The Influence of the Different Disposition Characteristics of Snake Toxins on the Pharmacokinetics of Snake Venom
Abstract
1. Introduction
2. Results
2.1. Intraveneous Bolus Venom Administration (“Best Field Case” Scenario)
2.2. The Depot Compartment Model (“Likely Field Case” Scenario)
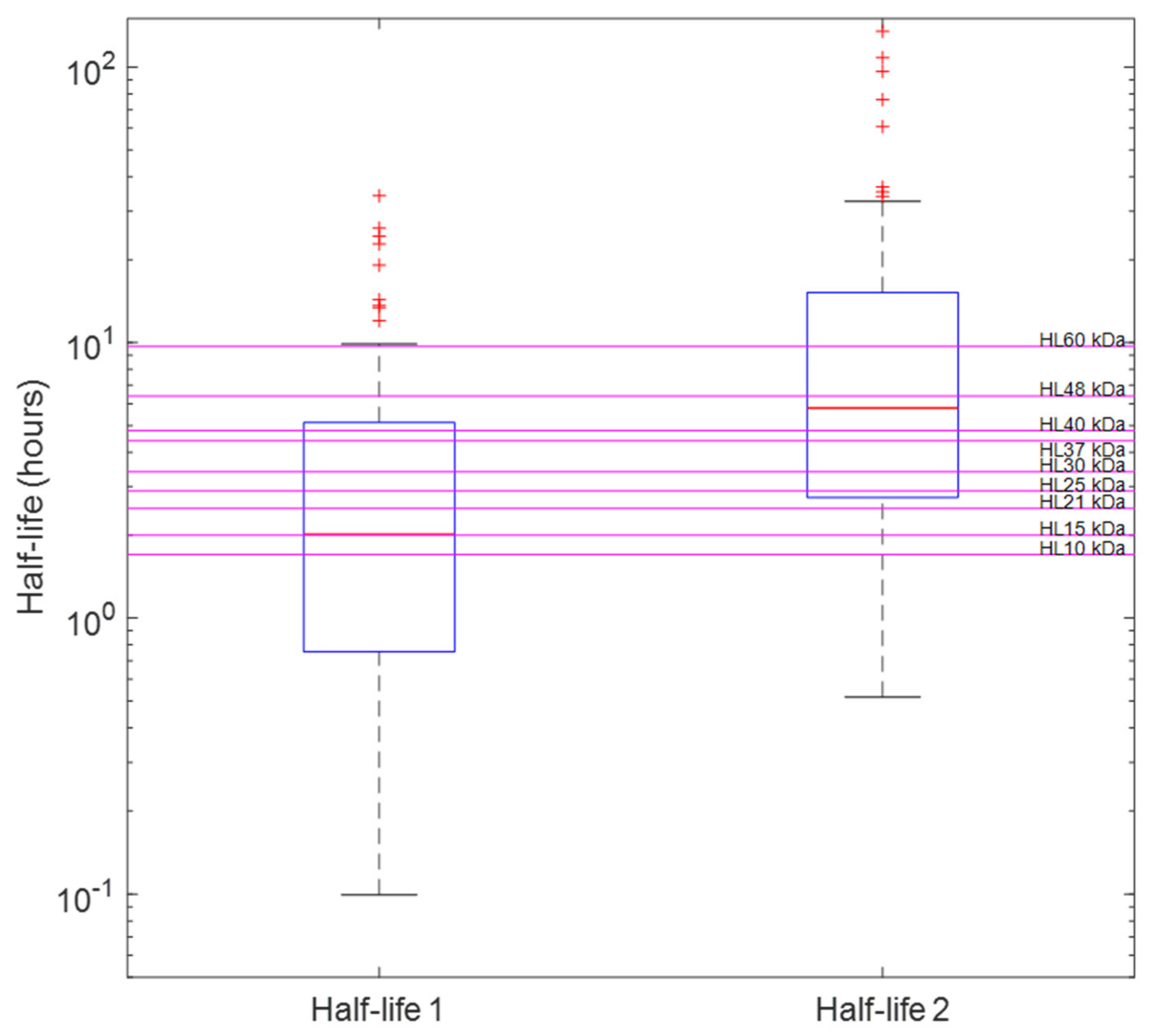
2.3. Distinguishing Individual Toxin Half-Lives from the Whole Venom
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Simulation of the Venom Profile
5.1.1. Establishing Prior PK Parameters for Characteristic Proteins
5.1.2. The Mixtures
5.1.3. The Simulation
Model for Population Parameters for Each Toxin Based on Molecular Weight
Model for the Individual Parameters for Each Toxin
Model for the Concentration–Time Data of Toxins
5.2. Pharmacokinetic Modelling
- i)
- An IV bolus considering using a 1- to - compartment model (best field case).
- ii)
- A first-order absorption using a 1- to - compartment model (likely field case).
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Compounds | Molecular Weight (kDa) | CL (L·h−1) | V (L) | References |
|---|---|---|---|---|
| Nesiritide | 3.5 | 38.6a | 13.3a | # |
| Glucagon recombinant | 3.8 | 56.7a | 17.5a | # |
| Abaloparatide | 4.0 | 20.4b | 50.0 | # |
| Relaxin | 6.0 | 10.5 | 17.4 | [23] |
| Neurotoxin (Naja sumatrana) | 6.5 | 2.36† | 67.6†c | [11] |
| Lepirudin | 7.0 | 9.30* | 14.5* | # |
| Cardiotoxin (N. sumatrana) | 7.0 | 2.50† | 69.3†c | [11] |
| Ecallantide | 7.1 | 9.18 | 26.4 | # |
| Phospholipase A2 (N. sumatrana) | 16 | 1.38† | 49.0†c | [11] |
| Filgrastim | 19 | 2.52a | 10.5a | # |
| rhGH | 20 | 9.14 | 4.1 | [23] |
| rCD4 | 50 | 3.42 | 4.9 | [23] |
| rt-PA | 63 | 37.2 | 8.1 | [23] |
| Albumin | 67 | 0.012b | 7.7 | +, [24] |
| CD4-IgG | 98 | 0.157 | 6.3 | [23] |
| Adalimumab | 144 | 0.012 | 5.4 | + |
| Eculizumab | 148 | 0.022 | 7.7 | # |
| Infliximab | 149 | 0.016* | 5.8* | [25,26,27] |
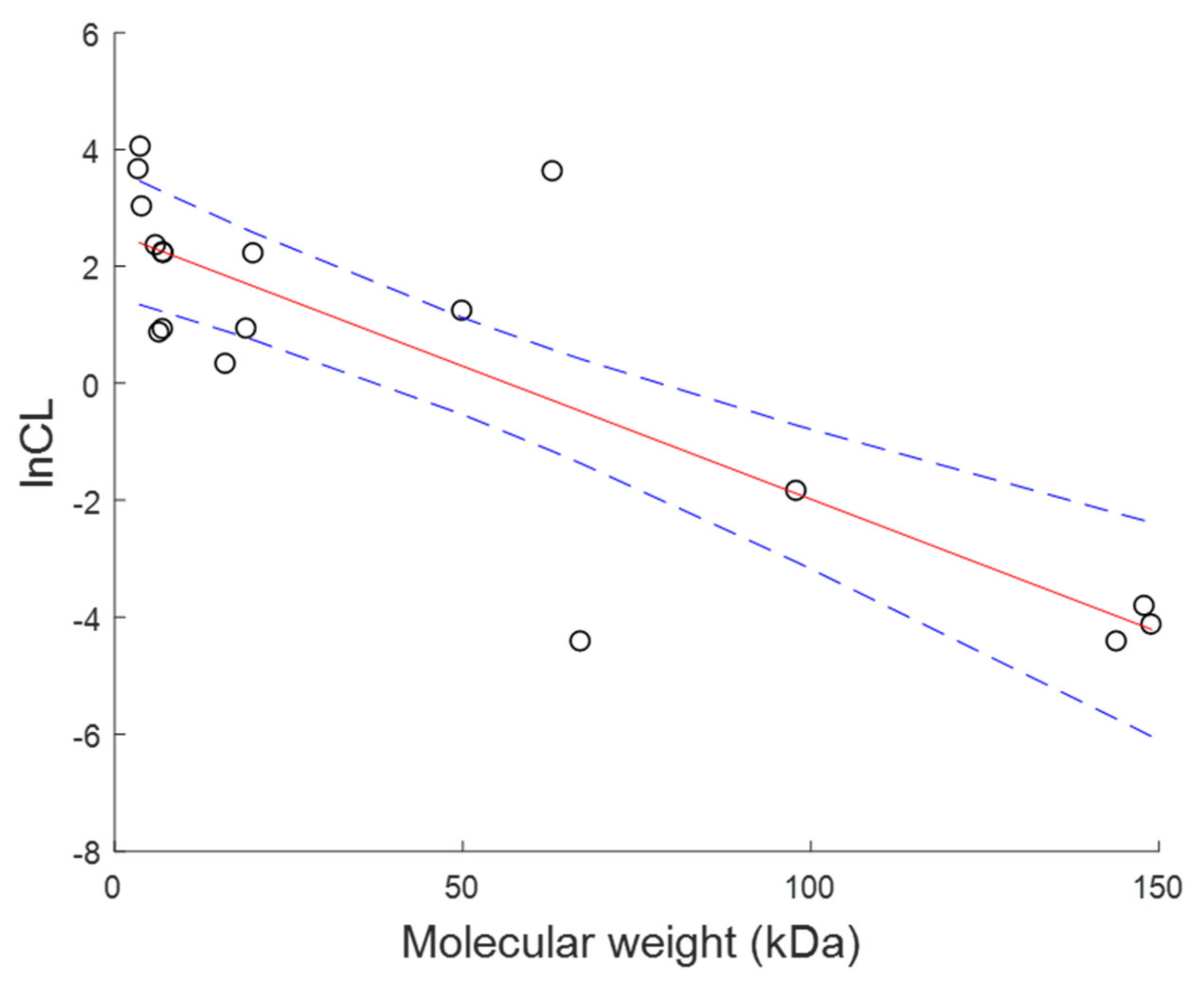
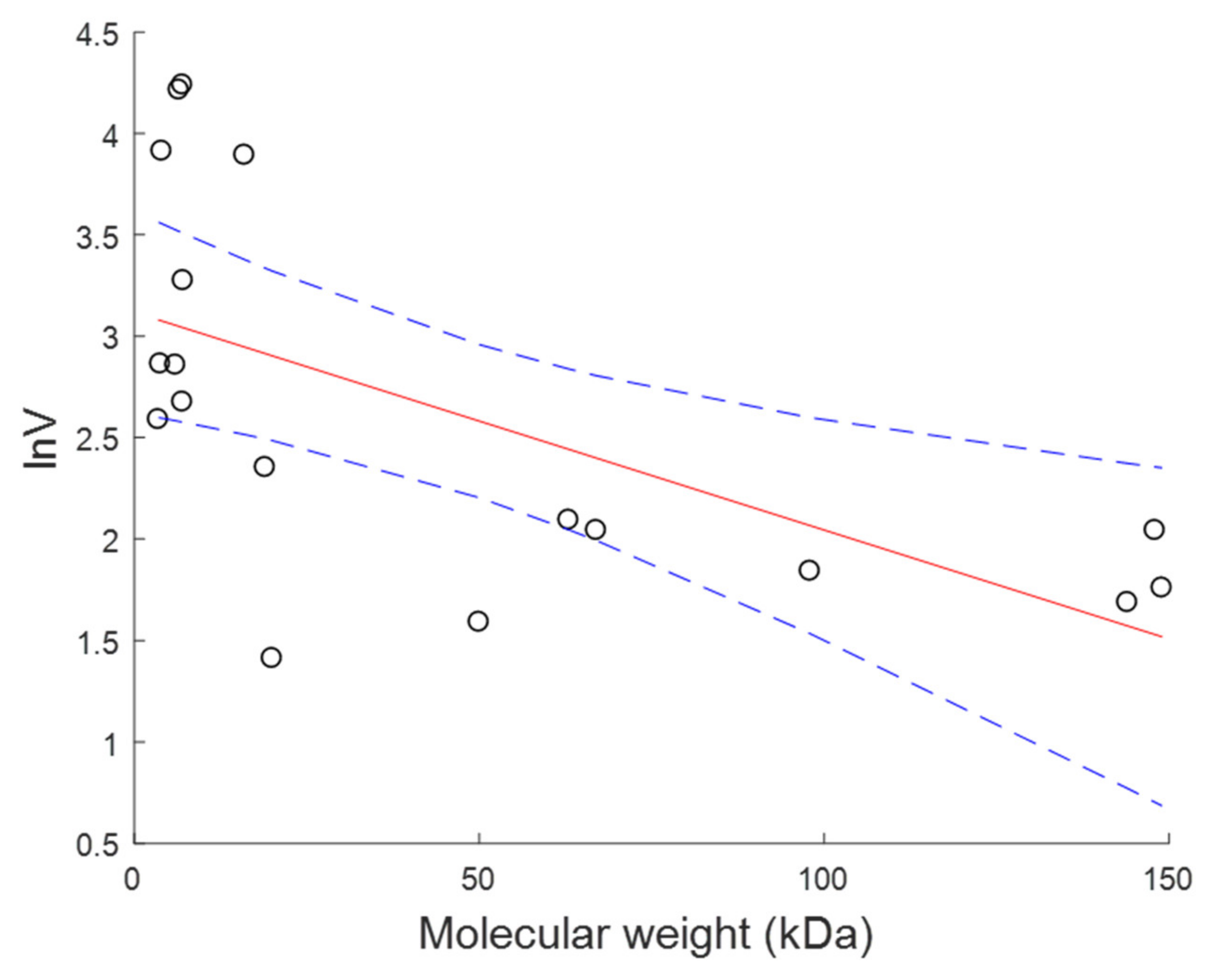
Appendix B
| Parameters | Parameter Values |
|---|---|
| 1.6528 | |
| 0.7525 | |
| 0.175 | |
| 0.0852 | |
| 0.217 | |
| 0.001 |
References
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef] [PubMed]
- Audebert, F.; Urtizberea, M.; Sabouraud, A.; Scherrmann, J.M.; Bon, C. Pharmacokinetics of vipera aspis venom after experimental envenomation in rabbits. J. Pharmacol. Exp. Ther. 1994, 268, 1512–1517. [Google Scholar] [PubMed]
- Guo, M.P.; Wang, Q.C.; Liu, G.F. Pharmacokinetics of cytotoxin from chinese cobra (naja naja atra) venom. Toxicon 1993, 31, 339–343. [Google Scholar] [CrossRef]
- Hart, A.J.; Hodgson, W.C.; O’Leary, M.; Isbister, G.K. Pharmacokinetics and pharmacodynamics of the myotoxic venom of pseudechis australis (mulga snake) in the anesthetised rat. Clin. Toxicol. 2014, 52, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Mello, S.M.; Linardi, A.; Renno, A.L.; Tarsitano, C.A.B.; Pereira, E.M.; Hyslop, S. Renal kinetics of bothrops alternatus (urutu) snake venom in rats. Toxicon 2010, 55, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Kinjoh, K.; Miyagi, C.; Oka, U.; Sunagawa, M.; Yamashita, S.; Kosugi, T. Pharmacokinetics of habutobin in rabbits. Toxicon 1995, 33, 1201–1206. [Google Scholar] [CrossRef]
- Paniagua, D.; Jimenez, L.; Romero, C.; Vergara, I.; Calderon, A.; Benard, M.; Bernas, M.J.; Rilo, H.; De Roodt, A.; D’Suze, G.; et al. Lymphatic route of transport and pharmacokinetics of micrurus fulvius (coral snake) venom in sheep. Lymphology 2012, 45, 144–153. [Google Scholar]
- Riviere, G.; Choumet, V.; Saliou, B.; Debray, M.; Bon, C. Absorption and elimination of viper venom after antivenom administration. J. Pharmacol. Exp. Ther. 1998, 285, 490–495. [Google Scholar]
- Sim, S.M.; Saremi, K.; Tan, N.H.; Fung, S.Y. Pharmacokinetics of cryptelytrops purpureomaculatus (mangrove pit viper) venom following intravenous and intramuscular injections in rabbits. Int. Immunopharmacol. 2013, 17, 997–1001. [Google Scholar] [CrossRef]
- Tan, C.H.; Sim, S.M.; Gnanathasan, C.A.; Fung, S.Y.; Tan, N.H. Pharmacokinetics of the sri lankan hump-nosed pit viper (hypnale hypnale) venom following intravenous and intramuscular injections of the venom into rabbits. Toxicon 2014, 79, 37–44. [Google Scholar] [CrossRef]
- Yap, M.K.; Tan, N.H.; Sim, S.M.; Fung, S.Y.; Tan, C.H. Pharmacokinetics of naja sumatrana (equatorial spitting cobra) venom and its major toxins in experimentally envenomed rabbits. [Erratum appears in Plos Negl. Trop. Dis. 2014 sep;8(9):E3277]. PLoS Negl. Trop. Dis. 2014, 8, e2890. [Google Scholar]
- Yap, M.K.K.; Tan, N.H.; Sim, S.M.; Fung, S.Y. Toxicokinetics of naja sputatrix (javan spitting cobra) venom following intramuscular and intravenous administrations of the venom into rabbits. Toxicon 2013, 68, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zheng, J.; Jiang, Z. Pharmacokinetics of thrombin-like enzyme from venom of agkistrodon halys ussuriensis emelianov determined by elisa in the rat. Toxicon 2001, 39, 1821–1826. [Google Scholar] [CrossRef]
- Allen, G.E.; Brown, S.G.A.; Buckley, N.A.; O’Leary, M.A.; Page, C.B.; Currie, B.J.; White, J.; Isbister, G.K. Clinical effects and antivenom dosing in brown snake (pseudonaja spp.) envenoming—australian snakebite project (asp-14). PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Audebert, F.; Grosselet, O.; Sabouraud, A.; Bon, C. Quantitation of venom antigens from european vipers in human serum or urine by elisa. J. Anal. Toxicol. 1993, 17, 236–240. [Google Scholar] [CrossRef]
- Audebert, F.; Sorkine, M.; Robbe-Vincent, A.; Bon, C. Viper bites in france: Clinical and biological evaluation; kinetics of envenomations. Hum. Exp. Toxicol. 1994, 13, 683–688. [Google Scholar] [CrossRef]
- Isbister, G.K.; Halkidis, L.; O’Leary, M.A.; Whitaker, R.; Cullen, P.; Mulcahy, R.; Bonnin, R.; Brown, S.G.A. Human anti-snake venom igg antibodies in a previously bitten snake-handler, but no protection against local envenoming. Toxicon 2010, 55, 646–649. [Google Scholar] [CrossRef]
- Sanhajariya, S.; Duffull, S.B.; Isbister, G.K. Pharmacokinetics of snake venom. Toxins 2018, 10, 73. [Google Scholar] [CrossRef]
- Hanvivatvong, O.; Mahasandana, S.; Karnchanachetanee, C. Kinetic study of russell’s viper venom in envenomed patients. Am. J. Trop. Med. Hyg. 1997, 57, 605–609. [Google Scholar] [CrossRef]
- McNally, T.; Conway, G.S.; Jackson, L.; Theakston, R.D.G.; Marsh, N.A.; Warrell, D.A.; Young, L.; Mackie, I.J.; Machin, S.J. Accidental envenoming by a gaboon viper (bitis gabonica): The haemostatic disturbances observed and investigation of in vitro haemostatic properties of whole venom. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 66–70. [Google Scholar] [CrossRef]
- Sano-Martins, I.S.; Tomy, S.C.; Campolina, D.; Dias, M.B.; de Castro, S.C.; de Sousa-e-Silva, M.C.; Amaral, C.F.; Rezende, N.A.; Kamiguti, A.S.; Warrell, D.A.; et al. Coagulopathy following lethal and non-lethal envenoming of humans by the south american rattlesnake (crotalus durissus) in brazil. QJM 2001, 94, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Tan, N.H.; Sim, S.M.; Fung, S.Y.; Gnanathasan, C.A. Proteomic investigation of sri lankan hump-nosed pit viper (hypnale hypnale) venom. Toxicon 2015, 93, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Mordenti, J.; Chen, S.A.; Moore, J.A.; Ferraiolo, B.L.; Green, J.D. Interspecies scaling of clearance and volume of distribution data for five therapeutic proteins. Pharm. Res. 1991, 8, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Rowland, M.; Tozer, T.N. Clinical Pharmacokinetics and Pharmacodynamics: Concepts and Applications, 4th ed.; Wolters Kluwer Health/Lippincott William & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Fasanmade, A.A.; Adedokun, O.J.; Ford, J.; Hernandez, D.; Johanns, J.; Hu, C.; Davis, H.M.; Zhou, H. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur. J. Clin. Pharmacol. 2009, 65, 1211–1228. [Google Scholar] [CrossRef]
- Klotz, U.; Teml, A.; Schwab, M. Clinical pharmacokinetics and use of infliximab. Clin. Pharmacokinet. 2007, 46, 645–660. [Google Scholar] [CrossRef]
- Ternant, D.; Ducourau, E.; Perdriger, A.; Corondan, A.; Le Goff, B.; Devauchelle-Pensec, V.; Solau-Gervais, E.; Watier, H.; Goupille, P.; Paintaud, G.; et al. Relationship between inflammation and infliximab pharmacokinetics in rheumatoid arthritis. Br. J. Clin. Pharmacol. 2014, 78, 118–128. [Google Scholar] [CrossRef]

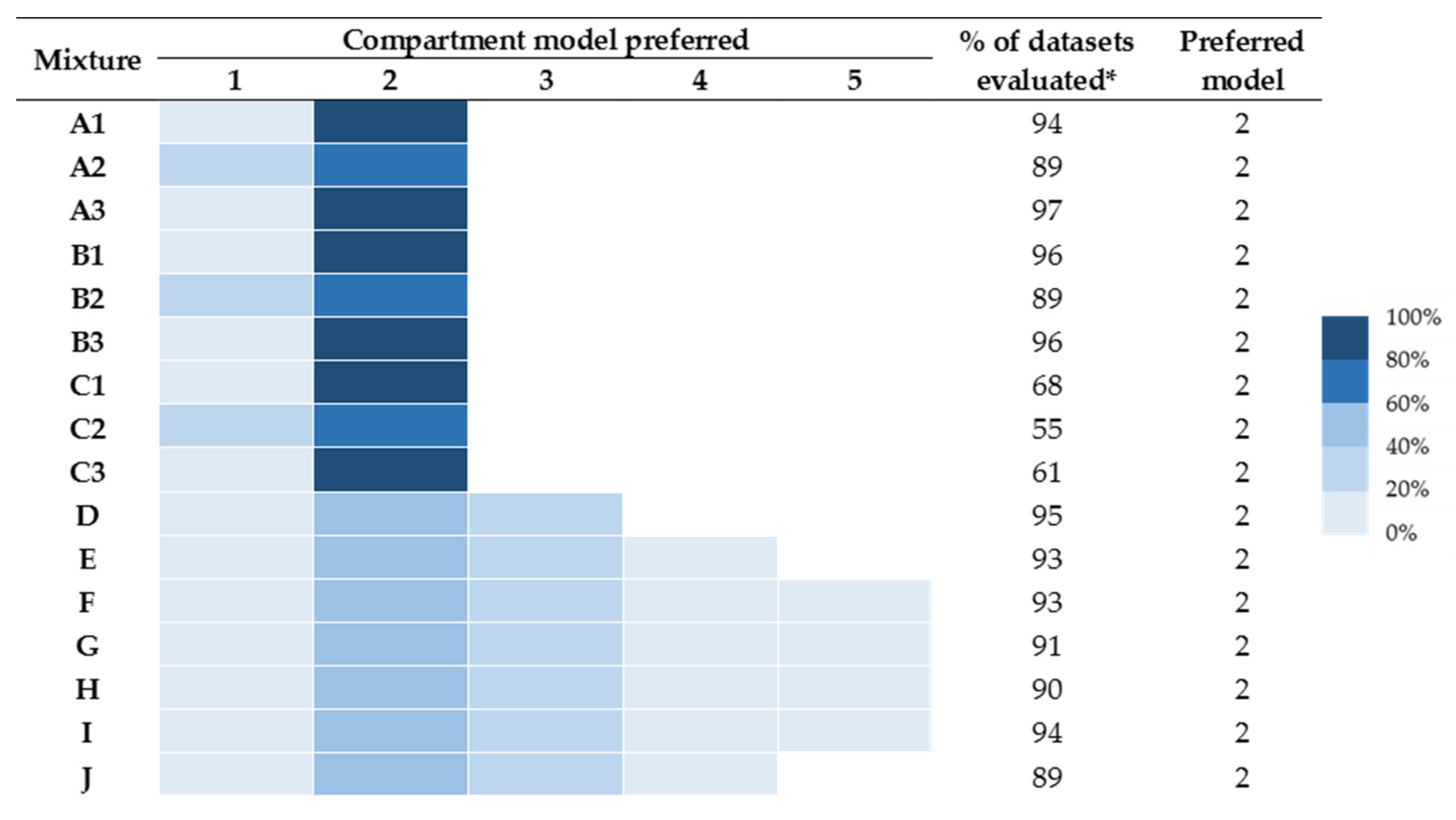
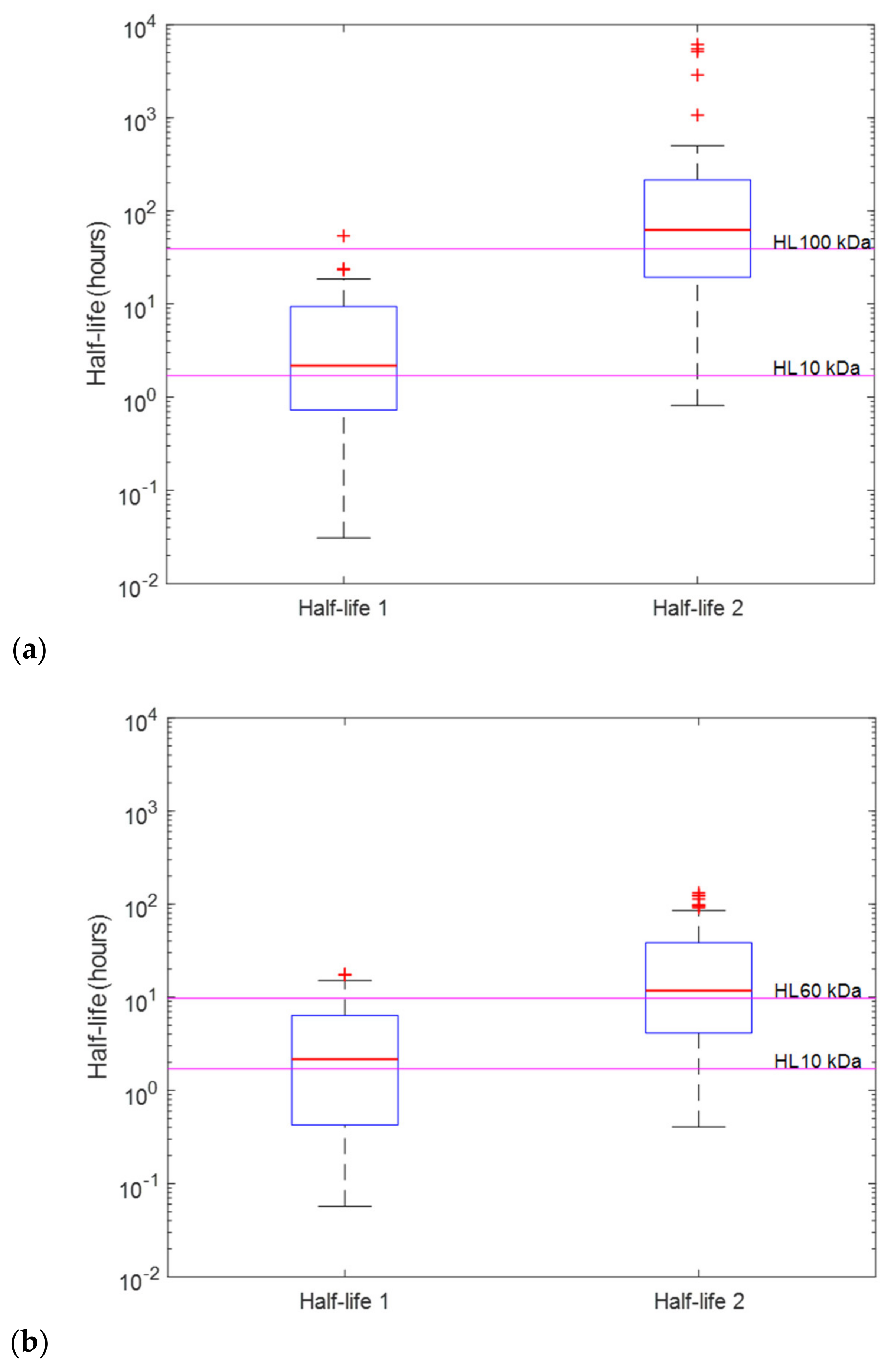
| Protein Components | Approximate Molecular Weight (kDa) | Relative Abundance |
|---|---|---|
| Phospholipase A2 (PLA2) | 14–15 | 40.1% |
| Zinc-dependent snake venom metalloprotease (SVMP) | 25 | 29.8% |
| 30 | 6.4% | |
| 40 | 0.7% | |
| L-amino acid (LAAO) | 60 | 11.9% |
| C-type lectin (CTL) | 14 | 5.5% |
| Snake venom serine protease (SVSP)—Thrombin-like enzyme ancrod | 37 | 0.5% |
| Snake venom serine protease (SVSP)—Thrombin-like enzyme | 48 | 2.8% |
| Not determined | 9–10 | 0.8% |
| 15 | 0.7% | |
| 21 | 0.8% |
| Mixture | Molecular Weights (kDa) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 15 | 21 | 25 | 30 | 37 | 40 | 48 | 60 | 100 | |
| A1 | 50 | . | . | . | . | . | . | . | . | 50 |
| A2 | 20 | . | . | . | . | . | . | . | . | 80 |
| A3 | 80 | . | . | . | . | . | . | . | . | 20 |
| B1 | 50 | . | . | . | . | . | . | . | 50 | . |
| B2 | 20 | . | . | . | . | . | . | . | 80 | . |
| B3 | 80 | . | . | . | . | . | . | . | 20 | . |
| C1 | 50 | . | . | . | 50 | . | . | . | . | . |
| C2 | 20 | . | . | . | 80 | . | . | . | . | . |
| C3 | 80 | . | . | . | 20 | . | . | . | . | . |
| D | . | 34 | . | 33 | . | . | . | . | 33 | . |
| E | . | 25 | . | 25 | 25 | . | . | . | 25 | . |
| F | . | 20 | . | 20 | 20 | . | . | 20 | 20 | . |
| G | 17 | 17 | . | 17 | 17 | . | . | 16 | 16 | . |
| H | 14 | 14 | 14 | 14 | 14 | . | . | 15 | 15 | . |
| I | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | . | 12.5 | 12.5 | 12.5 | . |
| J | 0.8 | 46.3 | 0.8 | 29.8 | 6.4 | 0.5 | 0.7 | 2.8 | 11.9 | . |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanhajariya, S.; Isbister, G.K.; Duffull, S.B. The Influence of the Different Disposition Characteristics of Snake Toxins on the Pharmacokinetics of Snake Venom. Toxins 2020, 12, 188. https://doi.org/10.3390/toxins12030188
Sanhajariya S, Isbister GK, Duffull SB. The Influence of the Different Disposition Characteristics of Snake Toxins on the Pharmacokinetics of Snake Venom. Toxins. 2020; 12(3):188. https://doi.org/10.3390/toxins12030188
Chicago/Turabian StyleSanhajariya, Suchaya, Geoffrey K. Isbister, and Stephen B. Duffull. 2020. "The Influence of the Different Disposition Characteristics of Snake Toxins on the Pharmacokinetics of Snake Venom" Toxins 12, no. 3: 188. https://doi.org/10.3390/toxins12030188
APA StyleSanhajariya, S., Isbister, G. K., & Duffull, S. B. (2020). The Influence of the Different Disposition Characteristics of Snake Toxins on the Pharmacokinetics of Snake Venom. Toxins, 12(3), 188. https://doi.org/10.3390/toxins12030188






