Recent Advances on Macrocyclic Trichothecenes, Their Bioactivities and Biosynthetic Pathway
Abstract
1. Introduction
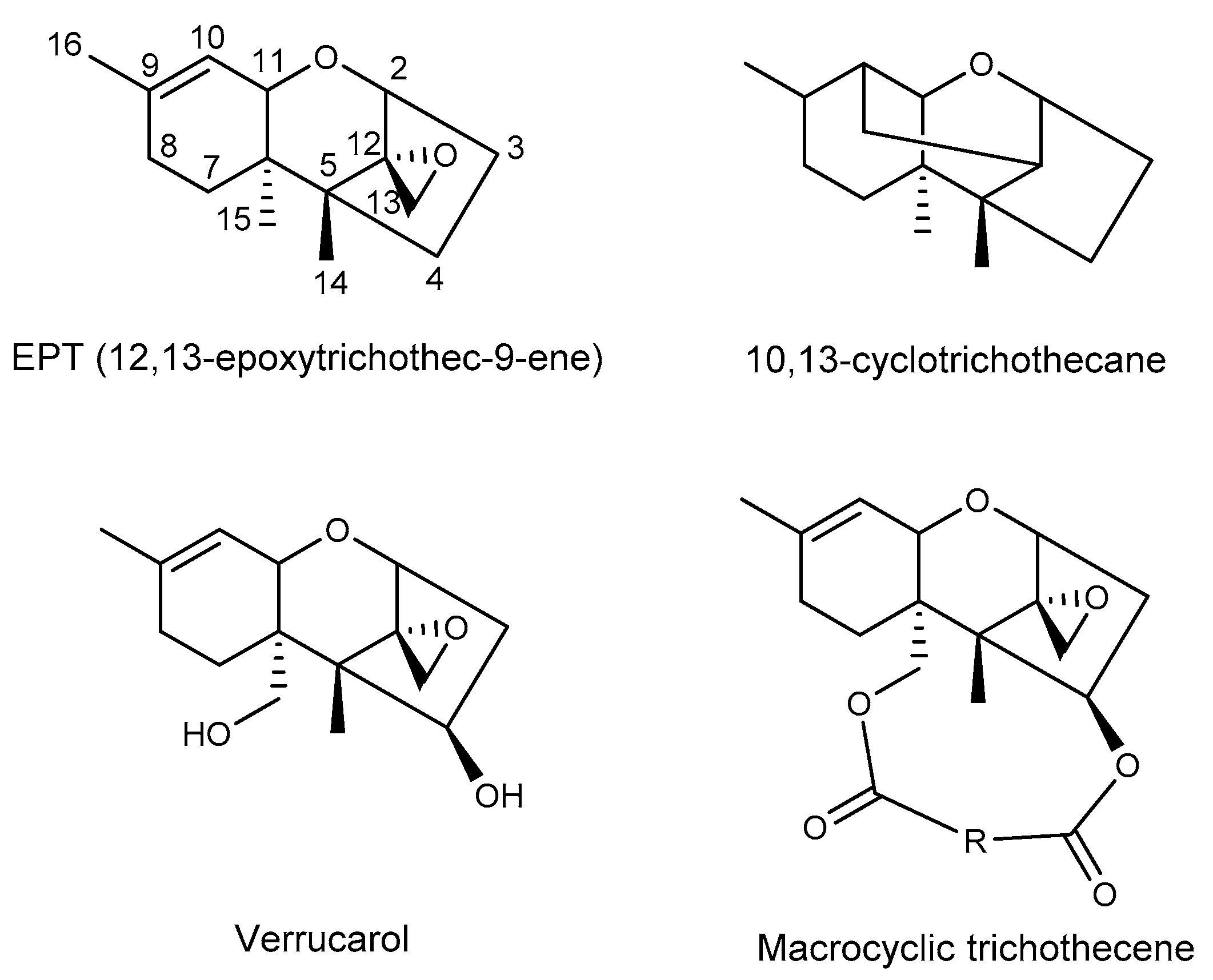
2. Macrocyclic Trichothecenes and the Producing Strains
3. Biological Activities of Macrocyclic Trichothecenes
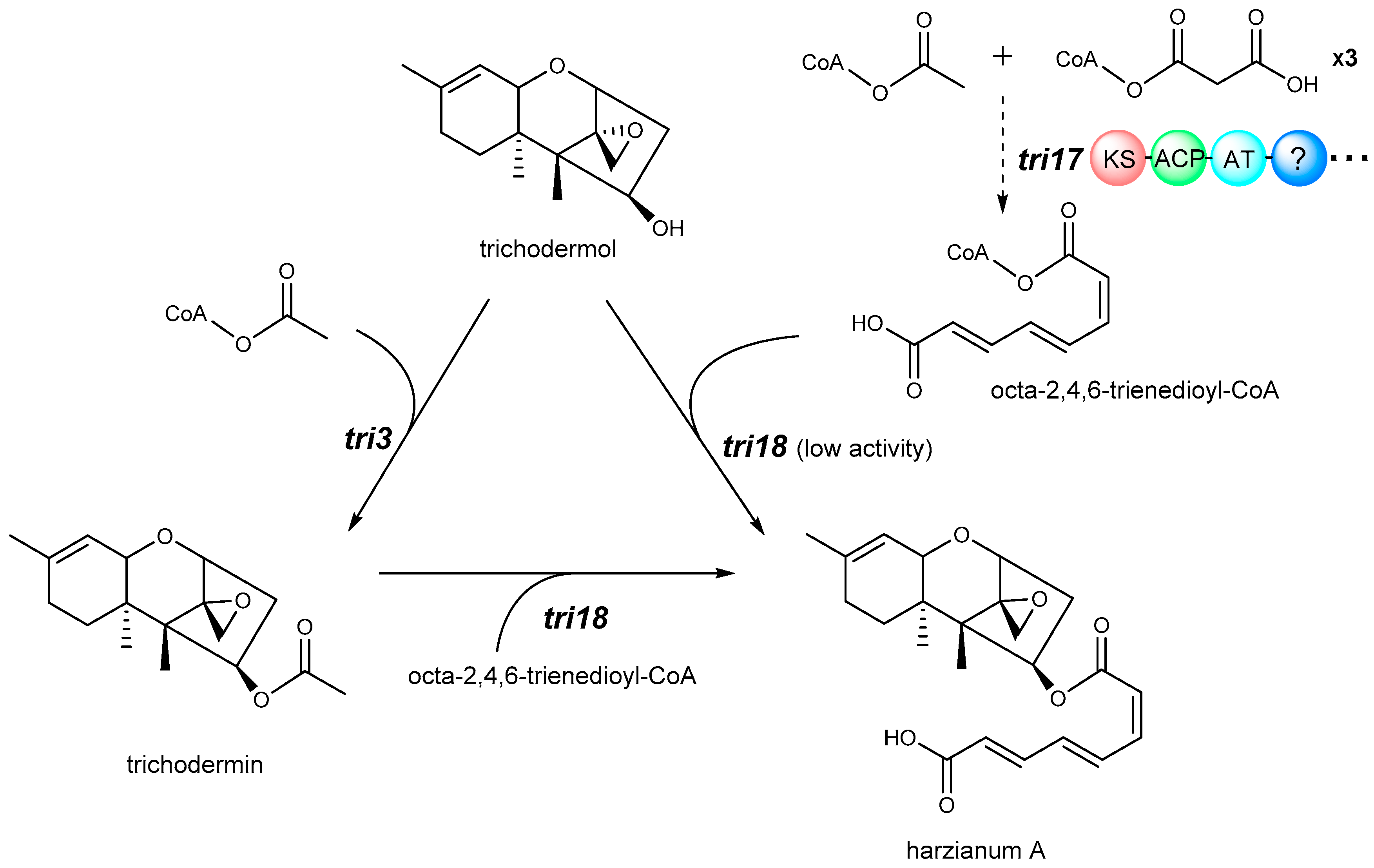
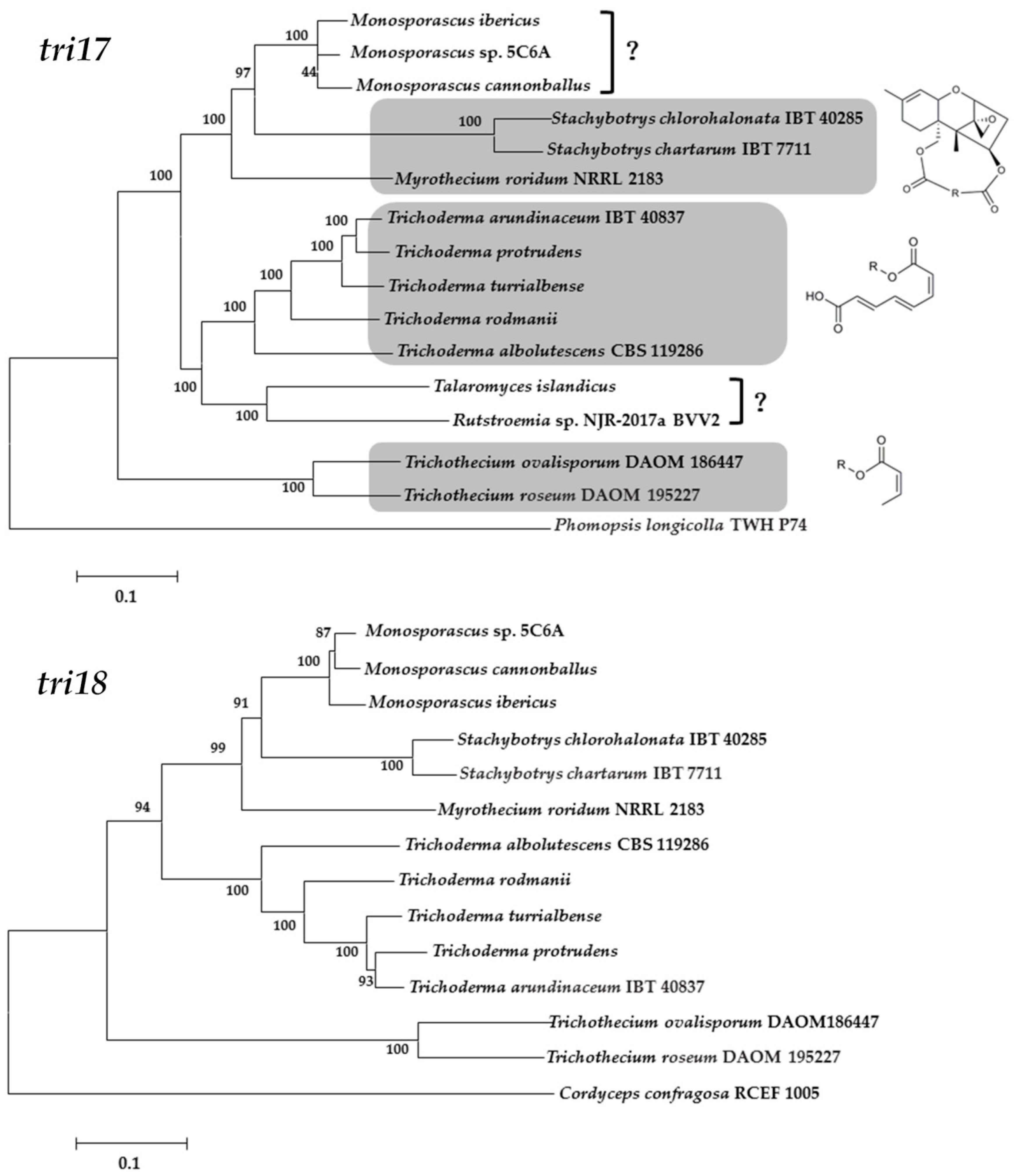
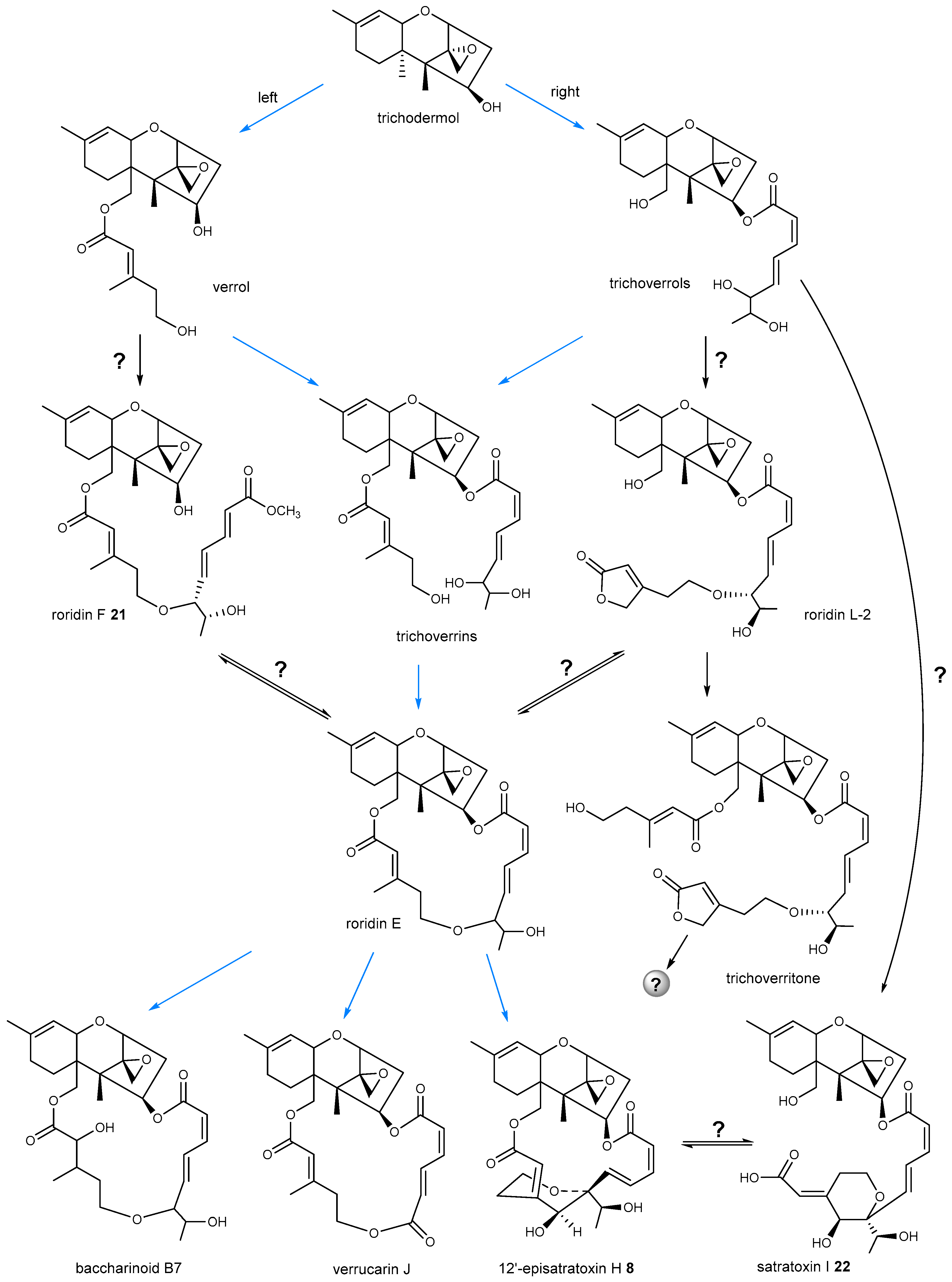
4. Biosynthetic Pathway
5. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wu, Q.H.; Dohnal, V.; Kuca, K.; Yuan, Z.H. Trichothecenes: Structure-toxic activity relationships. Curr. Drug Metab. 2013, 14, 641–660. [Google Scholar] [CrossRef] [PubMed]
- McMullen, M.; Jones, R.; Gallenberg, D. Scab of wheat and barley: A re-emerging disease of devastating impact. Plant Dis. 1997, 81, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, G.S.; Pettersson, H. Toxicological evaluation of trichothecenes in animal feed. Anim. Feed Sci. Technol. 2004, 114, 205–239. [Google Scholar] [CrossRef]
- Pascari, X.; Maul, R.; Kemmlein, S.; Marin, S.; Sanchis, V. The fate of several trichothecenes and zearalenone during roasting and enzymatic treatment of cereal flour applied in cereal-based infant food production. Food Control 2020, 114. [Google Scholar] [CrossRef]
- Habrowska-Gorczynska, D.E.; Kowalska, K.; Urbanek, K.A.; Dominska, K.; Sakowicz, A.; Piastowska-Ciesielska, A.W. Deoxynivalenol modulates the viability, ROS production and apoptosis in prostate cancer cells. Toxins 2019, 11, 265. [Google Scholar] [CrossRef]
- Opoku, N.; Back, M.A.; Edwards, S.G. Susceptibility of cereal species to Fusarium langsethiae under identical field conditions. Eur. J. Plant Pathol. 2018, 150, 869–879. [Google Scholar] [CrossRef]
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: From simple to complex mycotoxins. Toxins 2011, 3, 802–814. [Google Scholar] [CrossRef]
- Grove, J.F. The trichothecenes and their biosynthesis. Cheminform 2007, 38, 63–130. [Google Scholar] [CrossRef]
- Tamm, C.; Breitenstein, W. The Biosynthesis of Mycotoxins: A Study of Secondary Metabolism; Academic Press: New York, NY, USA, 1980; pp. 69–104. [Google Scholar]
- Proctor, R.H.; McCormick, S.P.; Kim, H.S.; Cardoza, R.E.; Stanley, A.M.; Lindo, L.; Kelly, A.; Brown, D.W.; Lee, T.; Vaughan, M.M.; et al. Evolution of structural diversity of trichothecenes, a family of toxins produced by plant pathogenic and entomopathogenic fungi. PLoS Path. 2018, 14, e1006946. [Google Scholar] [CrossRef]
- Kimura, M.; Tokai, T.; Takahashi-Ando, N.; Ohsato, S.; Fujimura, M. Molecular and genetic studies of Fusarium trichothecene biosynthesis: Pathways, genes, and evolution. Biosci. Biotechnol. Biochem. 2007, 71, 2105–2123. [Google Scholar] [CrossRef]
- Alexander, N.J.; Proctor, R.H.; McCormick, S.P. Genes, gene clusters, and biosynthesis of trichothecenes and fumonisins in Fusarium. Toxin Rev. 2009, 28, 198–215. [Google Scholar] [CrossRef]
- Brown, D.W.; Dyer, R.B.; McCormick, S.P.; Kendra, D.F.; Plattner, R.D. Functional demarcation of the Fusarium core trichothecene gene cluster. Fungal Genet. Biol. 2004, 41, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Garvey, G.S.; McCormick, S.P.; Alexander, N.J.; Rayment, I. Structural and functional characterization of TRI3 trichothecene 15-O-acetyltransferase from Fusarium sporotrichioides. Protein Sci. 2009, 18, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Hohn, T.M.; Krishna, R.; Proctor, R.H. Characterization of a transcriptional activator controlling trichothecene toxin biosynthesis. Fungal Genet. Biol. 1999, 26, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Malmierca, M.G.; Cardoza, R.E.; Alexander, N.J.; McCormick, S.P.; Collado, I.G.; Hermosa, R.; Monte, E.; Gutierrez, S. Relevance of trichothecenes in fungal physiology: Disruption of tri5 in Trichoderma arundinaceum. Fungal Genet. Biol. 2013, 53, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Garvey, G.S.; McCormick, S.P.; Rayment, I. Structural and functional characterization of the TRI101 trichothecene 3-O-acetyltransferase from Fusarium sporotrichioides and Fusarium graminearum—Kinetic insights to combating fusarium head blight. J. Biol. Chem. 2008, 283, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Cardoza, R.E.; Malmierca, M.G.; Hermosa, M.R.; Alexander, N.J.; McCormick, S.P.; Proctor, R.H.; Tijerino, A.M.; Rumbero, A.; Monte, E.; Gutierrez, S. Identification of loci and functional characterization of trichothecene biosynthesis genes in filamentous fungi of the genus Trichoderma. Appl. Environ. Microbiol. 2011, 77, 4867–4877. [Google Scholar] [CrossRef]
- McCormick, S.P.; Alexander, N.J.; Proctor, R.H. Fusarium Tri4 encodes a multifunctional oxygenase required for trichothecene biosynthesis. Can. J. Microbiol. 2006, 52, 636–642. [Google Scholar] [CrossRef]
- McCormick, S.P.; Alexander, N.J. Myrothecium roridum Tri4 encodes a multifunctional oxygenase required for three oxygenation steps. Can. J. Microbiol. 2007, 53, 572–579. [Google Scholar] [CrossRef]
- Tokai, T.; Koshino, H.; Takahashi-Ando, N.; Sato, M.; Fujimura, M.; Kimura, M. Fusarium Tri4 encodes a key multifunctional cytochrome P450 monooxygenase for four consecutive oxygenation steps in trichothecene biosynthesis. Biochem. Biophys. Res. Commun. 2007, 353, 412–417. [Google Scholar] [CrossRef]
- Lee, T.; Han, Y.K.; Kim, K.H.; Yun, S.H.; Lee, Y.W. Tri13 and Tri7 determine deoxynivalenol- and nivalenol-producing chemotypes of Gibberella zeae. Appl. Environ. Microbiol. 2002, 68, 2148–2154. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.W.; McCormick, S.P.; Alexander, N.J.; Proctor, R.H.; Desjardins, A.E. Inactivation of a cytochrome P450 is a determinant of trichothecene diversity in Fusarium species. Fungal Genet. Biol. 2002, 36, 224–233. [Google Scholar] [CrossRef]
- Alexander, N.J.; McCormick, S.P.; Waalwijk, C.; van der Lee, T.; Proctor, R.H. The genetic basis for 3-ADON and 15-ADON trichothecene chemotypes in Fusarium. Fungal Genet. Biol. 2011, 48, 485–495. [Google Scholar] [CrossRef]
- Villafana, R.T.; Ramdass, A.C.; Rampersad, S.N. Selection of Fusarium trichothecene toxin genes for molecular detection depends on TRI gene cluster organization and gene function. Toxins 2019, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Semeiks, J.; Borek, D.; Otwinowski, Z.; Grishin, N.V. Comparative genome sequencing reveals chemotype-specific gene clusters in the toxigenic black mold Stachybotrys. BMC Genom. 2014, 15. [Google Scholar] [CrossRef] [PubMed]
- Corley, D.G.; Millerwideman, M.; Durley, R.C. Isolation and Structure of Harzianum A: A New Trichothecene from Trichoderma harzianum. J. Nat. Prod. 1994, 57, 422–425. [Google Scholar] [CrossRef]
- Wang, A.R.; Xu, Y.B.; Gao, Y.X.; Huang, Q.; Luo, X.; An, H.M.; Dong, J.Y. Chemical and bioactive diversities of the genera Stachybotrys and Memnoniella secondary metabolites. Phytochem. Rev. 2015, 14, 623–655. [Google Scholar] [CrossRef]
- Liu, H.X.; Liu, W.Z.; Chen, Y.C.; Sun, Z.H.; Tan, Y.Z.; Li, H.H.; Zhang, W.M. Cytotoxic trichothecene macrolides from the endophyte fungus Myrothecium roridum. J. Asian Nat. Prod. Res. 2016, 18, 684–689. [Google Scholar] [CrossRef]
- Shen, L.; Wang, J.A.S.; Shen, H.J.; Song, Y.C.; Tan, R.X. A new cytotoxic trichothecene macrolide from the endophyte Myrothecium roridum. Planta Med. 2010, 76, 1004–1006. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, L.; Wang, N.; Wang, S.J.; Hu, J.C.; Gao, J.M. Potent Toxic Macrocyclic Trichothecenes from the Marine-Derived Fungus Myrothecium verrucaria Hmp-F73. Nat. Prod. Commun. 2011, 6, 1915–1916. [Google Scholar] [CrossRef]
- Lee, S.R.; Seok, S.; Ryoo, R.; Choi, S.U.; Kim, K.H. Macrocyclic trichothecene mycotoxins from a deadly poisonous mushroom, Podostroma cornu-damae. J. Nat. Prod. 2019, 82, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, D.; Cheng, Z.B.; Proksch, P.; Lin, W.H. Cytotoxic trichothecene-type sesquiterpenes from the sponge-derived fungus Stachybotrys chartarum with tyrosine kinase inhibition. RSC Adv. 2017, 7, 7259–7267. [Google Scholar] [CrossRef]
- Yu, N.J.; Guo, S.X.; Lu, H.Y. Cytotoxic macrocyclic trichothecenes from the mycelia of Calcarisporium arbuscula Preuss. J. Asian Nat. Prod. Res. 2002, 4, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Whyte, A.C.; Gloer, J.B.; Scott, J.A.; Malloch, D. Cercophorins A–C: Novel antifungal and cytotoxic metabolites from the coprophilous fungus Cercophora areolata. J. Nat. Prod. 1996, 59, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Minato, H.; Tori, K.; Ueyama, M. Structures of Isororidin E, Epoxyisororidin E, and Epoxy H and Diepoxyroridin H, New Metabolites Isolated from Cylindrocarpon Species Determined by C-13 and H-1 NMR Spectroscopy. Revision of C-2′:C-3′ Double-Bond Configuration of Roridin Group. Tetrahedron Lett. 1977, 18, 4093–4096. [Google Scholar] [CrossRef]
- Panozish, K.P.; Borovkov, A.V. Roridin a from Dendrodochium toxicum. Khimiya Prir. Soedin. 1974, 10, 404–405. [Google Scholar]
- Samples, D.; Hill, D.W.; Bridges, C.H.; Camp, B.J. Isolation of a mycotoxin (roridin A) from Phomopsis spp. Vet. Hum. Toxicol. 1984, 26, 21–23. [Google Scholar]
- Minato, H.; Katayama, T.; Tori, K. Vertisporin, a New Antibiotic from Verticimonosporium diffractum. Tetrahedron Lett. 1975, 16, 2579–2582. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Jarvis, B.B.; Dailey, R.J., Jr.; Bright, W.; Bryan, R.F.; Shizuri, Y. Baccharin, a novel potent antileukemic trichothecene triepoxide from Baccharis megapotamica. J. Am. Chem. Soc. 1976, 98, 7092–7093. [Google Scholar] [CrossRef]
- De Carvalho, M.P.; Weich, H.; Abraham, W.R. Macrocyclic trichothecenes as antifungal and anticancer compounds. Curr. Med. Chem. 2016, 23, 23–35. [Google Scholar] [CrossRef]
- Mondol, M.A.; Surovy, M.Z.; Islam, M.T.; Schuffler, A.; Laatsch, H. Macrocyclic trichothecenes from Myrothecium roridum strain M10 with motility inhibitory and zoosporicidal activities against Phytophthora nicotianae. J. Agric. Food Chem. 2015, 63, 8777–8786. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, B.B.; Stahly, G.P.; Pavanasasivam, G.; Midiwo, J.O.; Geoghegan, R.F. Isolation and characterization of the trichoverroids and new roridins and verrucarins. J. Org. Chem. 1981, 47, 1117–1124. [Google Scholar] [CrossRef]
- Shen, L.; Ai, C.Z.; Song, Y.C.; Wang, F.W.; Jiao, R.H.; Zhang, A.H.; Man, H.Z.; Tan, R.X. Cytotoxic trichothecene macrolides produced by the endophytic Myrothecium roridum. J. Nat. Prod. 2019, 82, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Lakornwong, W.; Kanokmedhakul, K.; Soytong, K.; Unartngam, A.; Tontapha, S.; Amornkitbamrung, V.; Kanokmedhakul, S. Types A and D trichothecene mycotoxins from the fungus Myrothecium roridum. Planta Med. 2019, 85, 774–780. [Google Scholar] [CrossRef]
- Lin, T.; Wang, G.H.; Zhou, Y.Q.; Zeng, D.Q.; Liu, X.X.; Ding, R.; Jiang, X.; Zhu, D.; Shan, W.J.; Chen, H.F. Structure elucidation and biological activity of two new trichothecenes from an endophyte, Myrothecium roridum. J. Agric. Food Chem. 2014, 62, 5993–6000. [Google Scholar] [CrossRef]
- Shen, L.; Zhu, L.; Tan, Q.W.; Wan, D.; Xie, J.; Peng, J.N. New cytotoxic trichothecene macrolide epimers from endophytic Myrothecium roridum IFB-E012. J. Antibiot. 2016, 69, 652–655. [Google Scholar] [CrossRef]
- Shen, L.; Jiao, R.H.; Ye, Y.H.; Wang, X.T.; Xu, C.; Song, Y.C.; Zhu, H.L.; Tan, R.X. Absolute configuration of new cytotoxic and other bioactive trichothecene macrolides. Chemistry 2006, 12, 5596–5602. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Li, Z.L.; Guan, L.P.; Wu, X.; Pan, H.Q.; Bai, J.; Hua, H.M. Structure determination of two new trichothecenes from a halotolerant fungus Myrothecium sp. GS-17 by NMR spectroscopy. Magn. Reson. Chem. 2012, 50, 632–636. [Google Scholar] [CrossRef]
- Piao, M.Z.; Shen, L.; Wang, F.W. A new trichothecene from Myrothecium roridum QDFE005, a symbiotic fungus isolated from Mactra chinensis. J. Asian Nat. Prod. Res. 2013, 15, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Minato, H.; Uotani, N.; Matsumoto, K.; Kondo, E. New antibiotics from Cylindrocarpon sp. J. Antibiot. 1977, 30, 681–682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Tamez, P.A.; Aydogmus, Z.; Tan, G.T.; Saikawa, Y.; Hashimoto, K.; Nakata, M.; Hung, N.V.; Xuan le, T.; Cuong, N.M.; et al. Antimalarial agents from plants. III. Trichothecenes from Ficus fistulosa and Rhaphidophora decursiva. Planta Med. 2002, 68, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.C.; Rosso, M.L.; Bertoni, M.D.; Maier, M.S.; Damonte, E.B. Evaluation of the antiviral activity against Junin virus of macrocyclic trichothecenes produced by the hypocrealean epibiont of Baccharis coridifolia. Planta Med. 2002, 68, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Amagata, T.; Rath, C.; Rigot, J.F.; Tarlov, N.; Tenney, K.; Valeriote, F.A.; Crews, P. Structures and cytotoxic properties of trichoverroids and their macrolide analogues produced by saltwater culture of Myrothecium verrucaria. J. Med. Chem. 2003, 46, 4342–4350. [Google Scholar] [CrossRef] [PubMed]
- Jayasooriya, R.G.P.T.; Moon, D.O.; Park, S.R.; Choi, Y.H.; Asami, Y.; Kim, M.O.; Jang, J.H.; Kim, B.Y.; Ahn, J.S.; Kim, G.Y. Combined treatment with verrucarin A and tumor necrosis factor-alpha sensitizes apoptosis by overexpression of nuclear factor-kappaB-mediated Fas. Environ. Toxicol. Pharmacol. 2013, 36, 303–310. [Google Scholar] [CrossRef]
- Brase, S.; Encinas, A.; Keck, J.; Nising, C.F. Chemistry and Biology of Mycotoxins and Related Fungal Metabolites. Chem. Rev. 2009, 109, 3903–3990. [Google Scholar] [CrossRef]
- Steinmetz, W.E.; Rodarte, C.B.; Lin, A. 3D QSAR study of the toxicity of trichothecene mycotoxins. Eur. J. Med. Chem. 2009, 44, 4485–4489. [Google Scholar] [CrossRef]
- Oda, T.; Xu, J.; Ukai, K.; Nakazawa, T.; Namikoshi, M. 12′-Hydroxyl group remarkably reduces Roridin E cytotoxicity. Mycoscience 2010, 51, 317–320. [Google Scholar] [CrossRef]
- Wu, Q.H.; Wang, X.; Nepovimova, E.; Miron, A.; Liu, Q.Y.; Wang, Y.; Su, D.X.; Yang, H.L.; Li, L.; Kuca, K. Trichothecenes: Immunomodulatory effects, mechanisms, and anti-cancer potential. Arch. Toxicol. 2017, 91, 3737–3785. [Google Scholar] [CrossRef]
- Smitka, T.A.; Bunge, R.H.; Bloem, R.J.; French, J.C. Two new trichothecenes, PD 113,325 and PD 113,326. J. Antibiot. 1984, 37, 823–828. [Google Scholar] [CrossRef]
- Matsumoto, M.; Nishiyama, M.; Maeda, H.; Tonouchi, A.; Konno, K.; Hashimoto, M. Structure-activity relationships of trichothecenes against COLO201 cells and Cochliobolus miyabeanus: The role of 12-epoxide and macrocyclic moieties. Bioorg. Med. Chem. Lett. 2019, 29, 982–985. [Google Scholar] [CrossRef]
- Lindo, L.; McCormick, S.P.; Cardoza, R.E.; Busman, M.; Alexander, N.J.; Proctor, R.H.; Gutierrez, S. Requirement of two acyltransferases for 4-O-acylation during biosynthesis of harzianum A, an antifungal trichothecene produced by Trichoderma arundinaceum. J. Agric. Food Chem. 2019, 67, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Weissman, K.J. Genetic engineering of modular PKSs: From combinatorial biosynthesis to synthetic biology. Nat. Prod. Rep. 2016, 33, 203–230. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.Q.; Garg, A.; Khosla, C.; Cane, D.E. Mechanism and stereochemistry of polyketide chain elongation and methyl group epimerization in polyether biosynthesis. J. Am. Chem. Soc. 2017, 139, 3283–3292. [Google Scholar] [CrossRef] [PubMed]
- Valenzano, C.R.; Lawson, R.J.; Chen, A.Y.; Khosla, C.; Cane, D.E. The biochemical basis for stereochemical control in polyketide biosynthesis. J. Am. Chem. Soc. 2009, 131, 18501–18511. [Google Scholar] [CrossRef]
- Liu, T.G.; Lin, X.; Zhou, X.F.; Deng, Z.X.; Cane, D.E. Mechanism of thioesterase-catalyzed chain release in the biosynthesis of the polyether antibiotic nanchangmycin. Chem. Biol. 2008, 15, 449–458. [Google Scholar] [CrossRef][Green Version]
- Al-Rawahi, A.Y.; Al-Mahmooli, I.H.; Al-Sadi, A.M.; Al-Sabahi, J.N.; Velazhahan, R. Toxin production by melon root rot fungus, Monosporascus cannonballus. Australas. Plant Path. 2018, 47, 543–546. [Google Scholar] [CrossRef]
- Wheeler, M.H.; Bruton, B.D.; Puckhaber, L.S.; Zhang, J.X.; Stipanovic, R.D. Identification of 1,8-dihydroxynaphthalene melanin in Monosporascus cannonballus and the analysis of hexaketide and pentaketide compounds produced by wild-type and pigmented isolates of the fungus. J. Agric. Food Chem. 2004, 52, 4113–4120. [Google Scholar] [CrossRef]
- Trapp, S.C.; Hohn, T.M.; McCormick, S.; Jarvis, B.B. Characterization of the gene cluster for biosynthesis of macrocyclic trichothecenes in Myrothecium roridum. Mol. Gen. Genet. 1998, 257, 421–432. [Google Scholar] [CrossRef]
- Degenkolb, T.; Dieckmann, R.; Nielsen, K.F.; Grafenhan, T.; Theis, C.; Zafari, D.; Chaverri, P.; Ismaiel, A.; Bruckner, H.; von Dohren, H.; et al. The Trichoderma brevicompactum clade: A separate lineage with new species, new peptaibiotics, and mycotoxins. Mycol. Prog. 2008, 7, 177–219. [Google Scholar] [CrossRef]
- Liu, L.; Tang, M.X.; Sang, X.N.; Chen, S.F.; Lu, X.J.; Wang, Y.B.; Si, Y.Y.; Wang, H.F.; Chen, G.; Pei, Y.H. Three new tetralol analogs from soil-derived fungus Myrothecium verrucaria with anti-inflammatory activity. J. Asian Nat. Prod. Res. 2018, 21, 33–42. [Google Scholar] [CrossRef]
- Tokai, T.; Takahashi-Ando, N.; Izawa, M.; Kamakura, T.; Yoshida, M.; Fujimura, M.; Kimura, M. 4-O-acetylation and 3-O-acetylation of trichothecenes by trichothecene 15-O-acetyltransferase encoded by Fusarium Tri3. Biosci. Biotechnol. Biochem. 2008, 72, 2485–2489. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, B.B.; Vrudhula, V.M.; Pavanasasivam, G. Trichoverritone and 16-hydroxyroridin L-2, new trichothecenes from Myrothecium roridum. Tetrahedron Lett. 1983, 24, 3539–3542. [Google Scholar] [CrossRef]
- Desjardins, A.E. Fusarium Mycotoxins: Chemistry, Genetics and Biology; APS Press: St. Paul, MN, USA, 2006; pp. 1–260. [Google Scholar]
- Pestka, J.J.; Forsell, J.H. Inhibition of Human-Lymphocyte Transformation by the Macrocyclic Trichothecenes Roridin A and Verrucarin A. Toxicol. Lett. 1988, 41, 215–222. [Google Scholar] [CrossRef]
- Dosen, I.; Andersen, B.; Phippen, C.B.W.; Clausen, G.; Nielsen, K.F. Stachybotrys mycotoxins: From culture extracts to dust samples. Anal. Bioanal. Chem. 2016, 408, 5513–5526. [Google Scholar] [CrossRef]
- Jarvis, B.B.; Mazzola, E.P. Macrocyclic and Other Novel Trichothecenes—Their Structure, Synthesis, and Biological Significance. Acc. Chem. Res. 1982, 15, 388–395. [Google Scholar] [CrossRef]
- Sy-Cordero, A.A.; Graf, T.N.; Wani, M.C.; Kroll, D.J.; Pearce, C.J.; Oberlies, N.H. Dereplication of macrocyclic trichothecenes from extracts of filamentous fungi through UV and NMR profiles. J. Antibiot. 2010, 63, 539–544. [Google Scholar] [CrossRef]
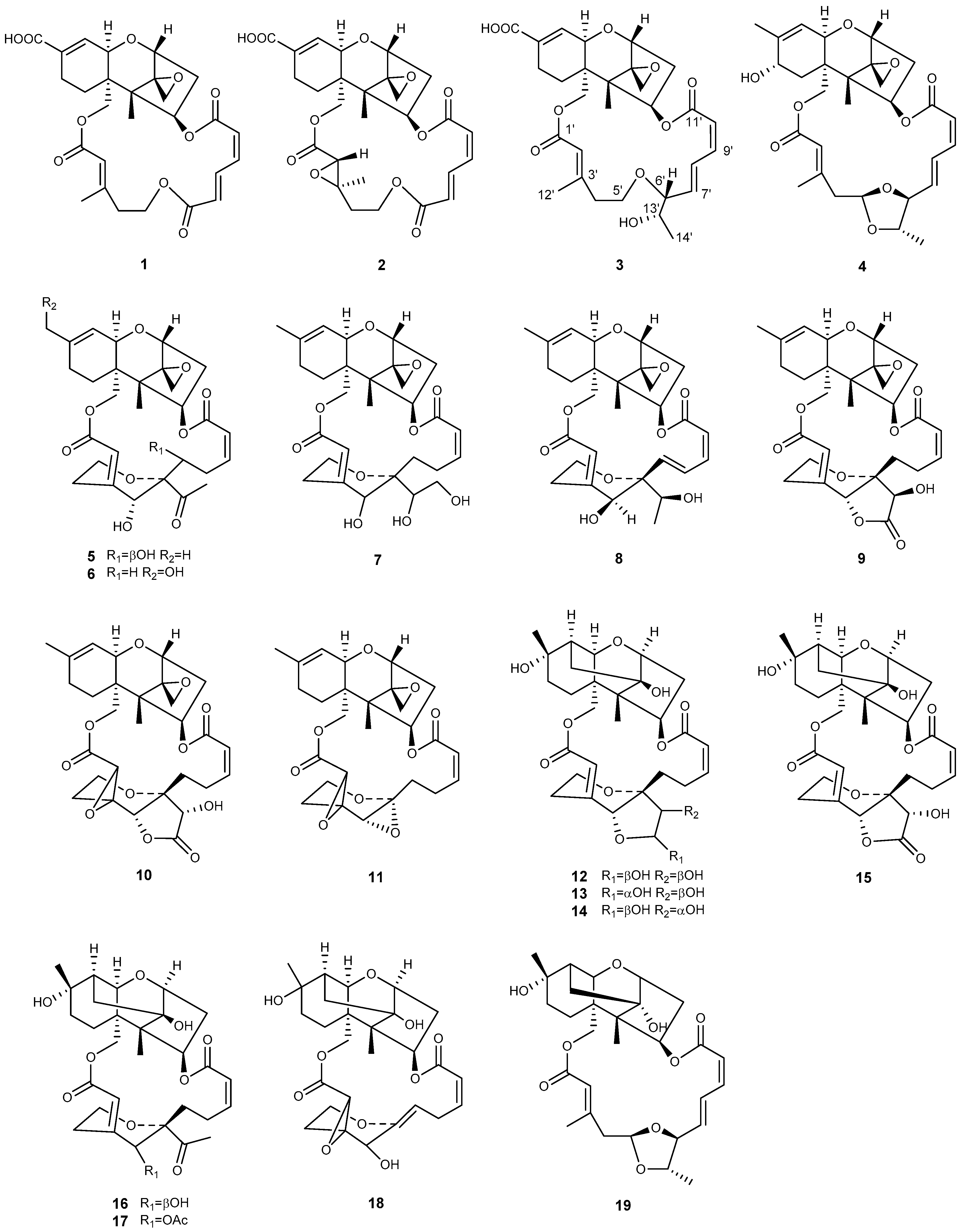
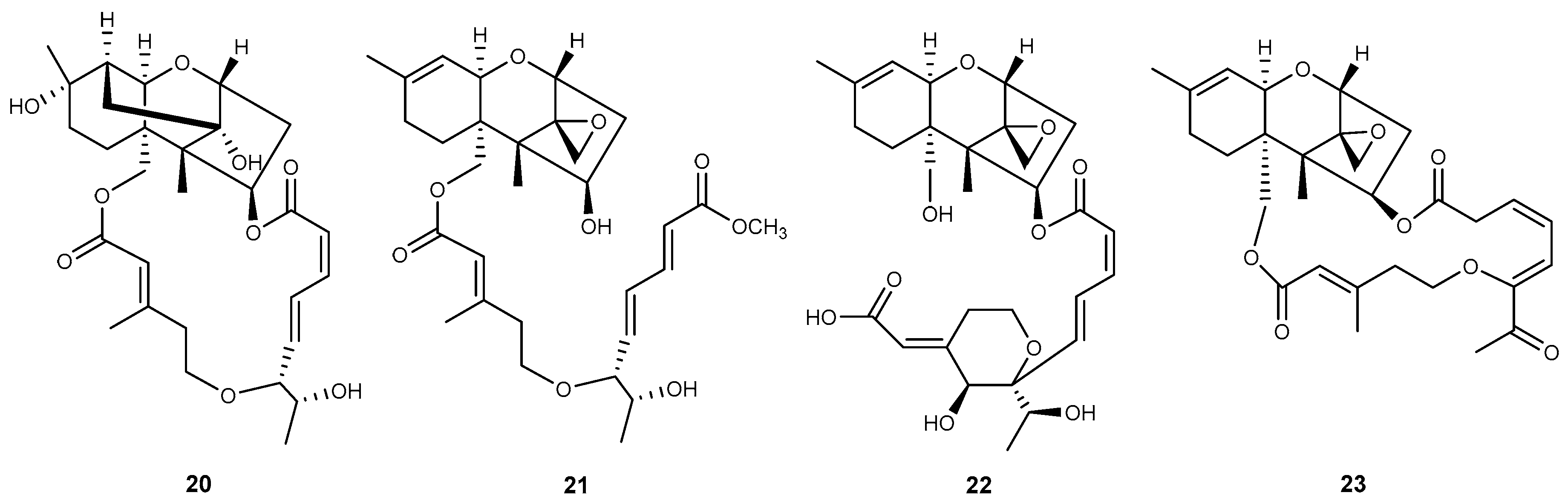
| Genera | No. | Compound | Ref. |
|---|---|---|---|
| Myrothecium | 1 | verrucarin Y | [42] |
| 2 | verrucarin Z | [42] | |
| 3 | epiroridin acid | [29] | |
| 4 | 8a-hydroxyroridin H | [49] | |
| 5 | 7′-hydroxymytoxin B | [45] | |
| 6 | 16-hydroxymytoxin B | [44] | |
| 7 | 13′,14′-hydroxymytoxin B | [46] | |
| 8 | 12′-episatratoxin H | [50] | |
| 9 | 14′-dehydrovertisporin | [44] | |
| 10 | roritoxin E | [30] | |
| 11 | 6′,12′-epoxymyrotoxin A | [45] | |
| 12 | dihydromyrothecine C, 1a | [47] | |
| 13 | dihydromyrothecine C, 1b | [47] | |
| 14 | myrothecine D | [44] | |
| 15 | myrothecine E | [44] | |
| 16 | myrothecine F | [44] | |
| 17 | myrothecine G | [44] | |
| 18 | 2′,3′-epoxymyrothecine A | [46] | |
| 19 | myrothecin A | [49] | |
| Podostroma | 20 | miophytocen D | [32] |
| 21 | roridin F | [32] | |
| 22 | satratoxin I | [32] | |
| Stachybotrys | 23 | chartarene D | [33] |
| Cytotoxicity (IC50) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | HepG-2 a | MCF-7 | SF-268 | K562 | SW1116 | KB | NCI-H187 | A549 | SMMC-7721 | SGC-7901 | Ref. |
| 3 | 0.380 ± 0.03 μM | 0.170 ± 0.01 μM | 0.751 ± 0.03 μM | 0.360 ± 0.05 μM b | [29] | ||||||
| 5 | 2.81 nM | 5.99 nM | [45] | ||||||||
| 6 | 2.87 μM | 0.18 μM | [44] | ||||||||
| 7 | 49 ± 3.35 nM | 63 ± 2.38 nM | 53 ± 3.36 nM | 46 ± 2.88 nM | [46] | ||||||
| 8 | 2.27 μM | 1.42 μM | [50] | ||||||||
| 9 | 56 nM | 200 nM | [44] | ||||||||
| 10 | 18.89 μM | 0.46 μM | [30] | ||||||||
| 11 | 0.63 nM | 0.79 nM | [45] | ||||||||
| 12 | 44.48 μM | [47] | |||||||||
| 14 | 8.2 μM | 0.57 μM | [44] | ||||||||
| 15 | 15.98 μM | 11.61 μM | [44] | ||||||||
| 16 | 0.97 μM | 10.62 μM | [44] | ||||||||
| 17 | 1.53 μM | 4.25 μM | [44] | ||||||||
| 18 | 32.03 ± 2.94 μM | 18.13 ± 3.89 μM | 36.45 ± 2.79 μM | 30.33 ± 9.71 μM | [46] | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Cen, Y.; Ye, W.; Li, S.; Zhang, W. Recent Advances on Macrocyclic Trichothecenes, Their Bioactivities and Biosynthetic Pathway. Toxins 2020, 12, 417. https://doi.org/10.3390/toxins12060417
Zhu M, Cen Y, Ye W, Li S, Zhang W. Recent Advances on Macrocyclic Trichothecenes, Their Bioactivities and Biosynthetic Pathway. Toxins. 2020; 12(6):417. https://doi.org/10.3390/toxins12060417
Chicago/Turabian StyleZhu, Muzi, Youfei Cen, Wei Ye, Saini Li, and Weimin Zhang. 2020. "Recent Advances on Macrocyclic Trichothecenes, Their Bioactivities and Biosynthetic Pathway" Toxins 12, no. 6: 417. https://doi.org/10.3390/toxins12060417
APA StyleZhu, M., Cen, Y., Ye, W., Li, S., & Zhang, W. (2020). Recent Advances on Macrocyclic Trichothecenes, Their Bioactivities and Biosynthetic Pathway. Toxins, 12(6), 417. https://doi.org/10.3390/toxins12060417





