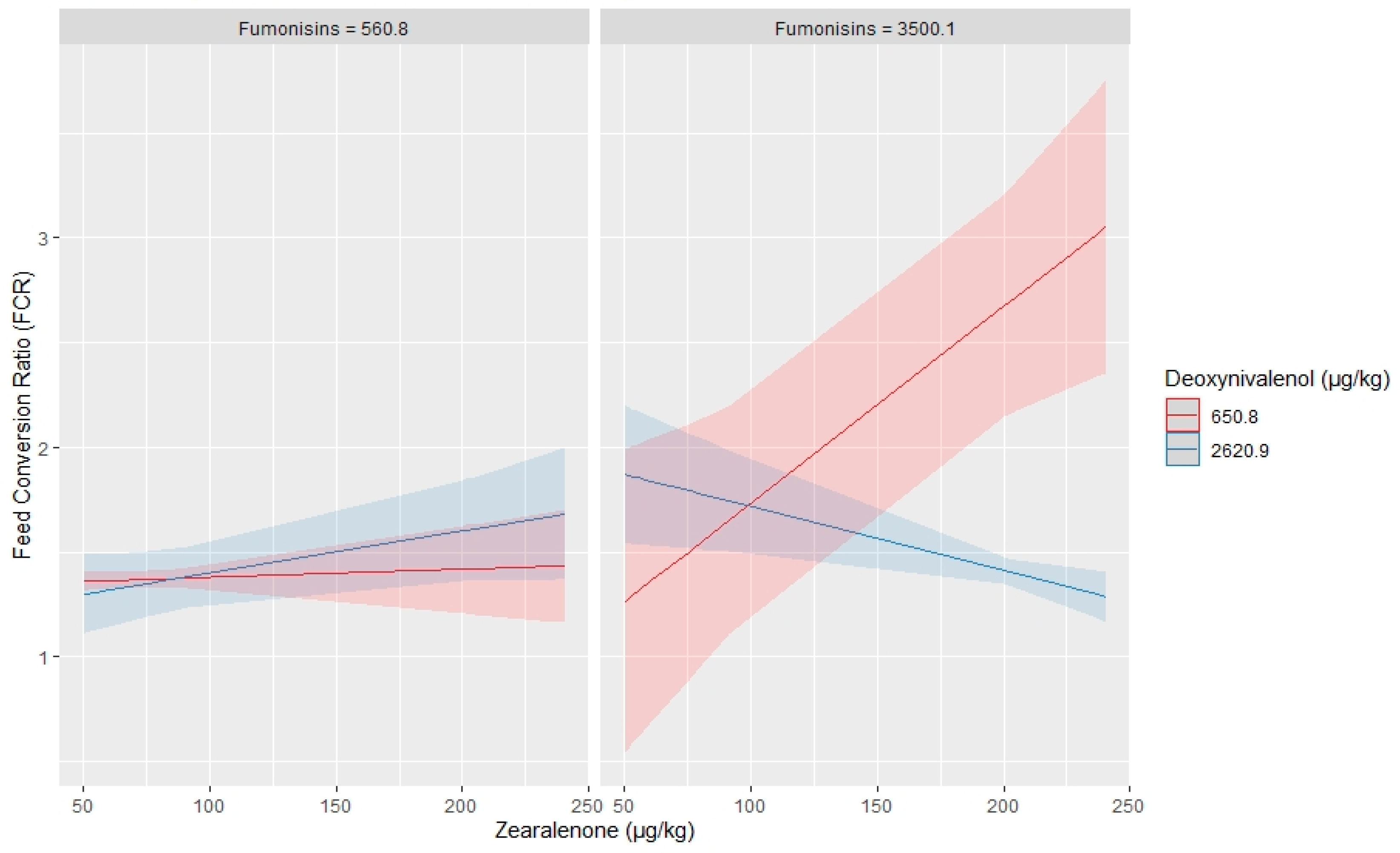
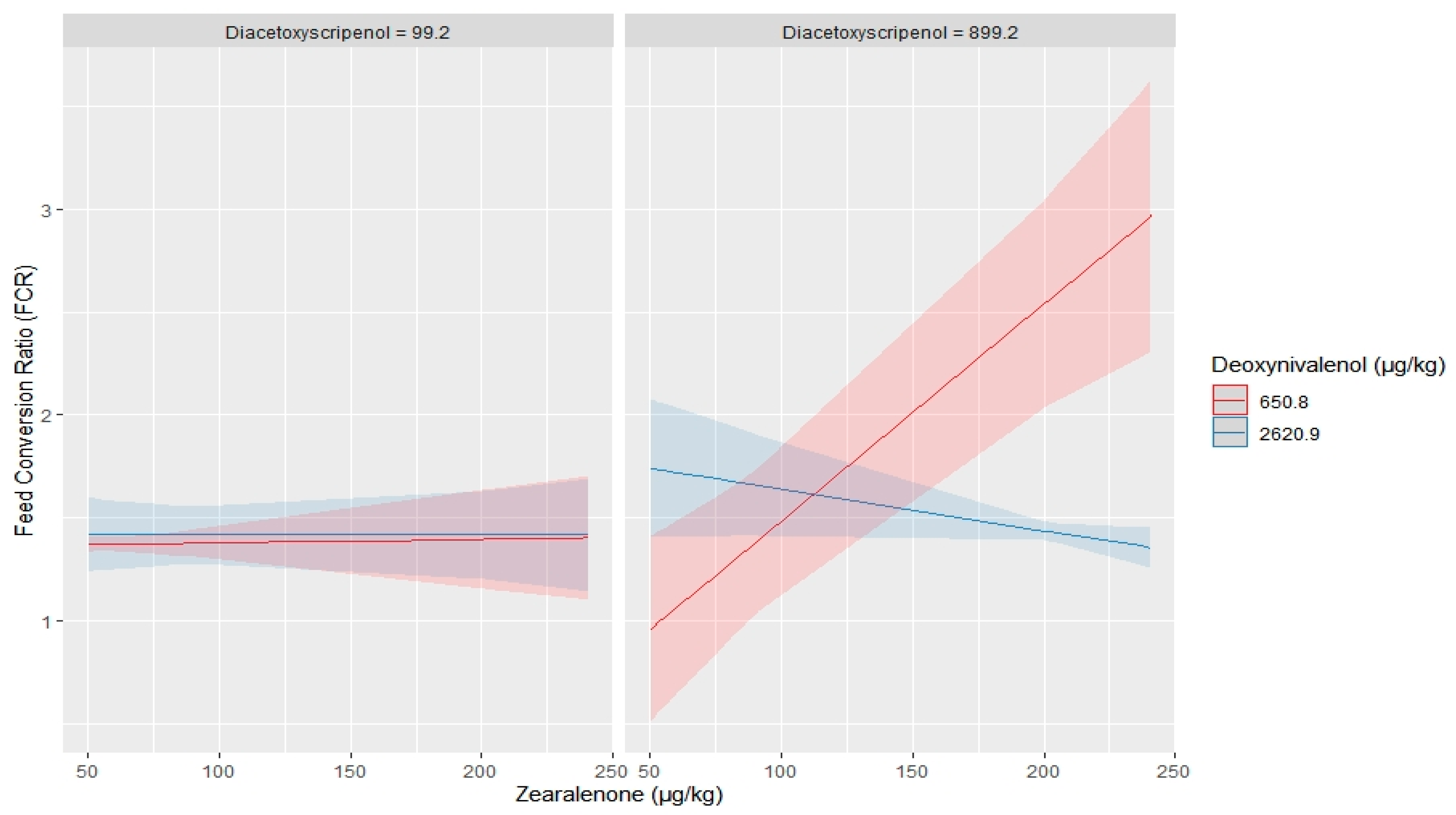
2.2. Impact of Multiple Mycotoxin on Broilers Performance
3. Discussion
4. Conclusions
5. Methods
5.1. Ethics and Animal Housing
5.2. Data and Sample Collection
5.3. Sample Preparation
5.4. UHPLC-MS/MS Parameters
5.5. Method Validation
5.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schader, C.; Muller, A.; Scialabba, N.; Hecht, J.; Isensee, A.; Erb, K.H.; Smith, P.; Makkar, H.; Klocke, P.; Leiber, F.; et al. Impacts of feeding less food-competing feedstuffs to livestock on global food system sustainability. J. R. Soc. Interface 2015, 12, 20150891. [Google Scholar] [CrossRef] [PubMed]
- Luciano, A.; Tretola, M.; Ottoboni, M.; Baldi, A.; Cattaneo, D.; Pinotti, L. potentials and challenges of former food products (food leftover) as alternative feed ingredients. Animals 2020, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Benford, D.; Boobis, A.; Eskola, M.; Fink-Gremmels, J.; Fürst, P.; Heppner, C.; Schlatter, J.; van Leeuwen, R. Risk assessment of contaminants in food and feed. EFSA J. 2012, 10, s1004. [Google Scholar] [CrossRef]
- Magnoli, A.; Poloni, V.; Cavaglieri, L. Impact of mycotoxin contamination in the animal feed industry. Curr. Opin. Food Sci. 2019, 29, 99–108. [Google Scholar] [CrossRef]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current situation of mycotoxin contamination and co-occurrence in animal feed—Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef]
- Balendres, K.; Karlovsky, M.; Cumagun, C. Mycotoxigenic fungi and mycotoxins in agricultural crop commodities in the philippines: A Review. Foods 2019, 8, 249. [Google Scholar] [CrossRef]
- Fremy, J.; Alassane-Kpembi, I.; Oswald, I.; Cottrill, B.; Van Egmond, H. A review on combined effects of moniliformin and co-occurring Fusarium toxins in farm animals. World Mycotoxin J. 2019, 12, 281–291. [Google Scholar] [CrossRef]
- Yang, C.; Song, G.; Lim, W. Effects of mycotoxin-contaminated feed on farm animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, N.; Yang, L.; Deng, Y.; Wang, J.; Song, S.; Lin, S.; Wu, A.; Zhou, Z.; Hou, J. Multi-mycotoxin analysis of animal feed and animal-derived food using LC–MS/MS system with timed and highly selective reaction monitoring. Anal. Bioanal. Chem. 2015, 407, 7359–7368. [Google Scholar] [CrossRef] [PubMed]
- Egmond, H.; Jonker, M. Current situation on regulations for mycotoxins. Mycotoxins 2004, 2003 (Suppl. 3), 1–15. [Google Scholar] [CrossRef]
- European Commission (EC). Commission Recommendation 576/2006/EC of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, L229, 7. [Google Scholar]
- Panasiuk, L.; Jedziniak, P.; Pietruszka, K.; Piatkowska, M.; Bocian, L. Frequency and levels of regulated and emerging mycotoxins in silage in Poland. Mycotoxin Res. 2019, 35, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Fraeyman, S.; Croubels, S.; Devreese, M.; Antonissen, G. Emerging Fusarium and Alternaria Mycotoxins: Occurrence, toxicity and toxicokinetics. Toxins 2017, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Dellafiora, L.; Dall’Asta, C. Masked mycotoxins: An emerging issue that makes renegotiable what is ordinary. Food Chem. 2016, 213, 534–535. [Google Scholar] [CrossRef] [PubMed]
- Bryła, M.; Waśkiewicz, A.; Ksieniewicz-Woźniak, E.; Szymczyk, K.; Jędrzejczak, R. Modified Fusarium mycotoxins in cereals and their products—Metabolism, occurrence, and toxicity: An updated review. Molecules 2018, 23, 963. [Google Scholar] [CrossRef] [PubMed]
- Boevre, M.; Graniczkowska, K.; Saeger, S. Metabolism of modified mycotoxins studied through in vitro and in vivo models: An overview. Toxicol. Lett. 2015, 233, 24–28. [Google Scholar] [CrossRef]
- Gratz, S.; Dinesh, R.; Yoshinari, T.; Holtrop, G.; Richardson, A.; Duncan, G.; MacDonald, S.; Lloyd, A.; Tarbin, J. Masked trichothecene and zearalenone mycotoxins withstand digestion and absorption in the upper GI tract but are efficiently hydrolyzed by human gut microbiota in vitro. Mol. Nutr. Food Res. 2017, 61, 1600680. [Google Scholar] [CrossRef]
- Kovalsky, P.; Kos, G.; Nährer, K.; Schwab, C.; Jenkins, T.; Schatzmayr, G.; Sulyok, M.; Krska, R. Co-Occurrence of regulated, masked and emerging mycotoxins and secondary metabolites in finished feed and maize—An extensive survey. Toxins 2016, 8, 363. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef]
- Khoshal, A.; Novak, B.; Martin, P.; Jenkins, T.; Neves, M.; Schatzmayr, G.; Oswald, I.P.; Pinton, P. Co-Occurrence of DON and emerging mycotoxins in worldwide finished pig feed and their combined toxicity in intestinal cells. Toxins 2019, 11, 727. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium toxins in cereals: Occurrence, legislation, factors promoting the appearance and their management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef]
- Girgis, G.; Barta, J.; Girish, C.; Karrow, N.; Boermans, H.; Smith, T. Effects of feed-borne Fusarium mycotoxins and an organic mycotoxin adsorbent on immune cell dynamics in the jejunum of chickens infected with Eimeria maxima. Vet. Immunol. Immunopathol. 2010, 138, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Mohaghegh, A.; Chamani, M.; Shivazad, M.; Sadeghi, A.; Afzali, N. Effect of esterified glucomannan on broilers exposed to natural mycotoxin-contaminated diets. J. Appl. Anim. Res. 2016, 45, 285–291. [Google Scholar] [CrossRef]
- Sobrane Filho, S.; Junqueira, O.; Laurentiz, A.; Filardi, R.; Rubio, M.; Duarte, K.; Laurentiz, R. Effects of mycotoxin adsorbents in aflatoxin B1- and fumonisin B1-contaminated broiler diet on performance and blood metabolite. Rev. Bras. De Zootec. 2016, 45, 250–256. [Google Scholar] [CrossRef]
- Pelyhe, C.; Kövesi, B.; Zándoki, E.; Kovács, B.; Erdélyi, M.; Kulcsár, S.; Mézes, M.; Balogh, K. Multi-trichothecene mycotoxin exposure activates glutathione-redox system in broiler chicken. Toxicon 2018, 153, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Northern Ireland Grain Trade Association, Food Fortress scheme. Available online: http://www.nigta.co.uk/food-fortress/106-feed-trade-backs-food-fortress (accessed on 10 April 2020).
- Commission, EC. Commission Decision of 14 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results (2002/657/EC). Off. J. Eur. Union 2002, 221, 8. [Google Scholar]
- Commission, EC. Commission Regulation (EC) No 401/ 2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union 2006, 70, 12–34. [Google Scholar]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Multi-mycotoxin screening of feed and feed raw materials from Africa. World Mycotoxin J. 2018, 11, 369–383. [Google Scholar] [CrossRef]
- Freire, L.; Sant’Ana, A. Modified mycotoxins: An updated review on their formation, detection, occurrence, and toxic effects. Food Chem. Toxicol. 2018, 111, 189–205. [Google Scholar] [CrossRef]
- Vismer, H.; Shephard, G.; van der Westhuizen, L.; Mngqawa, P.; Bushula-Njah, V.; Leslie, J. Mycotoxins produced by Fusarium proliferatum and F. pseudonygamai on maize, sorghum and pearl millet grains in vitro. Int. J. Food Microbiol. 2019, 296, 31–36. [Google Scholar] [CrossRef]
- Kosicki, R.; Błajet-Kosicka, A.; Grajewski, J.; Twarużek, M. Multiannual mycotoxin survey in feed materials and feedingstuffs. Anim. Feed Sci. Technol. 2016, 215, 165–180. [Google Scholar] [CrossRef]
- Meyer, H.; Skhosana, Z.; Motlanthe, M.; Louw, W.; Rohwer, E. Long Term Monitoring (2014–2018) of multi-mycotoxins in South African commercial maize and wheat with a locally developed and validated LC-MS/MS method. Toxins 2019, 11, 271. [Google Scholar] [CrossRef] [PubMed]
- Sell-Kubiak, E.; Wimmers, K.; Reyer, H.; Szwaczkowski, T. Genetic aspects of feed efficiency and reduction of environmental footprint in broilers: A review. J. Appl. Genet. 2017, 58, 487–498. [Google Scholar] [CrossRef]
- Amerah, A. Interactions between wheat characteristics and feed enzyme supplementation in broiler diets. Anim. Feed Sci. Technol. 2015, 199, 1–9. [Google Scholar] [CrossRef]
- Wu, Q.; Dohnal, V.; Kuca, K.; Yuan, Z. Trichothecenes: Structure-Toxic Activity Relationships. Curr. Drug Metab. 2013, 14, 641–660. [Google Scholar] [CrossRef] [PubMed]
- Voss, K.; Riley, R. Fumonisin toxicity and mechanism of action: Overview and current perspectives. Food Saf. 2013, 1, 2013006. [Google Scholar] [CrossRef]
- Antonissen, G.; Croubels, S.; Pasmans, F.; Ducatelle, R.; Eeckhaut, V.; Devreese, M.; Verlinden, M.; Haesebrouck, F.; Eeckhout, M.; De Saeger, S.; et al. Fumonisins affect the intestinal microbial homeostasis in broiler chickens, predisposing to necrotic enteritis. Vet. Res. 2015, 46, 55–60. [Google Scholar] [CrossRef]
- Liu, X.; Fan, L.; Yin, S.; Chen, H.; Hu, H. Molecular mechanisms of fumonisin B1-induced toxicities and its applications in the mechanism-based interventions. Toxicon 2019, 167, 1–5. [Google Scholar] [CrossRef]
- Awad, W.; Hess, M.; Twarużek, M.; Grajewski, J.; Kosicki, R.; Böhm, J.; Zentek, J. The Impact of the fusarium mycotoxin deoxynivalenol on the health and performance of broiler chickens. Int. J. Mol. Sci. 2011, 12, 7996–8012. [Google Scholar] [CrossRef]
- Lucke, A.; Doupovec, B.; Paulsen, P.; Zebeli, Q.; Böhm, J. Effects of low to moderate levels of deoxynivalenol on feed and water intake, weight gain, and slaughtering traits of broiler chickens. Mycotoxin Res. 2017, 33, 261–271. [Google Scholar] [CrossRef]
- Awad, W.; Ruhnau, D.; Hess, C.; Doupovec, B.; Schatzmayr, D.; Hess, M. Feeding of deoxynivalenol increases the intestinal paracellular permeability of broiler chickens. Arch. Toxicol. 2019, 93, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Keçi, M.; Lucke, A.; Paulsen, P.; Zebeli, Q.; Böhm, J.; Metzler-Zebeli, B. Deoxynivalenol in the diet impairs bone mineralization in broiler chickens. Toxins 2019, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Schwean-Lardner, K.; Hogan, N. Feed preference and feeding behaviours in grower broilers fed diets containing wheat naturally contaminated with fusarium mycotoxins. Br. Poult. Sci. 2019, 60, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Alassane-Kpembi, I.; Pinton, P.; Oswald, I. Effects of Mycotoxins on the Intestine. Toxins 2019, 11, 159. [Google Scholar] [CrossRef]
- Dietrich, B.; Neuenschwander, S.; Bucher, B.; Wenk, C. Fusarium mycotoxin-contaminated wheat containing deoxynivalenol alters the gene expression in the liver and the jejunum of broilers. Animal 2011, 6, 278–291. [Google Scholar] [CrossRef]
- Antonissen, G.; De Baere, S.; Devreese, M.; Van Immerseel, F.; Martel, A.; Croubels, S. Feed contamination with Fusarium mycotoxins induces a corticosterone stress response in broiler chickens. Poult. Sci. 2017, 96, 14–17. [Google Scholar] [CrossRef]
- Flannery, B.; Clark, E.; Pestka, J. Anorexia induction by the trichothecene deoxynivalenol (vomitoxin) is mediated by the release of the gut satiety hormone peptide YY. Toxicol. Sci. 2012, 130, 289–297. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Liu, S.; Wu, W.; Zhang, H. Comparison of anorectic potencies of type A trichothecenes T-2 Toxin, HT-2 toxin, diacetoxyscirpenol, and neosolaniol. Toxins 2018, 10, 179. [Google Scholar] [CrossRef]
- Terciolo, C.; Maresca, M.; Pinton, P.; Oswald, I. Review article: Role of satiety hormones in anorexia induction by trichothecene mycotoxins. Food Chem. Toxicol. 2018, 121, 701–714. [Google Scholar] [CrossRef]
- Fraeyman, S.; Croubels, S.; Devreese, M.; Ducatelle, R.; Rychlik, M.; Antonissen, G. Chronic dietary intake of enniatin B in broiler chickens has low impact on intestinal morphometry and hepatic histology, and shows limited transfer to liver tissue. Toxins 2018, 10, 45. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risks to human and animal health related to the presence of beauvericin and enniatins in food and feed. EFSA J. 2014, 12, 3802. [Google Scholar] [CrossRef]
- Yunus, A.; Ghareeb, K.; Twaruzek, M.; Grajewski, J.; Böhm, J. Deoxynivalenol as a contaminant of broiler feed: Effects on bird performance and response to common vaccines. Poult. Sci. 2012, 91, 844–851. [Google Scholar] [CrossRef]
- Yunus, A.; Blajet-Kosicka, A.; Kosicki, R.; Khan, M.; Rehman, H.; Böhm, J. Deoxynivalenol as a contaminant of broiler feed: Intestinal development, absorptive functionality, and metabolism of the mycotoxin. Poult. Sci. 2012, 91, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Grenier, B.; Dohnal, I.; Shanmugasundaram, R.; Eicher, S.; Selvaraj, R.; Schatzmayr, G.; Applegate, T.J. Susceptibility of broiler chickens to coccidiosis when fed subclinical doses of deoxynivalenol and fumonisins—Special emphasis on the immunological response and the mycotoxin interaction. Toxins 2016, 8, 231. [Google Scholar] [CrossRef]
- Metayer, V.; Travel, J.; Mika, O.; Bailly, C.; Cleva, L.; Boissieu, T.; Le Guennec, J.; Froment, P.; Albaric, O.; Labrut, S. Lack of toxic interaction between fusariotoxins in broiler chickens fed throughout their life at the highest level tolerated in the European Union. Toxins 2019, 11, 455. [Google Scholar] [CrossRef] [PubMed]
- Grenier, B.; Applegate, T. Modulation of intestinal functions following mycotoxin ingestion: Meta-Analysis of published experiments in animals. Toxins 2013, 5, 396–430. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Oswald, I. Effect of deoxynivalenol and other type B trichothecenes on the intestine: A review. Toxins 2014, 6, 1615–1643. [Google Scholar] [CrossRef]
- Maresca, M.; Mahfoud, R.; Garmy, N.; Fantini, J. The mycotoxin deoxynivalenol affects nutrient absorption in human intestinal epithelial cells. J. Nutr. 2002, 132, 2723–2731. [Google Scholar] [CrossRef]
- Matthäus, K.; Dänicke, S.; Strumpf, A.; Valenta, H.; Zieseniß, H.; Flachowsky, G. Progression of the mycotoxin and nutrient concentration in wheat after inoculation with Fusarium culmorum. Mycotoxin Res. 2002, 18, 60–64. [Google Scholar] [CrossRef]
- Zhu, Y.; Hassan, Y.; Watts, C.; Zhou, T. Innovative technologies for the mitigation of mycotoxins in animal feed and ingredients—A review of recent patents. Anim. Feed Sci. Technol. 2016, 216, 19–29. [Google Scholar] [CrossRef]
- Boudergue, C.; Burel, C.; Dragacci, S.; Favrot, M.C.; Fremy, J.M.; Massimi, C.; Prigent, P. Review of mycotoxin-detoxifying agents used as feed additives: Mode of action, efficacy and feed/food safety. EFSA Support. Publ. 2009, 6, 22E. [Google Scholar] [CrossRef]
- Elliott, C.; Connolly, L.; Kolawole, O. Potential adverse effects on animal health and performance caused by the addition of mineral adsorbents to feeds to reduce mycotoxin exposure. Mycotoxin Res. 2019, 36, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Prapapanpong, J.; Udomkusonsri, P.; Mahavorasirikul, W.; Choochuay, S.; Tansakul, N. In vitro studies on gastrointestinal monogastric and avian models to evaluate the binding efficacy of mycotoxin adsorbents by liquid chromatography-tandem mass spectrometry. J. Adv. Vet. Anim. Res. 2019, 6, 125. [Google Scholar] [CrossRef] [PubMed]
- Kolawole, O.; Meneely, J.; Greer, B.; Chevallier, O.; Jones, D.; Connolly, L.; Elliott, C. Comparative in vitro assessment of a range of commercial feed additives with multiple mycotoxin binding claims. Toxins 2019, 11, 659. [Google Scholar] [CrossRef]
- Oplatowska-Stachowiak, M.; Haughey, S.; Chevallier, O.; Galvin-King, P.; Campbell, K.; Magowan, E. Determination of the mycotoxin content in distiller’s dried grain with soluble using a multianalyte UHPLC–MS/MS method. J. Agric. Food Chem. 2015, 63, 9441–9451. [Google Scholar] [CrossRef]
| Mycotoxins | P/N (%) a | Minimum | 1st Quartile | Median | Mean | 3rd Quartile | Maximum |
|---|---|---|---|---|---|---|---|
| Deoxynivalenol | 77/78 (98) | 650.8 | 790.6 | 898.5 | 1387.4 | 2359.4 | 2620.9 |
| Zearalenone | 71/78 (91) | 50.3 | 53.7 | 78.4 | 116.1 | 201.5 | 240.9 |
| Fumonisin B1 | 78/78 (100) | 432.7 | 567.8 | 637.1 | 734.2 | 933.9 | 2300.2 |
| Fumonisin B2 | 78/78 (100) | 33.7 | 136.3 | 177.4 | 427.6 | 833.2 | 960.2 |
| Fumonisin B3 | 78/78 (100) | 32.9 | 50.5 | 66.5 | 115.5 | 173.8 | 320.1 |
| Diacetoxyscripenol | 60/78 (77) | 99.2 | 136.2 | 148.0 | 361.7 | 750.3 | 899.3 |
| Meleagrin | 50/78 (64) | 10.2 | 30.5 | 33.7 | 35.6 | 40.6 | 45.3 |
| Aurofurasin | 62/78 (79) | 5.5 | 12.2 | 26.5 | 34.2 | 42.6 | 115.9 |
| Tentoxin | 60/78 (77) | 11.9 | 28.9 | 32.1 | 30.6 | 33.4 | 59.1 |
| Equisetin | 55/78 (71) | 5.7 | 9.3 | 10.2 | 10.2 | 10.9 | 13.5 |
| Enniatin A | 78/78 (100) | 2.8 | 3.4 | 5.1 | 11.8 | 18.8 | 34.7 |
| Enniatin A1 | 78/78 (100) | 3.2 | 5.3 | 7.2 | 11.2 | 9.8 | 32.1 |
| Enniatin B | 78/78 (100) | 180.3 | 200.2 | 329.2 | 800.5 | 1511.3 | 2190.2 |
| Enniatin B1 | 78/78 (100) | 25.8 | 41.4 | 64.5 | 139.9 | 224.4 | 396.0 |
| Apicidin | 66/78 (85) | <LOD b | 10.3 | 10.6 | 19.3 | 30.6 | 64.2 |
| Deoxynivalenol-3-glucoside | 45/78 (58) | 12.3 | 16.9 | 33.8 | 46.5 | 49.2 | 145.7 |
| Beuvericin | 74/78 (95) | 30.9 | 35.2 | 41.5 | 167.9 | 394.8 | 474.9 |
| Roqufortine C | 20/78 (26) | 15.2 | 74.3 | 89.1 | 82.7 | 101.8 | 155.2 |
| Alternariol | 36/78 (46) | 9.8 | 36.5 | 99.2 | 124.4 | 191.7 | 390.6 |
| Citrinin | 47/78 (60) | <LOQ c | 10.5 | 31.7 | 79.7 | 155.2 | 255.2 |
| Emodin | 65/78 (83) | <LOQ c | 5.7 | 9.8 | 10.2 | 10.9 | 30.3 |
| Patulin | 39/78 (50) | 2.9 | 7.4 | 10.9 | 14.4 | 20.3 | 33.2 |
| Moniliformin | 60/78 (58) | 10.3 | 22.0 | 24.7 | 30.5 | 44.6 | 62.5 |
| 3-Acetyl deoxynivalenol | 40/78 (51) | <LOQ c | 13.5 | 35.6 | 42.1 | 68.4 | 90.1 |
| Day | Avg. FI a | Avg. BW b | Correlation (r) |
|---|---|---|---|
| 7 | 137.3 | 189.6 | 0.84 * |
| 14 | 500.9 | 504.1 | 0.58 * |
| 21 | 1153.5 | 994.6 | 0.45 * |
| 28 | 2031.1 | 1525.2 | 0.04 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolawole, O.; Graham, A.; Donaldson, C.; Owens, B.; Abia, W.A.; Meneely, J.; Alcorn, M.J.; Connolly, L.; Elliott, C.T. Low Doses of Mycotoxin Mixtures below EU Regulatory Limits Can Negatively Affect the Performance of Broiler Chickens: A Longitudinal Study. Toxins 2020, 12, 433. https://doi.org/10.3390/toxins12070433
Kolawole O, Graham A, Donaldson C, Owens B, Abia WA, Meneely J, Alcorn MJ, Connolly L, Elliott CT. Low Doses of Mycotoxin Mixtures below EU Regulatory Limits Can Negatively Affect the Performance of Broiler Chickens: A Longitudinal Study. Toxins. 2020; 12(7):433. https://doi.org/10.3390/toxins12070433
Chicago/Turabian StyleKolawole, Oluwatobi, Abigail Graham, Caroline Donaldson, Bronagh Owens, Wilfred A. Abia, Julie Meneely, Michael J. Alcorn, Lisa Connolly, and Christopher T. Elliott. 2020. "Low Doses of Mycotoxin Mixtures below EU Regulatory Limits Can Negatively Affect the Performance of Broiler Chickens: A Longitudinal Study" Toxins 12, no. 7: 433. https://doi.org/10.3390/toxins12070433
APA StyleKolawole, O., Graham, A., Donaldson, C., Owens, B., Abia, W. A., Meneely, J., Alcorn, M. J., Connolly, L., & Elliott, C. T. (2020). Low Doses of Mycotoxin Mixtures below EU Regulatory Limits Can Negatively Affect the Performance of Broiler Chickens: A Longitudinal Study. Toxins, 12(7), 433. https://doi.org/10.3390/toxins12070433







