Abstract
Evolution of insect resistance to Bt toxins challenges the use of Cry toxins to control agricultural pests. In lepidopterans, Cry toxin affinity towards multiple midgut epithelial receptors has become a matter of dispute. Cry1Ah toxin-binding proteins were identified in the larval midgut of susceptible (ACB-BtS) and resistant (ACB-AhR) strains of the Asian corn borer (ACB). A pull-down assay was performed using biotinylated Cry1Ah toxin, and the binding proteins were identified by employing liquid chromatography–tandem mass spectrometry (LC-MS/MS). This study aimed to find the binding consistency of the midgut epithelial protein to the Cry1Ah toxin. The binding proteins from different fractions of SDS-PAGE showed a different pattern. We observed an isoform of prophenoloxidase PPO1b (UniProt Acc No. A0A1Q1MKI0), which was found only in the ACB-AhR fractions. Prophenoloxidase (proPO) is an extraordinary defense molecule activated in insect species during pathogen invasion and the wound healing process. Importantly, this prophenoloxidase might have direct/indirect interaction with the Cry1Ah toxin. Our data also suggest that factors like techniques, enrichment of binding proteins in the sample and the reversible and irreversible nature of the brush border membrane vesicles (BBMVs) to Cry toxins could cause the inconsistency in the protein–protein interactions. Moreover, inside the larva midgut, the influence of the Cry toxins under physiological conditions might be different from the laboratory procedures.
Key Contribution:
In the current work, we analyzed the reliability of the Cry1Ah toxin-binding proteins extracted from the midgut of the ACB and compared the results with a previously published report using the same toxin. We observed an inconsistency in the proteins that bind to the Cry1Ah toxin. We conclude that there might be several factors influencing the binding ability; these factors including techniques, enrichment of binding proteins in the sample and the reversible and irreversible nature of the BBMVs to Cry toxins. Furthermore, it is reasonable to infer that the influence of the Cry toxins under physiological conditions might be quite different from the laboratory procedures.
1. Introduction
The Bacillus thuringiensis (Bt) insecticidal crystal (Cry) proteins are a diverse family of proteins with over 780 identified members [1]. Bt Cry proteins have insecticidal properties. The wide variety of these toxins, their effectiveness and relatively inexpensive processing have made Bt the world’s most commonly used biopesticide. These Cry proteins are used as sprays or as a Cry gene expressing transgenic crops, and are used mainly in the fight against lepidopteran and other agricultural crop pests [2]. However, the extensive use of transgenic crops raises the selection pressure to evolve insect resistance to the Bt proteins, thus decreasing the effectiveness of the Bt Cry proteins [3,4,5]. Several cases of insect resistance to Bt toxins both in the field and under laboratory conditions have been reported [4,5,6,7,8].
Hence, understanding the mechanism of insect resistance to Cry toxins is essential for the development of effective pest management strategies. Previously, a study showed that the reduction in toxin-binding to the midgut epithelium is associated with the resistance evolution mechanism in different insect populations [9]. Previously, two different models explained the modes of action delivered by the Cry toxin: (1) the pore formation model and (2) the signal transduction model [10]. The pro-toxins are cleaved by midgut proteases that bind to the membrane proteins in the brush border membrane vesicles (BBMVs) of the insect midgut. Eventually, these toxic oligomers promote insertion or activation of protein kinase A, which primes to osmotic imbalance or cytotoxicity and ultimately leading to cell death [11,12,13,14,15]. Several approaches have been shown to be successful in dealing with insect resistance, such as the acquisition of novel Cry proteins with different modes of action [6].
In Lepidoptera, different proteins have been previously identified as Cry1A toxin receptors, like cadherin (CAD) [12,16,17,18], aminopeptidase-N (APN) [19,20,21] and alkaline phosphatase (ALP) [22,23,24]. In addition, other different proteins have been reported, such as the ATP-binding cassette (ABC—ABCC2, ABCC3 and ABCA2) transporter [25,26,27,28], actin, Hsp70 and V-ATPase [29,30,31]. In addition to all these membrane proteins, it was reported that immune-related proteins might also contribute to the development of resistance [32,33,34,35,36]. Recently, a study brought evidence of the possible direct binding of the Cry1Ah toxin to phenoloxidase (PO) and other immune-related proteins identified in BBMV samples extracted from susceptible and resistant strains of ACB [36]. However, additional proteins may still be involved in the interaction with the Cry1A toxin.
The Asian corn borer (ACB), Ostrinia furnacalis (Guenée) (Lepidoptera: Crambidae), is an important lepidopteran pest, with larval caterpillars causing the most destructive damage to maize throughout China [37,38,39]. The larvae infest most parts of the plant, but the ears and stalk are the primary victims [40]. Managing this pest is a difficult task for the farmers due to the cost of insecticides, resistance development, environmental concerns and uncertainty about the effectiveness of the pest management strategies [41]. Shabbir et al. [39] proved that the Cry1Ah-expressing maize is effective against ACB larvae. However, in the same study, the ACB larvae gradually developed resistance against the Cry1Ah toxin (200-fold) as well as various levels of cross-resistance against the Cry1Ab, Cry1Ac and Cry1Fa toxins under laboratory conditions. The exact mechanism for the development of resistance in the ACB remains unknown.
In this study, we have determined the consistency of the Cry1Ah toxin-binding to BBMV proteins extracted from susceptible (ACB-BtS) and resistant (ACB-AhR) strains of the ACB. We employed a different protocol to analyze the consistency of the Cry1Ah toxin-binding protein and compared the pattern of the ACB proteins interacting with the Cry1Ah toxin. The Cry1Ah affinity towards the BBMV proteins was analyzed via a pull-down assay and LC-MS/MS analysis.
2. Results
2.1. APN and ALP Activities in ACB Larval Midgut
The APN and ALP activities in the BBMV samples were approximately three- to four-fold higher than in the midgut homogenate (Table 1). Enrichment of BBMV proteins was indicated by the high ratios of APN and ALP activities in the BBMV sample extracted from the midgut homogenate.

Table 1.
Enzymatic activities of aminopeptidase-N (APN) and alkaline phosphatase (ALP) in the midgut homogenate and brush border membrane vesicles (BBMVs) from resistant (ACB-AhR) and susceptible (ACB-BtS) Asian corn borer (ACB) larvae.
2.2. Cry1Ah-Binding Proteins from BBMV of ACB
The biotinylated Cry1Ah interacted with the BBMV proteins extracted from the ACB-BtS and ACB-AhR strains and were analyzed using 12% Gel (Figure 1). The ACB proteins that are bound to Cry1Ah are shown from Lanes 3 and 4 (Figure 1). Lanes 1 and 2 show the extracted BBMV proteins of the ACB-BtS (7.85 mg/mL) and ACB-AhR (9.20 mg/mL) strains, respectively. Biotinylated Cry1Ah interacted with the BBMVs of both the ACB-BtS and ACB-AhR strains, and showed a similar binding pattern in Lanes 3 and 4 (Figure 1).
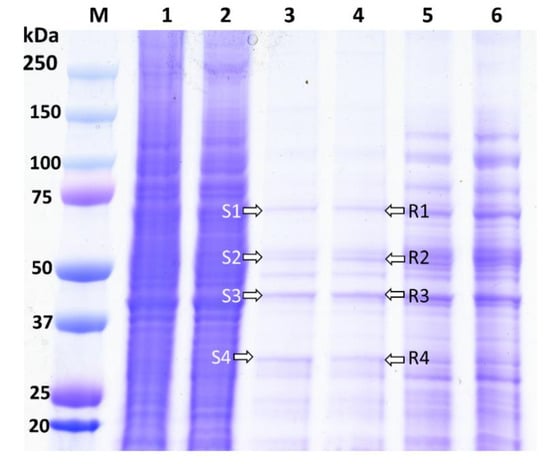
Figure 1.
Pull-down Cry1Ah-binding proteins from the BBMVs of the ACB-BtS and ACB-AhR strains. M: Precession plus, dual color marker (Biorad, Hercules, CA, USA); Lane 1 and 2: BBMV from ACB-BtS and ACB-AhR strains; Lane 3 and 4: Cry1Ah-binding proteins finally eluted from the BBMVs of the ACB-BtS and ACB-AhR strains; Lane 5 and 6: Non-treated control proteins from the ACB-BtS and ACB-AhR strains. S1–S4 and R1–R4, bands selected for LC-MS/MS analysis.
2.3. Cry1Ah-Binding Proteins Identified from the BBMVs of the ACB
The Cry1Ah-binding proteins of the ACB from Lanes 3 and 4 were divided into four fractions (Figure 1). To identify the Cry1Ah-binding proteins, these fragments of the gel were subjected to LC-MS/MS analysis. The proteins identified in each fraction of the gel showed a positive hit with a higher score, more than the threshold (>0.05) level (Table 2). The corresponding accession numbers obtained from the UniProt database are shown in Table 2. LC-MS/MS results revealed 32 different proteins binding Cry1Ah in the BBMV from ACB-BtS and 33 proteins from ACB-AhR (Table 2). Among these proteins, 24 Cry1Ah-binding proteins were common in both populations (Table 2). Besides the commonly reported Cry-binding proteins, such as APN, ALP, cadherin, Hsp70 and V-ATPase, we also detected prophenoloxidase, prophenoloxidase PPO1a, serine proteinase inhibitor 2 and serpin 5 from the S3, R3 and S4 fractions (Table 2). Especially, there were six proteins, namely carboxylesterase, ryanodine receptor, beta-hexosaminidase, NADH-ubiquinone oxidoreductase, acyl-CoA delta-9 desaturase and ALP, found only in the ACB-BtS (Table 2). On the other hand, integrin beta, prophenoloxidase and trehalase were detected only in the ACB-AhR samples (Table 2, Supplementary Table S1). ALP was identified only in the ACB-BtS samples, and thus not detected in the ACB-AhR samples (Supplementary Table S1).

Table 2.
The LC-MS/MS analysis results of the Cry1Ah-binding proteins of the BBMVs from susceptible and resistant strains of Ostrinia furnacalis.
3. Discussion
In the present study, we analyzed the consistency of Cry1Ah toxin-binding to specific BBMV proteins extracted from the susceptible ACB-BtS and resistant ACB-AhR strains of O. furnacalis. The list of Cry1Ah-binding proteins obtained from the four fractions (Figure 1) with their respective molecular weight was compared with the Cry1Ah-binding proteins previously reported by Shabbir et al. [36]. The Cry toxins that bind to the BBMV protein receptors are very important for understanding the toxic nature of the Cry toxins [42]. Interaction between the Bt toxins and BBMVs is associated with the evolution of resistance mechanisms [43,44,45,46,47].
In this study, we found some proteins that were previously reported as Cry-binding proteins, namely APN, ALP, cadherin, Hsp70 and V-ATPase [29,30,31,48] (Table 2). We compared the list of Cry1Ah-binding proteins of the ACB-BtS and ACB-AhR strains. In total, six proteins, namely carboxylesterase, ryanodine receptor, beta-hexosaminidase, NADH-ubiquinone oxidoreductase, acyl-CoA delta-9 desaturase and ALP, were only detected in the ACB-BtS samples (Table 2). Integrin beta, prophenoloxidase and trehalase were only detected in the ACB-AhR fractions (Table 2). Although most of the identified proteins seem to be similar to the binding protein reported [36], we observed few proteins which were different from that list. Likewise, in our present study, prophenoloxidase PPO1b (UniProt ID: A0A1Q1MKI0) and trehalase (UniProt ID-A0A1B2AQF4) were found only in the ACB-AhR fractions (Table 2, Supplementary Table S1). This inconsistency in binding might be associated with receptors on the BBMVs, including both reversible and irreversible binding steps [49,50].
On the other hand, we selected four fractions from the SDS-PAGE that had similar molecular weights as reported by Shabbir et al. [36] (Figure 1) and compared with the list of proteins from the fractions S3, S4, S6, S7–R3, R4, R6 and R7. Firstly, our protein list from the S1 and R1 fractions was compared with S3 and R3. Shabbir et al. [36] reported 14 proteins from the ACB-BtS samples and 11 from the ACB-AhR samples; however, we found 18 binding proteins from the ACB-BtS samples and 16 from the ACB-AhR samples. Arginine kinase, APN, actin, Hsp 7090, prophenoloxidase PPO1a and V-ATPase were the common proteins identified from both studies. Besides, proteins like trypsin, juvenile hormone and prophenoloxidase PPO1b were not found in our fractions. Inconsistency in binding may be related to the experimental setup or chemicals that were used for the study. Shabbir et al. [36] used the NHS-activated sepharose (GE Healthcare, Uppsala, Sweden), which was different from our pull-down assay protocol. An earlier report, based on a heterologous competition binding assay, proposed that Cry1Ab and Cry1Ac share the same binding sites in L. dispar [51], but the results suggested by Wolfersberger are contradictory to the ligand blotting results [52]. In Manduca sexta, partially purified Cry1Ac binding APN has been used in competition binding assays, surface plasmon resonance experiments and liposome reconstitution experiments [53,54,55]. Competition binding assays and liposome reconstitution experiments showed the affinity of Cry1Ac to APN was relatively low. Conversely, surface plasmon resonance results differed between the reports. This matter created a huge confusion among the researchers to adapt the right experiment to find the Cry toxin-binding protein. Such differences can also correlate in these experiments with the enrichment of binding proteins at different degrees that are subject to the Cry1A toxins [56].
Comparing the other fractions (S2 to S4), the pattern of the binding protein seems to differ from Shabbir et al. [36] (Table 2, Supplementary Table S2) Several factors might affect the interaction between the Cry1Ah toxin and binding protein, like using different kit chemicals, enrichment of binding proteins after the extraction process and the reversible and irreversible nature of the BBMVs to Cry toxins [49,50,56]. Moreover, inside the larva midgut, the influence of the Cry toxins under physiological conditions might be different from the experiments carried out in the laboratory. At this point, understanding the mechanism of the midgut receptors towards Cry toxins will be a vital part to maintain the right toxin under field conditions. However, based on these results, we cannot conclude the exact list of binding proteins from the BBMVs. Remarkably, in accordance with Shabbir et al. [36], ALP was detected only in the BBMV samples of the ACB-BtS strain, suggesting that reduced levels of ALP expression may contribute to the development of resistance in the ACB-AhR strain. Heliothis virescens and H. armigera showed resistance to Cry1Ac, and Spodoptera frugiperda developed resistance against the Cry1Fa toxin, suggesting that reduced levels of ALP protein promote the resistance development in these lepidopteran pests [57,58].
However, the expression of the ALP gene was 6-fold higher in ACB-AhR than in ACB-BtS. Similarly, ALP enzymatic activity in the BBMV samples of the ACB-AhR strain was two-fold higher than for the ACB-BtS strain [36]. Interestingly, we did not find any ALP isoforms in the pull-down samples from ACB-AhR. Based on our pull-down results, we suspect a possible mutation might occur in the ALP gene. In H. virescens, a mutation in the midgut cadherin protein inactivated the 12-cadherin domain and also showed resistance to Cry1A toxins [59]. Similarly, combined mutation in cadherin and ABCC2 (ATP Binding Cassette Subfamily C Member 2) proteins in H. virescens promoted a high level of resistance to Cry1Ac and eliminated the binding affinity to Cry1Aa, Cry1Ab and Cry1Ac [59]. Gene mapping and sequence techniques revealed a mutation in the homologue of ABCC2 in Plutella xylostella [60]. Vice versa, a Cry1Ab-β16-L511A mutation affected the binding of the Cry1Ab toxin to ALP in M. sexta larvae [23]. The occurrence of single-site mutations in the conserved arginine region of CryIAa affect the formation of the ionic channel [61]. Altogether, the transgenic method delivers convincing evidence that even a small structural change in the Cry toxin-binding protein can have a great impact on Cry toxicity [62]. These facts highlight the importance of knowledge on mutagenesis and its possible contribution for disabling the protein–protein interactions and enhance the development of resistance in the target pests.
In a different view, we observed certain immune-related proteins were in the binding protein list. We detected prophenoloxidase, prophenoloxidase PPO1a, serine proteinase inhibitor 2 and serpin 5 from the different fractions. The effectiveness of certain bioinsecticides may be correlated to the activation of proPO [63]. PO levels of Helicoverpa armigera and S. frugiperda were elevated after the larvae were exposed to Cry1Ac toxin [32,34]. In the event of Cry toxicity, the midgut epithelium was gradually breached by the Cry toxins. Activated phenoloxidase (PO) was regulated by the isoforms of the serine proteases and activated the formation of quinones, and these quinones are reactive intermediates for repairing tissue damage [64,65,66]. Our results show a possibility that the Cry1Ah toxin may interact with proPO and other regulating proteins like serine proteinase inhibitor 2 and serpin 5 that were identified in samples from both susceptible and resistant strains of the ACB. A further in-depth study would render the importance of these immune-related proteins and its action towards Cry toxicity. However, we cannot conclude that these proPO molecules possess an affinity to bind directly to the Cry1Ah toxin. Intensive studies are needed to confirm the direct interactions. Protein expression, the yeast two-hybrid system and other techniques could be helpful to determine the direct interaction of the proPO/related proteins with the Cry1Ah toxin.
Overall, our results suggest the presence of a binding inconsistency between the BBMV binding proteins and Cry1Ah toxin after the pull-down assay. There might be several factors influencing the binding ability, these factors including techniques, enrichment of binding proteins in the sample and the reversible and irreversible nature of the BBMVs to Cry toxins. These factors may play a major role in protein–protein interactions. Furthermore, another important point must be accounted for during Cry toxicity in insects: After the model pest is subjected to the Cry toxin, the actual events take place under physiological conditions, which might differ from the experiments that we employed to understand the Cry toxin interactions with the BBMVs. This work would provide good interest to the readers; for example, readers can compare the patterns of the gel pictures from this work and from that of Shabbir et al. [36] as well. The patterns and lists of binding proteins would render them a new idea regarding these Cry toxin-binding proteins. On the other hand, ALP was not detected in the ACB-AhR samples; this finding might provide interest to further research the ALP mutation in the ACB-AhR strain. At this point, a precise method is needed to assess the Cry toxin mode of action. A pull-down assay alone cannot conclude the binding mechanism of the Cry toxin, and there are multiple proteins reported for their affinity towards the Cry toxin. Among them, tracking a high-affinity protein to a particular Cry toxin would enable a novel way to control or delay the resistance development in a given crop pest. To achieve this, engaging the purified binding proteins with Cry toxins by employing multiple conformational experiments would render a clear understanding on Cry toxicity.
4. Conclusions
Taken together, up-to-date the Cry toxin mode of action remains unclear. We investigated and observed the inconsistency in the Cry1Ah toxin-binding proteins from the BBMVs of the ACB. There remain important factors that might influence the binding specificity of midgut BBMV proteins towards Cry toxins. These inconsistencies may have arisen due to several factors influencing their binding ability, and these factors include techniques employed to find protein–protein interactions, enrichment of binding proteins in the sample and the reversible and irreversible nature of the BBMVs to Cry toxins. Understanding Cry toxicity will remain difficult, until a crucial or precise method is introduced to demonstrate the correct receptor(s) interacting with the Cry toxin.
5. Materials and Methods
5.1. Insect Strains
Susceptible (ACB-BtS) and Cry1Ah (ACB-AhR) resistant strains of O. furnacalis used in this study were obtained from the Institute of Plant Protection (IPP), Chinese Academy of Agricultural Sciences (CAAS), Beijing, and reared under laboratory conditions. Larvae were reared at 27 ± 1 °C, 70–80% relative humidity (RH), with a photoperiod of 16:8 h light:dark (L:D). Fourth instar second-day larvae were used in this study. Purified Cry1Ah (trypsin-activated) toxin was purchased from Beijing General pest Biotech Research Co., Ltd., Beijing, China. The Cry1Ah toxin was expressed in B. thuringiensis, a crystalliferous mutant (HD73-).
5.2. Preparation of BBMV
Midgut tissue was dissected from the ACB-BtS and ACB-AhR strains. About 200 larvae were used for BBMV extraction for each strain. The midgut tissue was excised from the foregut and hindgut, and a longitudinal slit was made in the midgut tissue to remove the food and other debris. Dissected midgut tissue was rinsed with ice-cold Mannitol buffer (300 mM Mannitol, 17 mM Tris-HCl, 5 mM EGTA, 2 mM DDT and 0.5 mM PMSF, pH 7.4) and the tissue was picked with fine forceps and put on filter paper to remove the excess liquid. The BBMV was prepared according to the Wolfersberger method [67]. Briefly, 200 mg of midgut tissue sample was weighed and transferred into the Dounce homogenizer (7 mL), and a five-fold volume (w/v) of Mannitol buffer was added and homogenized on ice for five minutes and allowed for incubation on ice for two minutes; this process was repeated three times. The homogenate was diluted with an equal volume of MgCl2 (24 mM, pH 7.6) and incubated on ice for 15 min. Initially, this mixture was centrifuged at 4500 rpm for 15 min at 4 °C to remove the cell debris; the pellet was discarded, and the supernatant was retained. Further, the supernatant was centrifuged at 16,000 rpm for 30 min at 4 °C, and the pellet corresponds to the BBMV. The resulting pellet was suspended in 0.5 mL Mannitol buffer, and an equal volume MgCl2 (24 mM, pH 7.6) was added. This sample was centrifuged under the same conditions mentioned above and this process was repeated twice. The pellet (BBMV) was solubilized using 1 mL of solubilization buffer (Octyl β-d-glucopyranoside 1%, 50 mM Na2HPO4/NaH2PO4, 5 mM EGTA, 50 mM NaCl, 5 mM EDTA, 0.5 mM PMSF, pH 7.5) and incubated at 4 °C for 1 h and centrifuged at 50,000 rpm for 30 min. The supernatant was recovered, and the protein concentration in the BBMV was determined by the Bradford method using BSA as standard [68]. To determine the purity of BBMV, the APN and ALP enzymatic activities were assessed in the midgut tissue homogenate and the BBMV samples extracted from the ACB-BtS and ACB-AhR strains [69].
5.3. Pull-Down Assay
The purified Cry1Ah toxin was biotinylated using EZ-Link NHS-LC-Biotin (Thermofisher, Waltham, MA, USA). NHS-LC-Biotin was dissolved in 300 µL of N,N-Dimethylformamide (DMF) and then added to 4.7 mL of activated Cry1Ah toxin (2 mg/mL) in sodium carbonate buffer (50 mM, pH 10). The mixture was incubated for overnight at 4 °C and dialyzed (Biotopped tubing, 15 kDa) in sodium carbonate buffer (50 mM, pH 10) for 6 h at 4 °C. The Pierce™ Pull-Down Biotinylated Protein: Protein Interaction Kit (Thermoscientific, Waltham, MA, USA) was used to perform the pull-down assay following the manufacture’s protocol. To a spin column, 100 μL of biotinylated bait protein (NHS-LC-Biotin coupled with Cry1Ah) and 50 μL of streptavidin gel slurry was added and incubated at 4 °C for 45 min and centrifuged (1250× g, 4 °C, 1 min). After the incubation, 250 μL of biotin blocking solution was added to the column and incubated at RT for 5 min and centrifuged (1250× g, 4 °C, 1 min); this step is to block the available streptavidin sites. A total of 100 μL of BBMV (prey protein) was added and incubated for 2 h at 4 °C and centrifuged (1250× g, 4 °C, 1 min). Following the incubation, 250 μL of wash buffer was added to the column and mix by gently inverting the columns 5–7 times and centrifuged (1250× g, 4 °C, 1 min). A total of 10 μL of the neutralization buffer was added to the column, which will neutralize the pH of the contents upon elution. Finally, the Cry1Ah-binding proteins were eluted by adding 250 μL of elution buffer into the column and incubated at RT for 5 min and centrifuged (1250× g, 4 °C, 1 min). A non-treated gel control (without bait protein) was maintained. The columns were sealed with bottom plugs during the incubation or adding of buffers. Three technical replications were maintained per sample.
5.4. Identification of Cry1Ah-Binding Proteins
These eluted proteins were analyzed in a 12% Express Plus TM SDS-PAGE (Genscript, Piscataway, NJ, USA). The gel was stained using EZ blue™ Gel staining reagent (Sigma, St. Louis, MO, USA). The binding proteins of the ACB-BtS and ACB-AhR strains from Lanes 3 and 4 with different molecular weights were selected and cut into four fractions. Three technical replications were maintained per sample. All the fractions were sent to the Beijing Bio-Tech Pack Technology Company Ltd. (Beijing, China). for protein identification by liquid chromatography–tandem mass spectrometry (LC-MS/MS). The following parameters were set during the LC-MS/MS analysis: the protein modifications were carbamidomethylation (C) (fixed), oxidation (M) (variable), enzyme specificity was set to trypsin, the maximum missed cleavages were set to 2, the precursor ion mass tolerance was set to 10 ppm and the MS/MS tolerance was set to 0.6 Da. The peptides identified with high confidence were chosen for the downstream protein identification analysis. The raw mass spectrometry files were analyzed using Maxquant (1.5.6.5) and matched against the protein databases. Identifications of proteins were accepted if they could be established at a probability of >95% and contained at least two of the peptides identified.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6651/12/6/418/s1, Table S1: List of different Cry1Ah binding protein BBMVs from susceptible and resistant strains of Ostrinia furnacalis, Table S2: List of common Cry1Ah binding protein of BBMVs from susceptible and resistant strains of Ostrinia furnacalis.
Author Contributions
Conceptualization, K.H. and S.P.; investigation, S.P. and M.Z.S.; analysis, S.P., K.H., M.Z.S.; data curation, S.P., M.Z.S.; writing—original draft, S.P.; writing—review and editing, K.H., S.P., Z.W. and M.Z.S.; supervision, K.H. and Z.W.; project administration, K.H. and Z.W.; funding acquisition, K.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Major Science and Technology Project of China, 2016ZX08003-001.
Acknowledgments
The authors would like to acknowledge the National Major Science and Technology Project of China for their funding support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Crickmore, N.; Baum, J.; Bravo, A.; Lereclus, D.; Narva, K.; Sampson, K.; Schnepf, E.; Sun, M.; Zeigler, D.R. Bacillus Thuringiensis Toxin Nomenclature. 2019. Available online: http://www.btnomenclature.info/ (accessed on 10 February 2020).
- Sanchis, V.; Bourguet, D. Bacillus thuringiensis: Applications in agriculture and insect resistance management. A review. Agron. Sustain. Dev. 2008, 28, 11–20. [Google Scholar] [CrossRef]
- Bagla, P. Hardy cotton-munching pests are latest blow to GM crops. Science 2010, 327, 1439. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Brévault, T.; Carrière, Y. Insect resistance to Bt crops: Lessons from the first billion acres. Nat. Biotechnol. 2013, 31, 510–521. [Google Scholar] [CrossRef]
- Gassmann, A.J.; Petzold-Maxwell, J.L.; Clifton, E.H.; Dunbar, M.W.; Hoffmann, A.M.; Ingber, D.A.; Keweshan, R.S. Field-evolved resistance by western corn rootworm to multiple Bacillus thuringiensis toxins in transgenic maize. Proc. Natl. Acad. Sci. USA 2014, 111, 5141–5146. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Gassmann, A.J.; Crowder, D.W.; Carriére, Y. Insect resistance to Bt crops: Evidence versus theory. Nat. Biotechnol. 2008, 26, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Moar, W.; Roush, R.; Shelton, A.; Ferré, J.; MacIntosh, S.; Leonard, B.R.; Abel, C. Field-evolved resistance to Bt toxins. Nat. Biotechnol. 2008, 26, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Jakka, S.R.K.; Gong, L.; Hasler, J.; Banerjee, R.; Sheets, J.J.; Narva, K. Field evolved mode 1 resistance of the fall armyworm to transgenic Cry1Fa-expressing corn associated with reduced Cry1Fa toxin binding and midgut alkaline phosphatase expression. Appl. Environ. Microbiol. 2015, 82, 1023–1034. [Google Scholar] [CrossRef]
- Ferré, J.; Van Rie, J.; Macintosh, S.C. Insecticidal Genetically Modified Crops and Insect Resistance Management (IRM). In Integration of Insect-Resistant Genetically Modified Crops within IPM Programs; Romeis, J., Shelton, A.M., Kennedy, G.G., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 5, pp. 41–85. [Google Scholar]
- Soberón, M.; Gill, S.S.; Bravo, A. Signaling versus punching hole: How do Bacillus thuringiensis toxins kill insect midgut cells? Cell. Mol. Life Sci. 2009, 66, 1337–1349. [Google Scholar] [CrossRef]
- Daniel, A.; Sangadala, S.; Dean, D.H.; Adang, M.J. Denaturation of either Manduca sexta aminopeptidase N or Bacillus thuringiensis Cry1A toxins exposes binding epitopes hidden under nondenaturing conditions. Appl. Environ. Microbiol. 2002, 68, 2106–2112. [Google Scholar] [CrossRef][Green Version]
- Xu, X.; Yu, L.; Wu, Y. Disruption of a cadherin gene associated with resistance to Cry1Ac {delta}-endotoxin of Bacillus thuringiensis in Helicoverpa armigera. Appl. Environ. Microbiol. 2005, 71, 948–954. [Google Scholar] [CrossRef]
- Bravo, A.; Soberon, M. How to cope with insect resistance to Bt toxins? Trends Biotechnol. 2008, 26, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Tiewsiri, K.; Wang, P. Differential alteration of two aminopeptidases N associated with resistance to Bacillus thuringiensis toxin Cry1Ac in cabbage looper. Proc. Natl. Acad. Sci. USA 2011, 108, 14037–14042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Candas, M.; Griko, N.B.; Taussig, R.; Bulla, L.A., Jr. A mechanism of cell death involving an adenylyl cyclase/PKA signaling pathway is induced by the Cry1Ab toxin of Bacillus thuringiensis. Proc. Natl. Acad. Sci. USA 2006, 103, 9897–9902. [Google Scholar] [CrossRef] [PubMed]
- Candas, M.; Francis, B.R.; Griko, N.B.; Midboe, E.G.; Bulla, L.A., Jr. Proteolytic cleavage of the developmentally important cadherin BT-R1 in the midgut epithelium of Manduca sexta. Biochemistry 2002, 41, 13717–13724. [Google Scholar] [CrossRef]
- Griko, N.; Zhang, X.; Ibrahim, M.; Midboe, E.G.; Bulla, L.A., Jr. Susceptibility of Manduca sexta to Cry1Ab toxin of Bacillus thuringiensis correlates directly to developmental expression of the cadherin receptor BT-R1. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 151, 59–63. [Google Scholar] [CrossRef]
- Hua, G.; Park, Y.; Adang, M.J. Cadherin AdCad1 in Alphitobius diaperinus larvae is a receptor of Cry3Bb toxin from Bacillus thuringiensis. Insect Biochem. Mol. Biol. 2014, 45, 11–17. [Google Scholar] [CrossRef]
- Nakanishi, K.; Yaoi, K.; Nagino, Y.; Hara, H.; Kitami, M.; Atsumi, S.; Miura, N.; Sato, R. Aminopeptidase N isoforms from the midgut of Bombyx mori and Plutella xylostella their classification and the factors that determine their binding specificity to Bacillus thuringiensis Cry1A toxin. FEBS Lett. 2002, 519, 215–220. [Google Scholar] [CrossRef]
- Rajagopal, R.; Agrawal, N.; Selvapandiyan, A.; Sivakumar, S.; Ahmad, S.; Bhatnagar, R.K. Recombinantly expressed isoenzymic aminopeptidases from Helicoverpa armigera (American cotton bollworm) midgut display differential interaction with closely related Bacillus thuringiensis insecticidal proteins. Biochem. J. 2003, 370, 971–978. [Google Scholar] [CrossRef]
- Bravo, A.; Gómez, I.; Conde, J.; Muñoz-Garay, C.; Sánchez, J.; Miranda, R.; Zhuang, M.; Gill, S.S.; Soberón, M. Oligomerization triggers binding of a Bacillus thuringiensis Cry1Ab pore-forming toxin to aminopeptidase N receptor leading to insertion into membrane microdomains. Biochim. Biophys. Acta Biomembr. 2004, 1667, 38–46. [Google Scholar] [CrossRef]
- Jurat-Fuentes, J.L.; Adang, M.J. Characterization of a Cry1Ac-receptor alkaline phosphatase in susceptible and resistant Heliothis virescens larvae. Eur. J. Biochem. 2004, 271, 3127–3135. [Google Scholar] [CrossRef]
- Arenas, I.; Bravo, B.; Soberón, M.; Gómez, I. Role of alkaline phosphatase from Manduca sexta in the mechanism of action of Bacillus thuringiensis Cry1Ab toxin. J. Biol. Chem. 2010, 285, 12497–12503. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga-Navarrete, F.; Gómez, I.; Peña, G.; Bravo, A.; Soberón, M.A. Tenebrio molitor GPI-anchored alkaline phosphatase is involved in binding of Bacillus thuringiensis Cry3Aa to brush border membrane vesicles. Peptides 2013, 41, 81–86. [Google Scholar] [CrossRef]
- Heckel, D.G. Learning the ABCs of Bt: ABC transporters and insect resistance to Bacillus thuringiensis provide clues to a crucial step in toxin mode of action. Pestic. Biochem. Phys. 2010, 104, 103–110. [Google Scholar] [CrossRef]
- Gahan, L.J.; Pauchet, Y.; Vogel, H.; Heckel, D.G. An ABC transporter mutation is correlated with insect resistance to Bacillus thuringiensis Cry1Ac toxin. PLoS Genet. 2010, 6, e1001248. [Google Scholar] [CrossRef]
- Park, Y.; Gonza´lez-Martı´nez, R.M.; Navarro-Cerrillo, G.; Chakroun, M.; Kim, Y.; Ziarsolo, P.; Blanca, J.; Cañizares, J.; Ferré, J.; Herrero, S. ABCC transporters mediate insect resistance to multiple Bt toxins revealed by bulk segregant analysis. BMC Biol. 2014, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Tay, W.T.; Mahon, R.J.; Heckel, D.G.; Walsh, T.K.; Downes, S.; James, W.J.; Sui-Fai, L.; Reineke, A.; Williams, A.K.; Gordon, K.H.J. Insect resistance to Bacillus thuringiensis toxin Cry2Ab is conferred by mutations in an ABC transporter subfamily A protein. PLoS Genet. 2015, 11, e1005534. [Google Scholar] [CrossRef]
- McNall, R.J.; Adang, M.J. Identification of novel Bacillus thuringiensis Cry1Ac binding proteins in Manduca sexta midgut through proteomic analysis. Insect Biochem. Mol. Biol. 2003, 33, 999–1010. [Google Scholar] [CrossRef]
- Krishnamoorthy, M.; Jurat-Fuentes, J.L.; McNall, R.J.; Andacht, T.; Adang, M.J. Identification of novel Cry1Ac binding proteins in midgut membranes from Heliothis virescens using proteomic analyses. Insect Biochem. Mol. Biol. 2007, 37, 189–201. [Google Scholar] [CrossRef]
- Xu, L.; Ferry, N.; Wang, Z.; Zhang, J.; Edwards, M.G.; Gatehouse, A.M.R.; He, K.L. A proteomic approach to study the mechanism of tolerance to Bt toxins in Ostrinia furnacalis larvae selected for resistance to Cry1Ab. Transgen. Res. 2013, 22, 1155–1166. [Google Scholar] [CrossRef]
- Ma, G.; Roberts, H.; Sarjan, M.; Featherstone, N.; Lahnstein, J.; Akhurst, R.; Schmidt, O. Is the mature endotoxin Cry1Ac from Bacillus thuringiensis inactivated by a coagulation reaction in the gut lumen of resistant Helicoverpa armigera larvae? Insect Biochem. Mol. Biol. 2005, 35, 729–739. [Google Scholar] [CrossRef]
- Liu, S.; Niu, H.; Xiao, T.; Xue, C.; Liu, Z.; Luo, W. Does phenoloxidase contributed to the resistance? Selection with butane-fipronil enhanced its activities from diamondback moths. Open Biochem. J. 2009, 3, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Cunha, F.M.; Wanderley-Teixeira, V.; Torres, J.B.; Álvaro, A.C.; Teixeira, T.J.S.; Alves, T.J.S.; Brayner, F.A. Impact of Bt cotton on the immune system and histology of the midgut of the fall armyworm Spodoptera frugiperda (Smith, J.E.) (Lepidoptera: Noctuidae). Anim. Biol. 2013, 63, 185–197. [Google Scholar] [CrossRef]
- Prabu, S.; Jing, D.; Shabbir, M.Z.; Yuan, W.; Wang, Z.; He, K.L. Contribution of phenoloxidase activation mechanism to Bt insecticidal protein resistance in Asian corn borer. Int. J. Biol. Macromol. 2010, 153, 88–99. [Google Scholar] [CrossRef]
- Shabbir, M.Z.; Zhang, T.; Prabu, S.; Wang, Y.; Wang, Z.; Bravo, A.; Soberón, M.; He, K.L. Identification of Cry1Ah-binding proteins through pull down and gene expression analysis in Cry1Ah-resistant and susceptible strains of Ostrinia furnacalis. Pestic. Biochem. Phys. 2020, 163, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Ren, Y.; Liu, Y.; Liang, G.; Song, F.; Bai, S.; Wang, J.; Wang, G. Overexpression of a novel Cry1Ie gene confers resistance to Cry1Ac-resistant cotton bollworm in transgenic lines of maize. Plant Cell Tissue Organ Cult. 2013, 115, 151–158. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, T.; Bai, S.; Wang, Z.; He, K.L. Evaluation of Bt corn with pyramided genes on efficacy and insect resistance management for the Asian corn borer in China. PLoS ONE 2016, 11, e0168442. [Google Scholar] [CrossRef]
- Shabbir, M.Z.; Quan, Y.; Wang, Z.; Bravo, A.; Soberón, M.; He, K.L. Characterization of the Cry1Ah resistance in Asian corn borer and its cross-resistance to other Bacillus thuringiensis toxins. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- He, K.L.; Wang, Z.Y.; Zhou, D.; Wen, L.; Song, Y. Evaluation of transgenic Bt corn for resistance to the Asian corn borer (Lepidoptera: Pyralidae). J. Econ. Entomol. 2003, 96, 935–940. [Google Scholar] [CrossRef]
- Zhou, D.R.; He, K.L.; Wang, Z.Y.; Ye, Z.H.; Wen, L.P.; Gao, Y.X.; Song, Y.Y. Asian Corn Borer and Its Integrated Management; Golden Shield Press: Beijing, China, 1995; p. 102. (In Chinese) [Google Scholar]
- Zhuang, M.; Oltean, D.I.; Gómez, I.; Pullikuth, A.K.; Soberón, M.; Bravo, A.; Gill, S.S. Heliothis virescens and Manduca sexta lipid rafts are involved in Cry1A toxin binding to the midgut epithelium and subsequent pore formation. J. Biol. Chem. 2002, 277, 13863–13872. [Google Scholar] [CrossRef]
- Ferré, J.; Van Rie, J. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 2002, 47, 501–533. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Walters, F.S.; Hart, H.; Palekar, N.; Chen, J. The mode of action of the Bacillus thuringiensis vegetative insecticidal protein vip3a differs from that of Cry1ab δ-endotoxin. Biochem. Pharmacol. 2003, 69, 4648–4657. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, H.A.A.; González-Cabrera, J.; Ferré, J.; Flannagan, R.; Siegfried, B.D. Analyses of Cry1Ab binding in resistant and susceptible strains of the European corn borer, Ostrinia nubilalis (Hübner) (Lepidoptera: Crambidae). Appl. Environ. Microbiol. 2006, 72, 5318–5324. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.J.G.; Siqueira, H.A.A.; Zhuang, M.; Storer, N.P.; Siegfried, B.D. Measurements of Cry1F binding and activity of luminal gut proteases in susceptible and Cry1F resistant Ostrinia nubilalis larvae (Lepidoptera: Crambidae). J. Invertebr. Pathol. 2010, 103, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Crespo, A.L.B.; Rodrigo-simón, A.; Siqueira, H.A.A.; Pereira, E.J.G.; Ferré, J.; Siegfried, B.D. Cross-resistance and mechanism of resistance to Cry1Ab toxin from Bacillus thuringiensis in a field-derived strain of European corn borer, Ostrinia nubilalis. J. Invertebr. Pathol. 2011, 107, 185–192. [Google Scholar] [CrossRef]
- Bravo, A.; Gómez, I.; Porta, H.; García-Gómez, B.I.; Rodriguez-Almazan, C.; Pardo, L.; Soberón, M. Evolution of Bacillus thuringiensis Cry toxins insecticidal activity. Microb. Biotechnol. 2013, 6, 17–26. [Google Scholar] [CrossRef]
- Liang, Y.; Patel, S.S.; Dean, D.H. Irreversible binding kinetics of Bacillus thuringiensis CryIA δ-endotoxins to gypsy moth brush border membrane vesicles is directly correlated to toxicity. J. Biol. Chem. 1995, 270, 24719–24724. [Google Scholar] [CrossRef]
- Wu, S.J.; Dean, D.H. Functional Significance of Loops in The Receptor Binding Domain of Bacillus thuringiensis CryIIIA δ-Endotoxin. J. Mol. Biol. 1996, 255, 628–640. [Google Scholar] [CrossRef]
- Wolfersberger, M.G. The toxicity of two Bacillus thuringiensis δ-endotoxins to gypsy moth larvae is inversely related to the affinity of binding sites on midgut brush border membranes for the toxins. Experientia 1990, 46, 475–477. [Google Scholar] [CrossRef]
- Lee, M.K.; Dean, D.H. Inconsistencies in determining Bacillus thuringiensis toxin binding sites relationship by comparing competition assays with ligand blotting. Biochem. Biophys. Res. Commun. 1996, 220, 575–580. [Google Scholar] [CrossRef]
- Sangadala, S.; Walters, F.S.; English, L.H.; Adang, M.J. A mixture of Manduca sexta aminopeptidase and phosphatase enhances Bacillus thuringiensis insecticidal CryIA(c) toxin binding and 86Rb-K1 efflux in vitro. J. Biol. Chem. 1994, 269, 10088–10092. [Google Scholar]
- Masson, L.; Lu, J.Y.; Mazza, A.; Brousseau, R.; Adang, M.J. The CryIA(c) receptor purified from Manduca sexta displays multiple specificities. J. Biol. Chem. 1995, 270, 20309–20315. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.L.; Lu, Y.J.; Söhnlein, P.; Brousseau, R.; Laprade, R.; Masson, L.; Adang, M.J. Ion channels formed in planar lipid bilayers by Bacillus thuringiensis toxins in the presence of Manduca sexta midgut receptors. FEBS Lett. 1997, 412, 270–276. [Google Scholar] [CrossRef]
- Keeton, T.P.; Francis, B.R.; Maaty, W.S.; Bulla, L.A., Jr. Effects of midgut-protein-preparative and ligand binding procedures on the toxin binding characteristics of BT-R1, a common high-affinity receptor in Manduca sexta for Cry1A Bacillus thuringiensis toxins. Appl. Environ. Microbiol. 1998, 64, 2158–2165. [Google Scholar] [CrossRef] [PubMed]
- Jurat-Fuentes, J.L.; Karumbaiah, L.; Jakka, S.R.; Ning, C.; Liu, C.; Wu, K.; Jackson, J.; Gould, F.; Blanco, C.; Portilla, M.; et al. Reduced levels of membrane-bound alkaline phosphatase are common to lepidopteran strains resistant to cry toxins from Bacillus thuringiensis. PLoS ONE 2001, 6, e17606. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, C.; Xiao, Y.; Zhang, D.; Zhang, Y.; Li, X.; Tabashnik, B.E.; Wu, K. A toxin-binding alkaline phosphatase fragment synergizes Bt toxin Cry1Ac against susceptible and resistant Helicoverpa armigera. PLoS ONE 2015, 10, e0126288. [Google Scholar] [CrossRef]
- Gahan, L.J.; Gould, F.; Heckel, D.G. Identification of a gene associated with Bt resistance in Heliothis virescens. Science 2001, 293, 857–860. [Google Scholar] [CrossRef]
- Baxter, S.W.; Badenes-Pérez, F.R.; Morrison, A.; Vogel, H.; Crickmore, N.; Kain, W.; Wang, P.; Heckel, W.G.; Jiggins, C.D. Parallel evolution of Bacillus thuringiensis toxin resistance in lepidoptera. Genetics 2011, 189, 675–679. [Google Scholar] [CrossRef]
- Aronson, A.I.; Wu, D.; Zhang, C. Mutagenesis of specificity and toxicity regions of a Bacillus thuringiensis protoxin gene. J. Bacteriol. 1995, 177, 4059–4065. [Google Scholar] [CrossRef]
- Atsumi, S.; Miyamato, K.; Yamamoto, K.; Narukawa, J.; Kawai, S.; Sezutsu, H.; Kobayashi, I.; Uchino, K.; Tamura, T.; Mita, K.; et al. Single amino acid mutation in an ATP-binding cassette transporter causes resistance to Bt toxin Cry1Ab in the silkworm, Bombyx mori. Proc. Natl Acad. Sci. USA 2012, 109, 1591–1598. [Google Scholar] [CrossRef]
- Valadez-Lira, J.A.; Alcocer-Gonzalez, J.M.; Damas, G.; Nuñez-Mejía, G.; Oppert, B.; Rodriguez-Padilla, C.; Tamez-Guerra, P. Comparative evaluation of phenoloxidase activity in different larval stages of four lepidopteran pests after exposure to Bacillus thuringiensis. J. Insect Sci. 2012, 12, 1–11. [Google Scholar] [CrossRef]
- Ashida, M.; Dohke, K. Activation of pro-phenol oxidase by the activating enzyme of the silkworm, Bombyx mori. Insect Biochem. 1980, 10, 37–47. [Google Scholar] [CrossRef]
- Nappi, A.J.; Vass, E. Cytotoxic reactions associated with insect immunity. Adv. Exp. Med. Biol. 2001, 484, 329–348. [Google Scholar] [PubMed]
- Bidla, G.; Hauling, T.; Dushay, M.S.; Theopold, U. Activation of insect phenoloxidase after injury: Endogenous versus foreign elicitors. J. Innate Immun. 2009, 1, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Wolfersberger, M.; Lüthy, P.; Maurer, A.; Parenti, P.; Sacchi, F.; Giordana, B.; Hanozet, G. Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae). Comp. Biochem. Phys. A Comp. Phys. 1987, 86, 301–308. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Rodrigo-Simón, A.; Caccia, S.; Ferré, J. Bacillus thuringiensis Cry1Ac toxin-binding and pore-forming activity in brush border membrane vesicles prepared from anterior and posterior midgut regions of lepidopteran larvae. Appl. Environ. Microbiol. 2008, 74, 1710–1716. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).