PLA2 Inhibitor Varespladib as an Alternative to the Antivenom Treatment for Bites from Nikolsky’s Viper Vipera berus nikolskii
Abstract
:1. Introduction
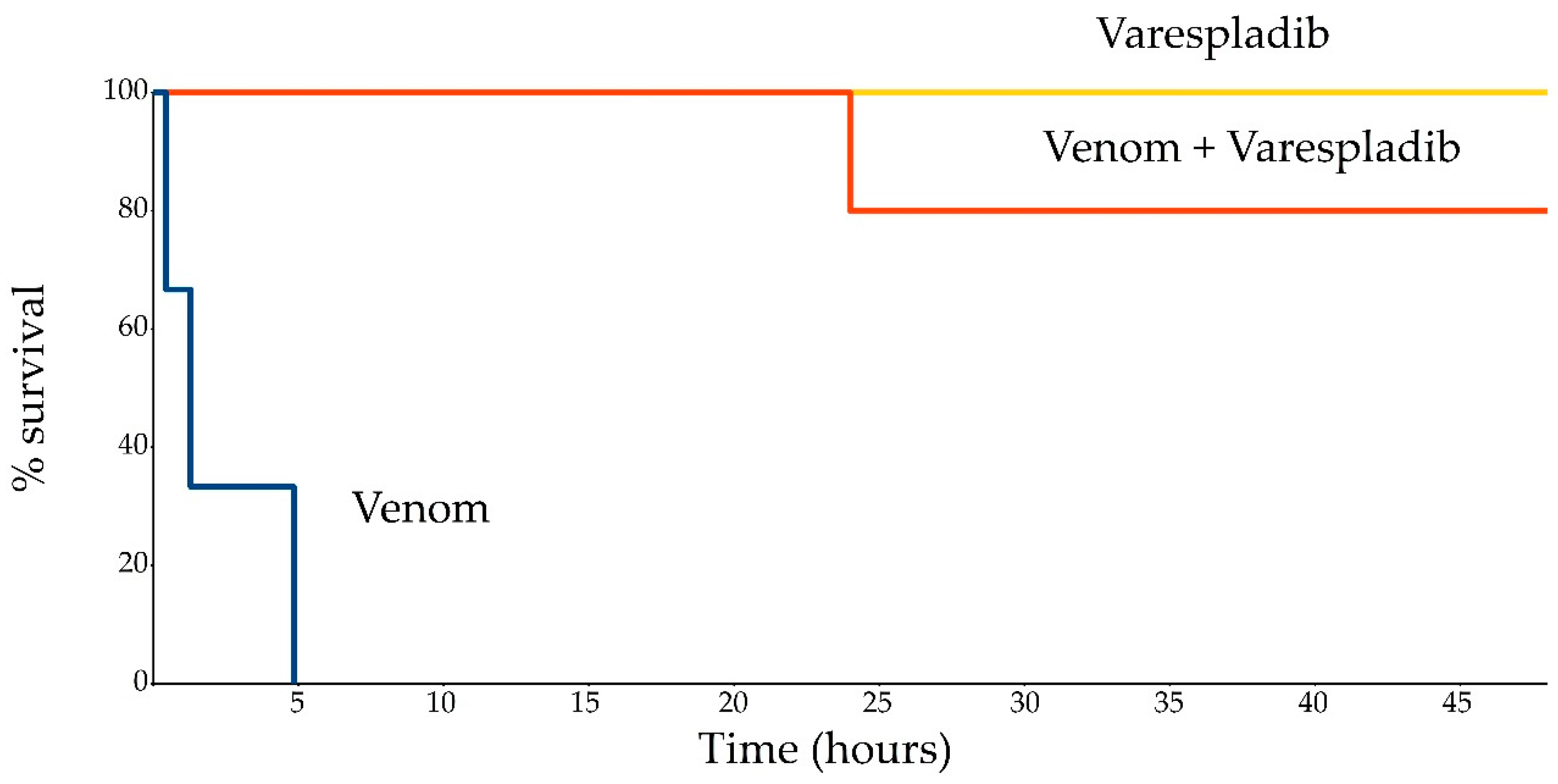
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals and Reagents
5.2. Treatment Conditions
5.3. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins. Available online: http://www.who.int/bloodproducts/snake_antivenoms/en (accessed on 19 March 2019).
- Theakston, R.D.G.; Warrell, D.A. Crisis in snake antivenom supply for Africa. Lancet 2000, 356, 2104. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, A.; Engmark, M.; Milbo, C.; Johannesen, J.; Lomonte, B.; Gutiérrez, J.M.; Lohse, B. From fangs to pharmacology: The future of snakebite envenoming therapy. Curr. Pharm. Des. 2016, 22, 5270–5293. [Google Scholar] [CrossRef] [PubMed]
- Lewin, M.; Samuel, S.; Merkel, J.; Bickler, P. Varespladib (LY315920) Appears to Be a Potent, Broad-Spectrum, Inhibitor of Snake Venom Phospholipase A2 and a Possible Pre-Referral Treatment for Envenomation. Toxins 2016, 8, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewin, M.R.; Gutiérrez, J.M.; Samuel, S.P.; Herrera, M.; Bryan-Quirós, W.; Lomonte, B.; Bickler, P.E.; Bulfone, T.C.; Williams, D.J. Delayed Oral LY333013 Rescues Mice from Highly Neurotoxic, Lethal Doses of Papuan Taipan (Oxyuranus scutellatus) Venom. Toxins 2018, 10, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewin, M.R.; Gilliam, L.L.; Gilliam, J.; Samuel, S.P.; Bulfone, T.C.; Bickler, P.E.; Gutiérrez, J.M. Delayed LY333013 (Oral) and LY315920 (Intravenous) Reverse Severe Neurotoxicity and Rescue Juvenile Pigs from Lethal Doses of Micrurus fulvius (Eastern Coral Snake) Venom. Toxins 2018, 10, 479. [Google Scholar]
- Wang, Y.; Zhang, J.; Zhang, D.; Xiao, H.; Xiong, S.; Huang, C. Exploration of the inhibitory potential of Varespladib for snakebite envenomation. Molecules 2018, 23, 391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bittenbinder, M.A.; Zdenek, C.N.; Op den Brouw, B.; Youngman, N.J.; Dobson, J.S.; Naude, A.; Vonk, F.J.; Fry, B.G. Coagulotoxic cobras: Clinical implications of strong anticoagulant actions of african spitting Naja venoms that are not neutralised by antivenom but are by LY315920 (Varespladib). Toxins 2018, 10, 516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, G.M.; Lim-Wilby, M. Molecular docking. Methods Mol. Biol. 2008, 443, 365–382. [Google Scholar] [PubMed]
- Milto, K.D.; Zinenko, O.I. Distribution and morphological variance of Vipera berus in Eastern Europe. In Herpetologia Petropolitana, Proceedings of the 12th Ordinary General Meeting of the Societas Europaea Herpetologica, Saint Peterburg, Russia, 12–16 August 2003; Ananjeva, N., Tsinenko, O., Eds.; SEH: Saint Peterburg, Russia, 2005; pp. 64–73. [Google Scholar]
- Zinenko, O.; Ţurcanu, V.; Strugariu, A. Distribution and morphological variation of Vipera berus nikolskii Vedmederja, Grubant et Rudaeva, 1986 in Western Ukraine, The Republic of Moldova and Romania. Amphib.-Reptil. 2010, 31, 51–67. [Google Scholar] [CrossRef]
- Zinenko, O.I.; Kotenko, T.I. Nikolsky’s viper, forest-steppe viper. Vipera nikolskii Vedmederja, Grubant et Rudaeva, 1986. In The Red Data Book of Ukraine. Animals; Akimov, I.A., Ed.; Global Consulting: Kyiv, Ukraine, 2009; p. 396. [Google Scholar]
- Danilov-Danilyan, V.I. (Ed.) The Red Data Book of Russian Federation. Animals; AST, Astrel: Moscow, Russia, 2001; p. 862. [Google Scholar]
- Kovalchuk, S.I.; Ziganshin, R.H.; Starkov, V.G.; Tsetlin, V.I.; Utkin, Y.N. Quantitative proteomic analysis of venoms from Russian vipers of Pelias group: Phospholipases A2 are the main venom components. Toxins 2016, 8, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, W.; Starkov, V.G.; Tsetlin, V.I.; Utkin, Y.N.; Lin, Z.; Bi, R. Isolation and preliminary crystallographic studies of two new phospholipases A 2 from Vipera nikolskii venom. Acta Cryst. 2005, 61, 189–192. [Google Scholar]
- Ramazanova, A.S.; Zavada, L.L.; Starkov, V.G.; Kovyazina, I.V.; Subbotina, T.F.; Kostyukhina, E.E.; Dementieva, I.N.; Ovchinnikova, T.V.; Utkin, Y.U. Heterodimeric neurotoxic phospholipases A2—The first proteins from venom of recently established species Vipera nikolskii: Implication of venom composition in viper systematics. Toxicon 2008, 51, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Dyachenko, I.A.; Murashev, A.N.; Andreeva, T.V.; Tsetlin, V.I.; Utkin, Y.N. Analysis of nociceptive effects of neurotoxic phospholipase A2 from Vipera nikolskii venom in mice. J. Venom Res. 2013, 4, 1–4. [Google Scholar] [PubMed]
- Malina, T. Venom Variation and Their Clinical Significance in Case of an Isolated Population of the Common Adder (Vipera berus) in Eastern Hungary. Ph.D. Thesis, Debrecen University, Debrecen, Hungary, 2015. [Google Scholar]
- Tasoulis, T.; Isbister, G.K. A Review and Database of Snake Venom Proteomes. Toxins 2017, 9, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderon, L.; Lomonte, B.; Gutierrez, J.M.; Tarkowski, A.; Hanson, L.A. Biological and biochemical activities of Vipera berus (European viper) venom. Toxicon 1993, 31, 743–753. [Google Scholar] [CrossRef]
- Bocian, A.; Urbanik, M.; Hus, K.; Łyskowski, A.; Petrilla, V.; Andrejčáková, Z.; Petrillová, M.; Legath, J. Proteome and Peptidome of Vipera berus berus Venom. Molecules 2016, 21, 1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adis R&D profile “Varespladib”. Am. J. Cardiovasc. Drugs 2011, 11, 137–143.
- Krasovsky, G.N.; Rakmanin, Y.A.; Egorova, N.A. Extrapolation of Toxicological Data from Animals to Man; Meditsina Publishers: Moscow, Russia, 2009; p. 208. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zinenko, O.; Tovstukha, I.; Korniyenko, Y. PLA2 Inhibitor Varespladib as an Alternative to the Antivenom Treatment for Bites from Nikolsky’s Viper Vipera berus nikolskii. Toxins 2020, 12, 356. https://doi.org/10.3390/toxins12060356
Zinenko O, Tovstukha I, Korniyenko Y. PLA2 Inhibitor Varespladib as an Alternative to the Antivenom Treatment for Bites from Nikolsky’s Viper Vipera berus nikolskii. Toxins. 2020; 12(6):356. https://doi.org/10.3390/toxins12060356
Chicago/Turabian StyleZinenko, Oleksandr, Igor Tovstukha, and Yevgen Korniyenko. 2020. "PLA2 Inhibitor Varespladib as an Alternative to the Antivenom Treatment for Bites from Nikolsky’s Viper Vipera berus nikolskii" Toxins 12, no. 6: 356. https://doi.org/10.3390/toxins12060356
APA StyleZinenko, O., Tovstukha, I., & Korniyenko, Y. (2020). PLA2 Inhibitor Varespladib as an Alternative to the Antivenom Treatment for Bites from Nikolsky’s Viper Vipera berus nikolskii. Toxins, 12(6), 356. https://doi.org/10.3390/toxins12060356




