Investigation of In Vitro Endocrine Activities of Microcystis and Planktothrix Cyanobacterial Strains
Abstract
1. Introduction
- Do cyanobacterial metabolites interfere with hormone receptors, especially with the estrogen receptor?H1-0: Extracts from cyanobacterial cultures do not show effects on the estrogen receptor (or other hormone receptors).
- Are there other modes of action that may result in endocrine disrupting activity of cyanobacterial metabolites, e.g., effects on cytochrome P450 mediated estradiol biotransformation?H2-0: Estradiol biotransformation is not affected by the presence of cyanobacterial culture extracts or MCs.
- If cyanobacterial metabolites show ED activity, are MCs responsible for any of the observed effects?H3-0: MCs do not show ED activity.
2. Results
2.1. Reporter Gene Assays (RGAs)
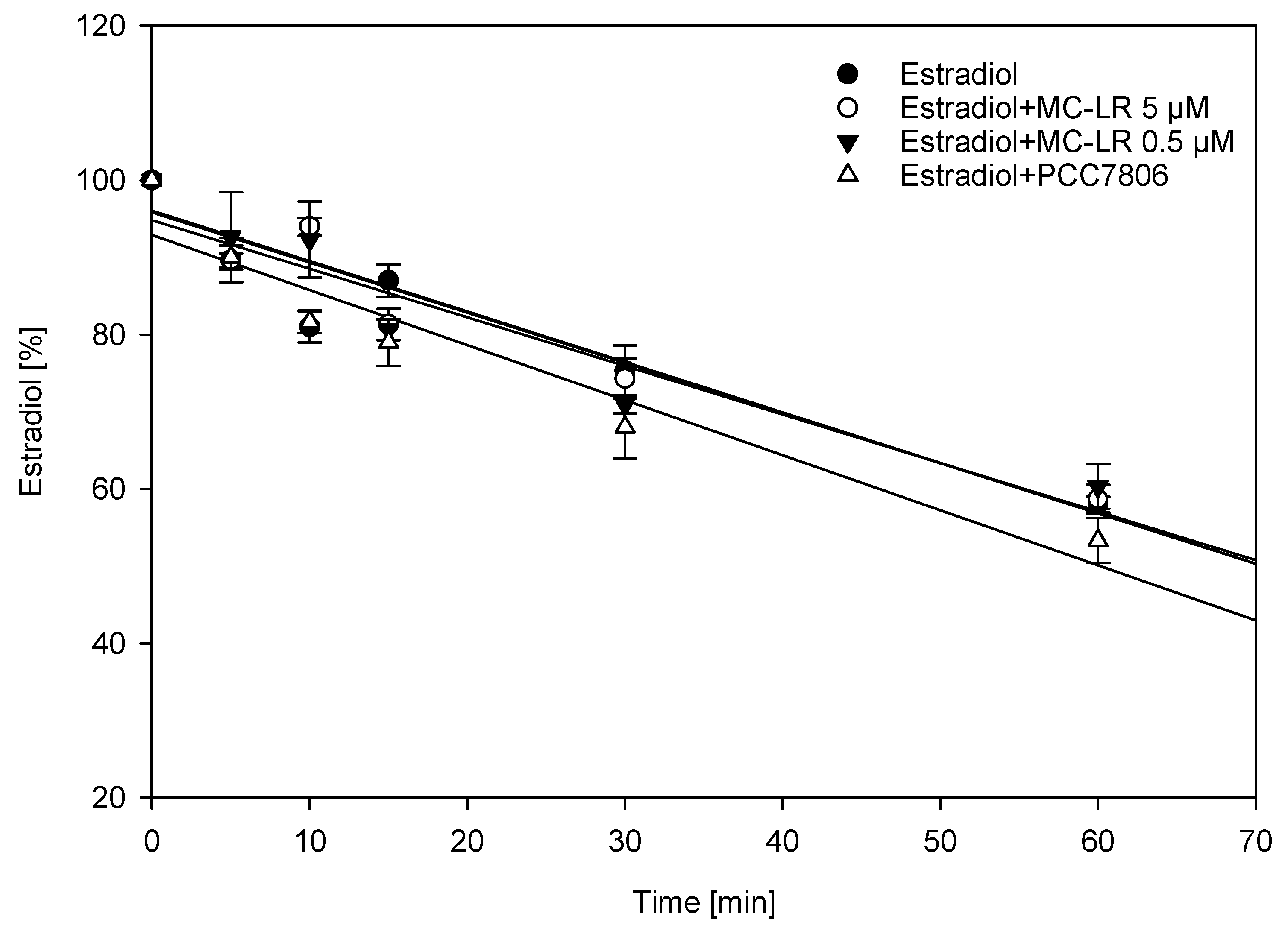
2.2. Interference of Cytochrome P450-Mediated Metabolism of Estradiol Using Human Liver Microsomes (HLM)
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Reagents
5.2. Cultivation of Cyanobacterial Strains and Extraction
5.3. Microcystin ELISA
5.4. Cell Culture
5.5. Reporter-Gene Assays (RGAs)
5.6. MTT Cell Viability Assay
5.7. Human Liver Microsome (HLM) Incubation
5.8. Quantification of Estradiol and Biotransformation Products Using LC–MS/MS
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W.; Azevedo, S.M.; An, J.S.; Molica, R.J.; Jochimsen, E.M.; Lau, S.; Rinehart, K.L.; Shaw, G.R.; Eaglesham, G.K. Human fatalities from cyanobacteria: Chemical and biological evidence for cyanotoxins. Environ. Health Perspect. 2001, 109, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Catherine, A.; Bernard, C.; Spoof, L.; Bruno, M. Microcystins and Nodularins. In Handbook of Cyanobacterial Monitoring and Cyanotoxins Analysis, 1st ed.; Meriluoto, J., Spoof, L., Cood, G.A., Eds.; John Wiley & Sons Ltd: Chichester, UK, 2017. [Google Scholar]
- Janssen, E.M. Cyanobacterial peptides beyond microcystins—A review on co-occurrence, toxicity, and challenges for risk assessment. Water Res. 2019, 151, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Chorus, I.; Bartram, J. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; E&FN Spon: New York, NY, USA, 1999. [Google Scholar]
- Paerl, H.W.; Hall, N.S.; Calandrino, E.S. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci. Total Environ. 2011, 409, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Otten, T.G. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality. 2017. Available online: https://www.who.int/water_sanitation_health/publications/drinking-water-quality-guidelines-4-including-1st-addendum/en/ (accessed on 3 April 2020).
- Falconer, I.R.; Humpage, A.R. Health risk assessment of cyanobacterial (blue-green algal) toxins in drinking water. Int. J. Environ. Res. Public Health 2005, 2, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef]
- Díez-Quijada, L.; Prieto, A.I.; Guzmán-Guillén, R.; Jos, A.; Camean, A.M. Occurrence and toxicity of microcystin congeners other than MC-LR and MC-RR: A review. Food Chem. Toxicol. 2019, 125, 106–132. [Google Scholar] [CrossRef]
- Botes, D.P.; Kruger, H.; Viljoen, C.C. Isolation and characterization of four toxins from the blue–green alga, Microcystis aeruginosa. Toxicon 1982, 20, 945–954. [Google Scholar] [CrossRef]
- Botes, D.P.; Viljoen, C.C.; Kruger, H.; Wessels, P.L.; Williams, D.H. Configuration assignments of the amino acid residues and the presence of N-methyldehydroalanine in toxins from the blue-green alga, Microcystis aeruginosa. Toxicon 1982, 20, 1037–1042. [Google Scholar] [CrossRef]
- Bouaicha, N.; Miles, C.O.; Beach, D.G.; Labidi, Z.; Djabri, A.; Benayache, N.Y.; Nguyen-Quang, T. Structural diversity, characterization and toxicology of microcystins. Toxins 2019, 11, 714. [Google Scholar] [CrossRef] [PubMed]
- International Practical Shooting Confederation. Global Assessment of the State-of-the-Science of Endocrine Disruptors; IPCS: Geneva, Switzerland, 2002; Available online: https://www.who.int/ipcs/publications/new_issues/endocrine_disruptors/en/ (accessed on 3 April 2020).
- United Nations Environment Programme; WHO. State of the Science of Endocrine Disrupting Chemicals–2012; WHO: Geneva, Switzerland, 2012; Available online: https://www.who.int/ceh/publications/endocrine/en (accessed on 3 April 2020).
- Giulivo, M.; Lopez de Alda, M.; Capri, E.; Barcelo, D. Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review. Environ. Res. 2016, 151, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.Y.; Aris, A.Z. Endocrine disrupting compounds in drinking water supply system and human health risk implication. Environ. Int. 2017, 106, 207–233. [Google Scholar] [CrossRef] [PubMed]
- Stepankova, T.; Ambrozova, L.; Blaha, L.; Giesy, J.P.; Hilscherova, K. In vitro modulation of intracellular receptor signaling and cytotoxicity induced by extracts of cyanobacteria, complex water blooms and their fractions. Aquat. Toxicol. 2011, 105, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Sychrova, E.; Stepankova, T.; Novakova, K.; Blaha, L.; Giesy, J.P.; Hilscherova, K. Estrogenic activity in extracts and exudates of cyanobacteria and green algae. Environ. Int. 2012, 39, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Chen, Q.; Crawford, S.E.; Song, L.; Chen, W.; Hammers-Wirtz, M.; Strauss, T.; Seiler, T.B.; Schaffer, A.; Hollert, H. Cyanobacterial blooms act as sink and source of endocrine disruptors in the third largest freshwater lake in China. Environ. Pollut. 2019, 245, 408–418. [Google Scholar] [CrossRef]
- Douma, M.; Ouahid, Y.; Loudiki, M.; Del Campo, F.F.; Oudra, B. The first detection of potentially toxic Microcystis strains in two Middle Atlas Mountains natural lakes (Morocco). Environ. Monit. Assess. 2017, 189, 39. [Google Scholar] [CrossRef]
- Graham, J.L.; Loftin, K.A.; Meyer, M.T.; Ziegler, A.C. Cyanotoxin mixtures and taste-and-odor compounds in cyanobacterial blooms from the Midwestern United States. Environ. Sci. Technol. 2010, 44, 7361–7368. [Google Scholar] [CrossRef]
- Hou, J.; Li, L.; Wu, N.; Su, Y.; Lin, W.; Li, G.; Gu, Z. Reproduction impairment and endocrine disruption in female zebrafish after long-term exposure to MC-LR: A life cycle assessment. Environ. Pollut. 2016, 208, 477–485. [Google Scholar] [CrossRef]
- Oziol, L.; Bouaicha, N. First evidence of estrogenic potential of the cyanobacterial heptotoxins the nodularin-R and the microcystin-LR in cultured mammalian cells. J. Hazard. Mater. 2010, 174, 610–615. [Google Scholar] [CrossRef]
- Zhao, Y.; Xie, L.; Yan, Y. Microcystin-LR impairs zebrafish reproduction by affecting oogenesis and endocrine system. Chemosphere 2015, 120, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Jonas, A.; Scholz, S.; Fetter, E.; Sychrova, E.; Novakova, K.; Ortmann, J.; Benisek, M.; Adamovsky, O.; Giesy, J.P.; Hilscherova, K. Endocrine, teratogenic and neurotoxic effects of cyanobacteria detected by cellular in vitro and zebrafish embryos assays. Chemosphere 2015, 120, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Rogers, E.D.; Henry, T.B.; Twiner, M.J.; Gouffon, J.S.; McPherson, J.T.; Boyer, G.L.; Sayler, G.S.; Wilhelm, S.W. Global gene expression profiling in larval zebrafish exposed to microcystin-LR and Microcystis reveals endocrine disrupting effects of cyanobacteria. Environ. Sci. Technol. 2011, 45, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, J.; Zhang, X.; Xie, P. A review of reproductive toxicity of microcystins. J. Hazard. Mater. 2016, 301, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shao, S.; Zhou, F.; Wen, S.; Chen, F.; Han, X. Reproductive toxicity on female mice induced by microcystin-LR. Environ. Toxicol. Pharmacol. 2014, 37, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Geng, Z.; Zhou, Y.; Chen, Y.; Wu, J.; Han, X. Distribution of microcystin-LR to testis of male Sprague-Dawley rats. Ecotoxicology 2013, 22, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Yan, W.; Wu, Q.; Liu, C.; Gong, X.; Hung, T.C.; Li, G. Parental exposure to microcystin-LR induced thyroid endocrine disruption in zebrafish offspring, a transgenerational toxicity. Environ. Pollut. 2017, 230, 981–988. [Google Scholar] [CrossRef]
- Liu, W.; Chen, C.; Chen, L.; Wang, L.; Li, J.; Chen, Y.; Jin, J.; Kawan, A.; Zhang, X. Sex-dependent effects of microcystin-LR on hypothalamic-pituitary-gonad axis and gametogenesis of adult zebrafish. Sci. Rep. 2016, 6, 22819. [Google Scholar] [CrossRef]
- Hecker, M.; Newsted, J.L.; Murphy, M.B.; Higley, E.B.; Jones, P.D.; Wu, R.; Giesy, J.P. Human adrenocarcinoma (H295R) cells for rapid in vitro determination of effects on steroidogenesis: Hormone production. Toxicol. Appl. Pharmacol. 2006, 217, 114–124. [Google Scholar] [CrossRef]
- Sanderson, J.T.; Boerma, J.; Lansbergen, G.W.; van den Berg, M. Induction and inhibition of aromatase (CYP19) activity by various classes of pesticides in H295R human adrenocortical carcinoma cells. Toxicol. Appl. Pharmacol. 2002, 182, 44–54. [Google Scholar] [CrossRef]
- Portmann, C.; Blom, J.F.; Gademann, K.; Juttner, F. Aerucyclamides A and B: Isolation and synthesis of toxic ribosomal heterocyclic peptides from the cyanobacterium Microcystis aeruginosa PCC 7806. J. Nat. Prod. 2008, 71, 1193–1196. [Google Scholar] [CrossRef] [PubMed]
- Portmann, C.; Blom, J.F.; Kaiser, M.; Brun, R.; Juttner, F.; Gademann, K. Isolation of aerucyclamides C and D and structure revision of microcyclamide 7806A: Heterocyclic ribosomal peptides from Microcystis aeruginosa PCC 7806 and their antiparasite evaluation. J. Nat. Prod. 2008, 71, 1891–1896. [Google Scholar] [CrossRef] [PubMed]
- Tonk, L.; Welker, M.; Huisman, J.; Visser, P.M. Production of cyanopeptolins, anabaenopeptins, and microcystins by the harmful cyanobacteria Anabaena 90 and Microcystis PCC7806. Harmful Algae 2009, 8, 219–224. [Google Scholar] [CrossRef]
- Haande, S.; Ballot, A.; Rohrlack, T.; Fastner, J.; Wiedner, C.; Edvardsen, B. Diversity of Microcystis aeruginosa isolates (Chroococcales, Cyanobacteria) from East-African water bodies. Arch. Microbiol. 2007, 188, 15–25. [Google Scholar] [CrossRef]
- Mallia, V.; Uhlig, S.; Rafuse, C.; Meija, J.; Miles, C.O. Novel microcystins from Planktothrix prolifica NIVA-CYA 544 Identified by LC–MS/MS, functional group derivatization and 15N-labeling. Mar. Drugs 2019, 17, 643. [Google Scholar] [CrossRef]
- Rohrlack, T.; Skulberg, R.; Skulberg, O.M. Distribution of oligopeptide chemotypes of the cyanobacterium Planktothrix and their persistence in selected lakes in Fennoscandia. J. Phycol. 2009, 45, 1259–1265. [Google Scholar] [CrossRef]
- Kotai, J. Instructions for Preparation of Modified Nutrient Solution Z8 for Algae; Norwegian Institute for Water Research: Oslo, Norway, 1972. [Google Scholar]
- Yamazaki, H.; Shaw, P.M.; Guengerich, F.P.; Shimada, T. Roles of cytochromes P450 1A2 and 3A4 in the oxidation of estradiol and estrone in human liver microsomes. Chem. Res. Toxicol. 1998, 11, 659–665. [Google Scholar] [CrossRef]
- Labrie, F.; Luu-The, V.; Lin, S.X.; Labrie, C.; Simard, J.; Breton, R.; Belanger, A. The key role of 17β-hydroxysteroid dehydrogenases in sex steroid biology. Steroids 1997, 62, 148–158. [Google Scholar] [CrossRef]
- Lee, A.J.; Cai, M.X.; Thomas, P.E.; Conney, A.H.; Zhu, B.T. Characterization of the oxidative metabolites of 17β-estradiol and estrone formed by 15 selectively expressed human cytochrome P450 isoforms. Endocrinology 2003, 144, 3382–3398. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Nakajima, M.; Yokoi, T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005, 227, 115–124. [Google Scholar] [CrossRef]
- Briand, E.; Bormans, M.; Gugger, M.; Dorrestein, P.C.; Gerwick, W.H. Changes in secondary metabolic profiles of Microcystis aeruginosa strains in response to intraspecific interactions. Environ. Microbiol. 2016, 18, 384–400. [Google Scholar] [CrossRef] [PubMed]
- Rohrlack, T.; Utkilen, H. Effects of nutrient and light availability on production of bioactive anabaenopeptins and microviridin by the cyanobacterium Planktothrix agardhii. Hydrobiologia 2007, 583, 231–240. [Google Scholar] [CrossRef]
- Tonk, L.; Visser, P.M.; Christiansen, G.; Dittmann, E.; Snelder, E.O.; Wiedner, C.; Mur, L.R.; Huisman, J. The microcystin composition of the cyanobacterium Planktothrix agardhii changes toward a more toxic variant with increasing light intensity. Appl. Environ. Microbiol. 2005, 71, 5177–5181. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ke, M.; Fan, X.; Zhang, M.; Zhu, Y.; Lu, T.; Sun, L.; Qian, H. Reproductive and endocrine-disrupting toxicity of Microcystis aeruginosa in female zebrafish. Chemosphere 2018, 192, 289–296. [Google Scholar] [CrossRef]
- Trinchet, I.; Djediat, C.; Huet, H.; Dao, S.P.; Edery, M. Pathological modifications following sub-chronic exposure of medaka fish (Oryzias latipes) to microcystin-LR. Reprod. Toxicol. 2011, 32, 329–340. [Google Scholar] [CrossRef]
- Hoogenboom, L.A.P.; de Haan, L.; Hooijerink, D.; Bor, G.; Murk, A.J.; Brouwer, A. Estrogenic activity of estradiol and its metabolites in the ER-CALUX assay with human T47D breast cells. APMIS 2001, 109, 101–107. [Google Scholar] [CrossRef][Green Version]
- Zhu, B.T.; Han, G.Z.; Shim, J.Y.; Wen, Y.; Jiang, X.R. Quantitative structure-activity relationship of various endogenous estrogen metabolites for human estrogen receptor α and β subtypes: Insights into the structural determinants favoring a differential subtype binding. Endocrinology 2006, 147, 4132–4150. [Google Scholar] [CrossRef]
- Cheng, Z.N.; Shu, Y.; Liu, Z.Q.; Wang, L.S.; Ou-Yang, D.S.; Zhou, H.H. Role of cytochrome P450 in estradiol metabolism in vitro. Acta Pharmacol. Sin. 2001, 22, 148–154. [Google Scholar]
- Lee, A.J.; Kosh, J.W.; Conney, A.H.; Zhu, B.T. Characterization of the NADPH-dependent metabolism of 17β-estradiol to multiple metabolites by human liver microsomes and selectively expressed human cytochrome P450 3A4 and 3A5. J. Pharmacol. Exp. Ther. 2001, 298, 420–432. [Google Scholar]
- Fontanillo, M.; Kohn, M. Microcystins: Synthesis and structure–activity relationship studies toward PP1 and PP2A. Bioorg. Med. Chem. 2018, 26, 1118–1126. [Google Scholar] [CrossRef]
- MacKintosh, C.; Beattie, K.A.; Klumpp, S.; Cohen, P.; Codd, G.A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990, 264, 187–192. [Google Scholar] [CrossRef]
- Runnegar, M.T.; Kong, S.; Berndt, N. Protein phosphatase inhibition and in vivo hepatotoxicity of microcystins. Am. J. Physiol. 1993, 265, G224–G230. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, S.; Matsushima, R.; Watanabe, M.F.; Harada, K.; Ichihara, A.; Carmichael, W.W.; Fujiki, H. Inhibition of protein phosphatases by microcystins and nodularin associated with hepatotoxicity. J. Cancer Res. Clin. Oncol. 1990, 116, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Samdal, I.A.; Ballot, A.; Lovberg, K.E.; Miles, C.O. Multihapten approach leading to a sensitive ELISA with broad cross-reactivity to microcystins and nodularin. Environ. Sci. Technol. 2014, 48, 8035–8043. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, P.; Scippo, M.L.; Kausel, G.; Figueroa, J.; Maghuin-Rogister, G.; Martial, J.A.; Muller, M. Use of reporter cell lines for detection of endocrine-disrupter activity. Anal. Bioanal. Chem. 2004, 378, 655–663. [Google Scholar]
- Frizzell, C.; Ndossi, D.; Verhaegen, S.; Dahl, E.; Eriksen, G.; Sorlie, M.; Ropstad, E.; Muller, M.; Elliott, C.T.; Connolly, L. Endocrine disrupting effects of zearalenone, alpha- and beta-zearalenol at the level of nuclear receptor binding and steroidogenesis. Toxicol. Lett. 2011, 206, 210–217. [Google Scholar] [CrossRef]


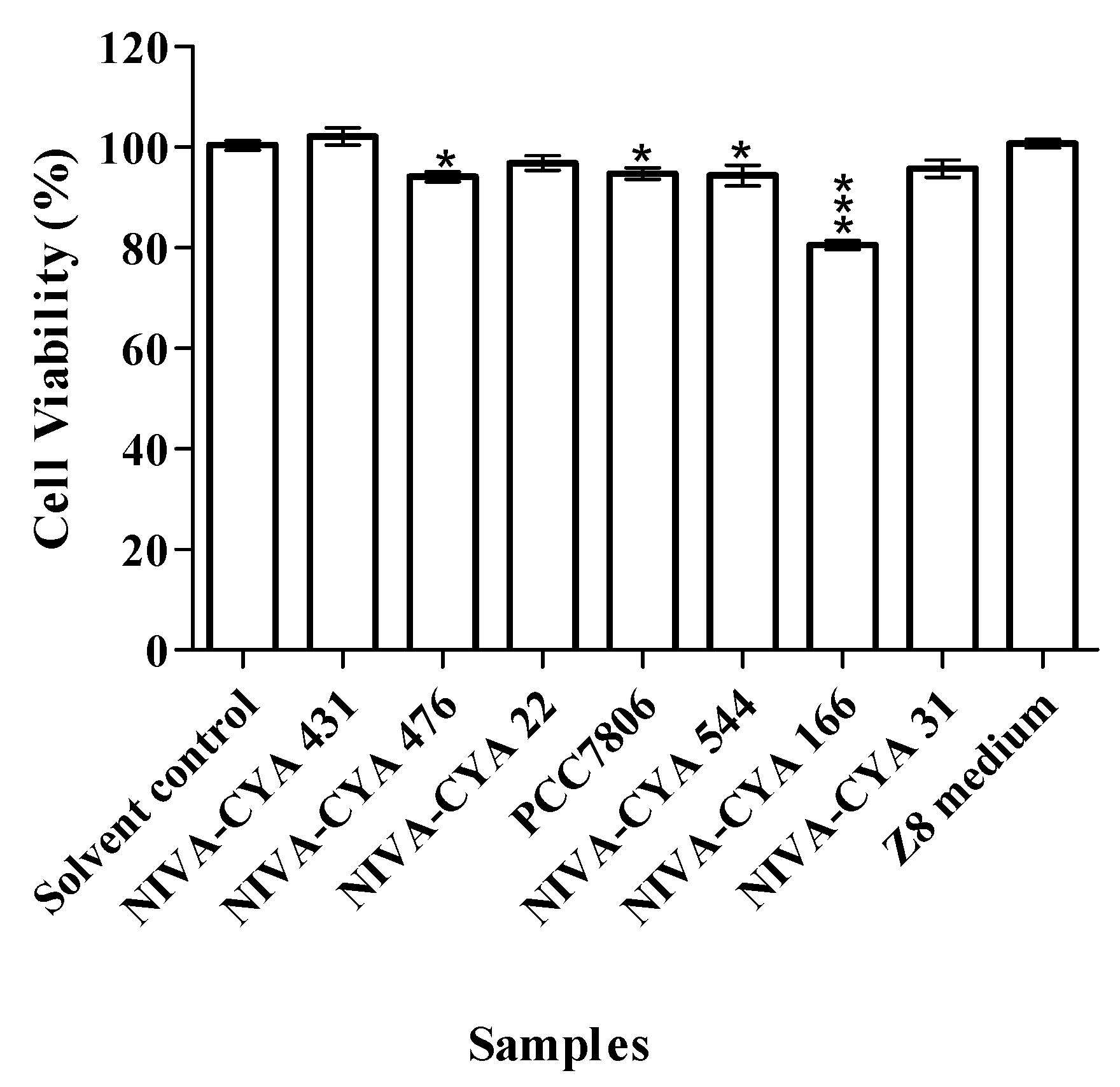

| Strain ID | Species | MCs a |
|---|---|---|
| NIVA-CYA 431 | Microcystis novacekii | No |
| NIVA-CYA 476 | Microcystis aeruginosa | No |
| NIVA-CYA 22 | Microcystis aeruginosa | No |
| PCC7806 | Microcystis aeruginosa | Yes |
| NIVA-CYA 544 | Planktothrix prolifica | Yes |
| NIVA-CYA 166 | Microcystis aeruginosa | No |
| NIVA-CYA 31 | Microcystis aeruginosa | Yes |
| Estradiol Metabolite | 5 µM Estradiol (%) | 5 µM Estradiol + 0.5 µM MC-LR (%) | 5 µM Estradiol + 5 µM MC-LR | 5 µM Estradiol + PCC7806 |
|---|---|---|---|---|
| 2-hydroxyestradiol | 100 | 71 (±5) * | 72 (±11) * | 72 (±12) * |
| estrone | 100 | 115 (±13) | 105 (±10) | 124 (±17) ** |
| Compound | Transition a [m/z] | CE b | FV c | CAV d | RT e |
|---|---|---|---|---|---|
| estriol | 271.2 → 133.0 (157.0) | 27 | 85 | 2 | 10.5 |
| 2-hydroxyestradiol | 271.2 → 175.0 (149.0) | 19 | 120 | 4 | 11.8 |
| 4-hydroxyestradiol | 271.2 → 175.0 (149.0) | 19 | 115 | 4 | 12.0 |
| estradiol | 255.2 → 159.0 (133.0) | 19 | 100 | 2 | 13.5 |
| estrone | 271.2 → 133.0 (157.0) | 31 | 70 | 4 | 15.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallia, V.; Ivanova, L.; Eriksen, G.S.; Harper, E.; Connolly, L.; Uhlig, S. Investigation of In Vitro Endocrine Activities of Microcystis and Planktothrix Cyanobacterial Strains. Toxins 2020, 12, 228. https://doi.org/10.3390/toxins12040228
Mallia V, Ivanova L, Eriksen GS, Harper E, Connolly L, Uhlig S. Investigation of In Vitro Endocrine Activities of Microcystis and Planktothrix Cyanobacterial Strains. Toxins. 2020; 12(4):228. https://doi.org/10.3390/toxins12040228
Chicago/Turabian StyleMallia, Vittoria, Lada Ivanova, Gunnar S. Eriksen, Emma Harper, Lisa Connolly, and Silvio Uhlig. 2020. "Investigation of In Vitro Endocrine Activities of Microcystis and Planktothrix Cyanobacterial Strains" Toxins 12, no. 4: 228. https://doi.org/10.3390/toxins12040228
APA StyleMallia, V., Ivanova, L., Eriksen, G. S., Harper, E., Connolly, L., & Uhlig, S. (2020). Investigation of In Vitro Endocrine Activities of Microcystis and Planktothrix Cyanobacterial Strains. Toxins, 12(4), 228. https://doi.org/10.3390/toxins12040228







