Commercial Biocontrol Agents Reveal Contrasting Comportments Against Two Mycotoxigenic Fungi in Cereals: Fusarium Graminearum and Fusarium Verticillioides
Abstract
1. Introduction
2. Results
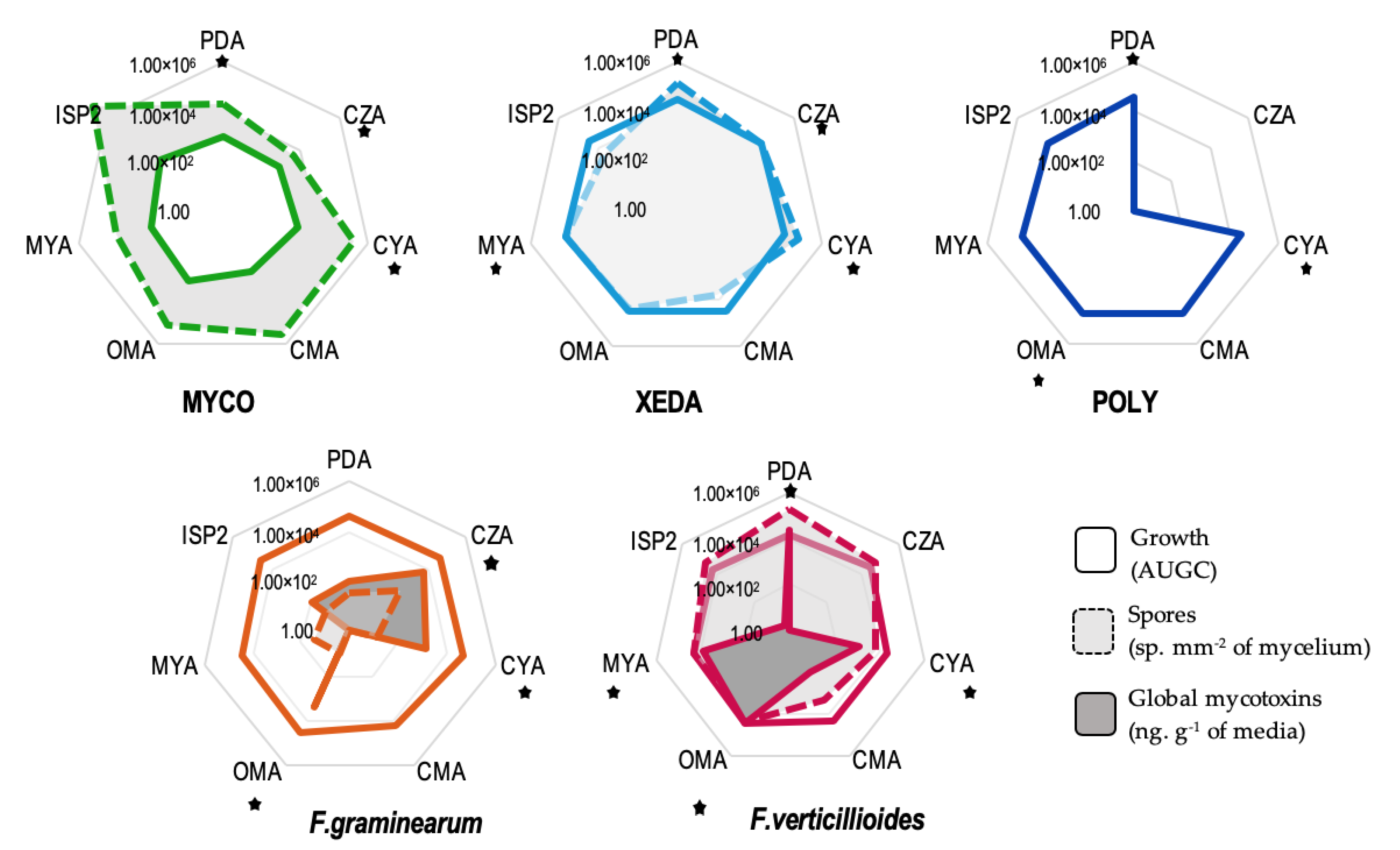
2.1. Comportments of Microorganisms and Culture Media Selection
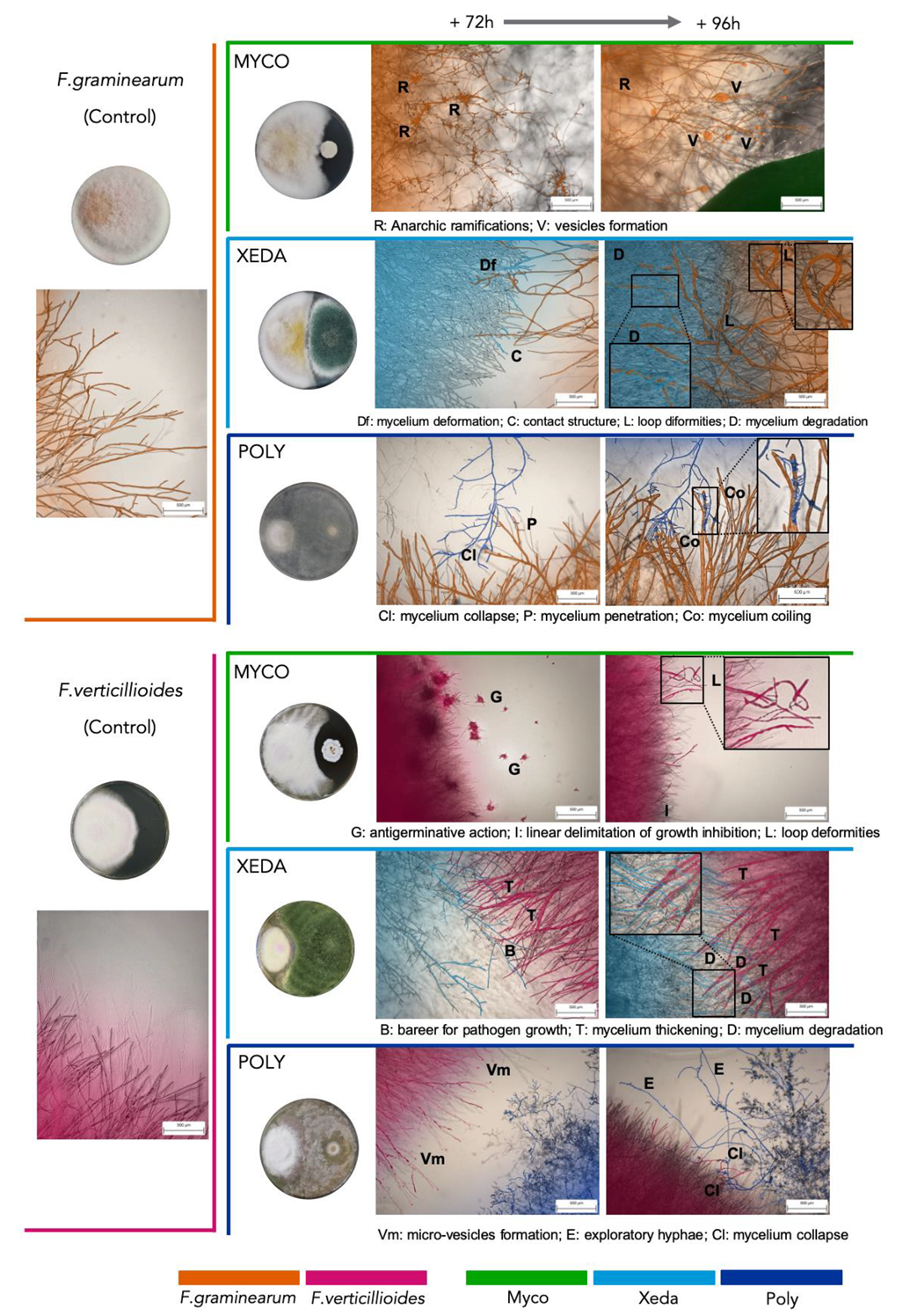
2.2. Macroscopic and Microscopic Interaction
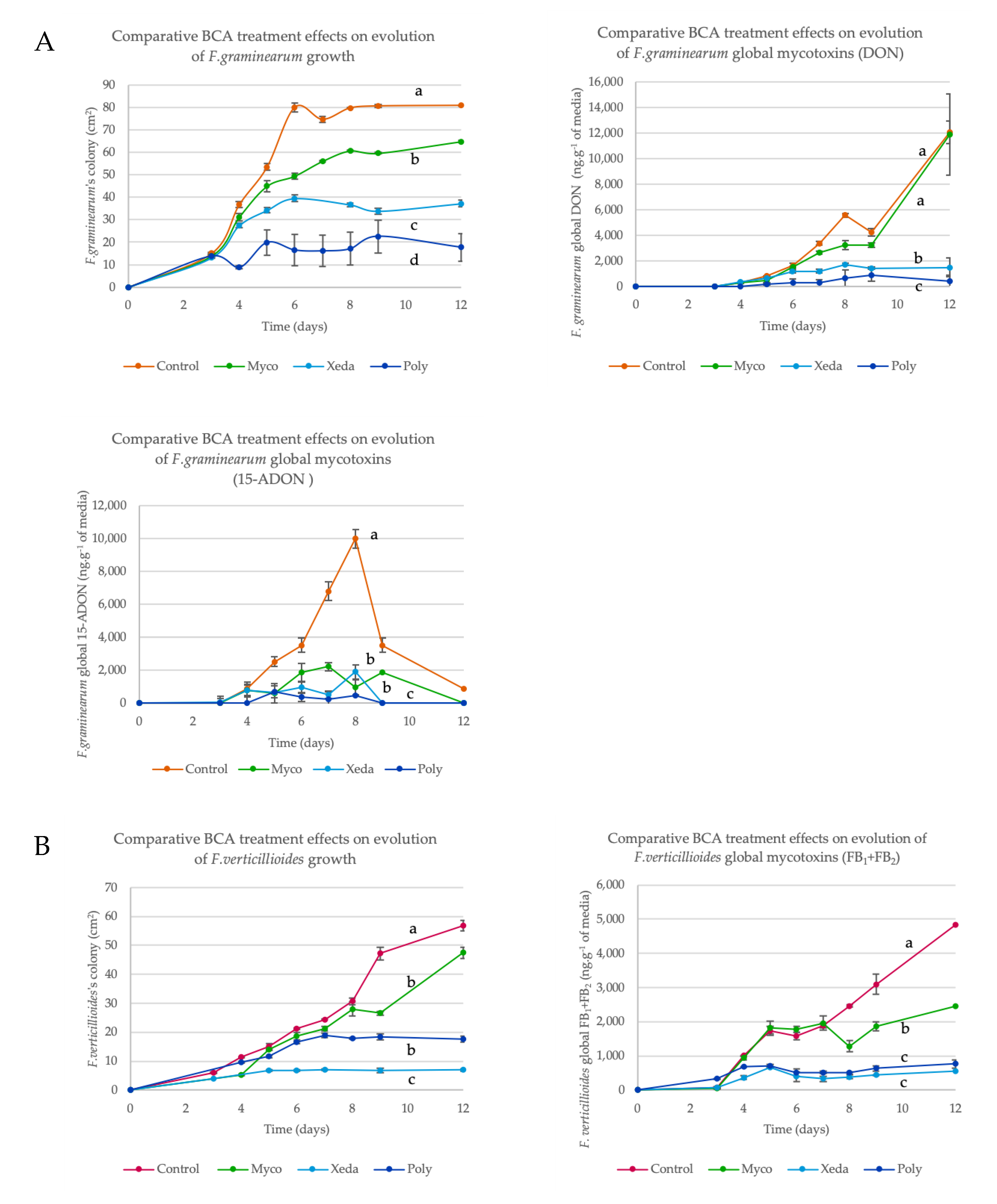
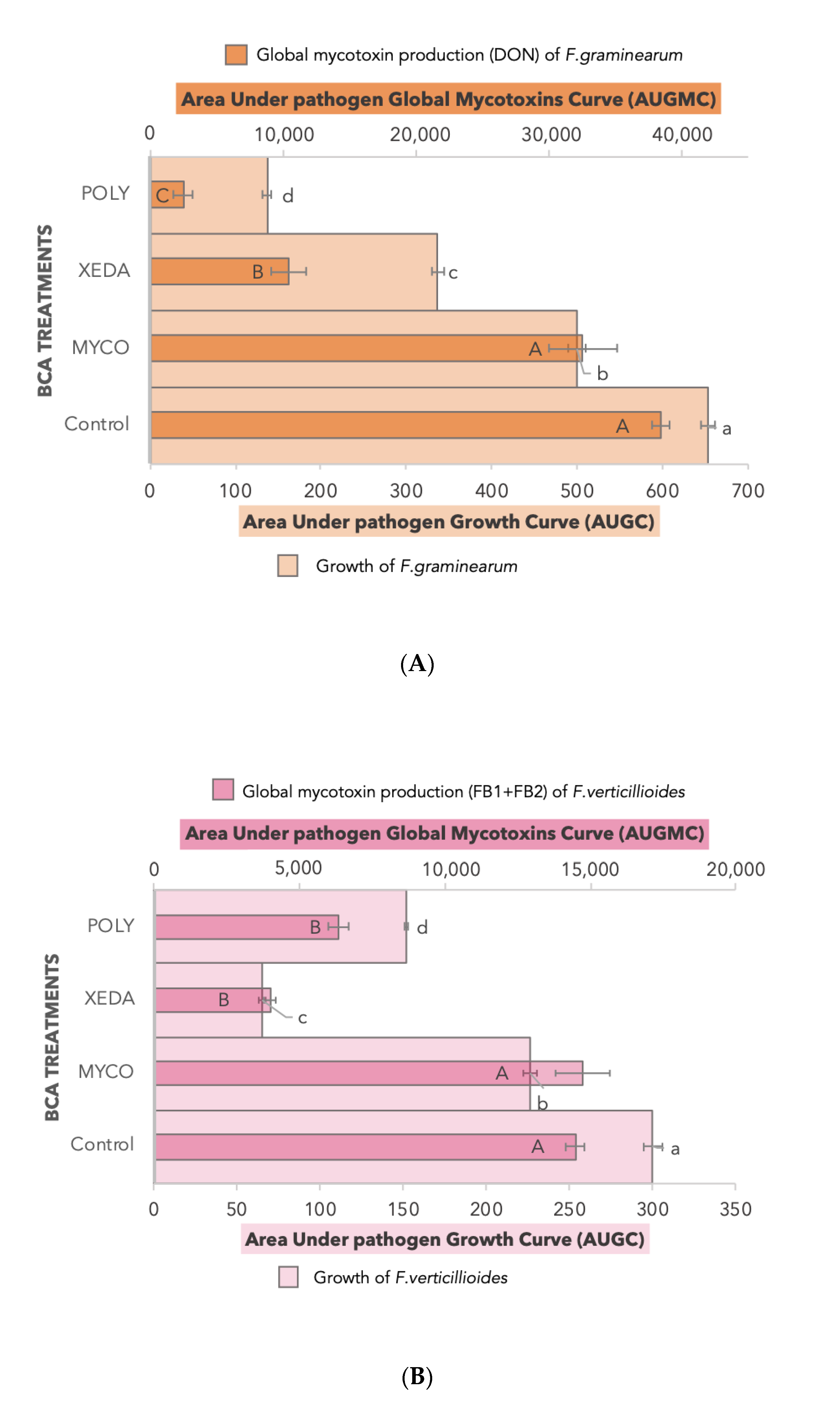
2.3. BCA Impact on Growth and Mycotoxin Production
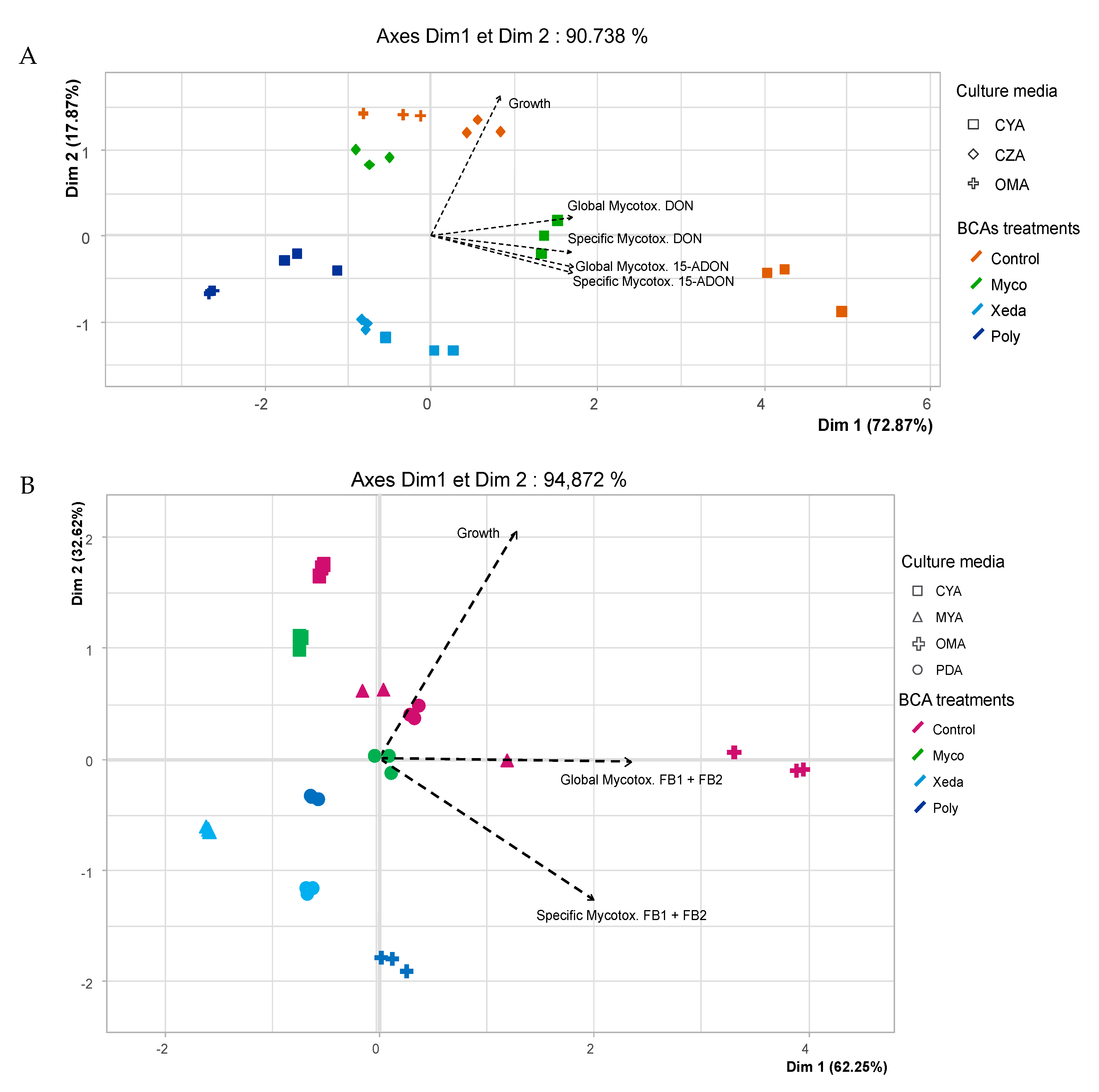
2.4. Impact of Media Variation on Pathogenic Factors during Dual Culture Bioassay (Pathogens–BCAs)
2.5. Nutrient Profiling and Nutritional Competition
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Micro-Organisms
5.1.1. Fusarium Species
5.1.2. Commercial Biological Control Agents (BCAs)
5.1.3. Behaviors on Different Media
5.2. Antagonist Activity in vitro on Selected Culture Media
5.2.1. Dual Culture Bioassay and Growth Evaluation
5.2.2. Mycotoxin Extraction and Analysis
5.3. Macroscopic and Microscopic Observations
5.4. Nutrient Profiling
5.5. Data Expression and Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
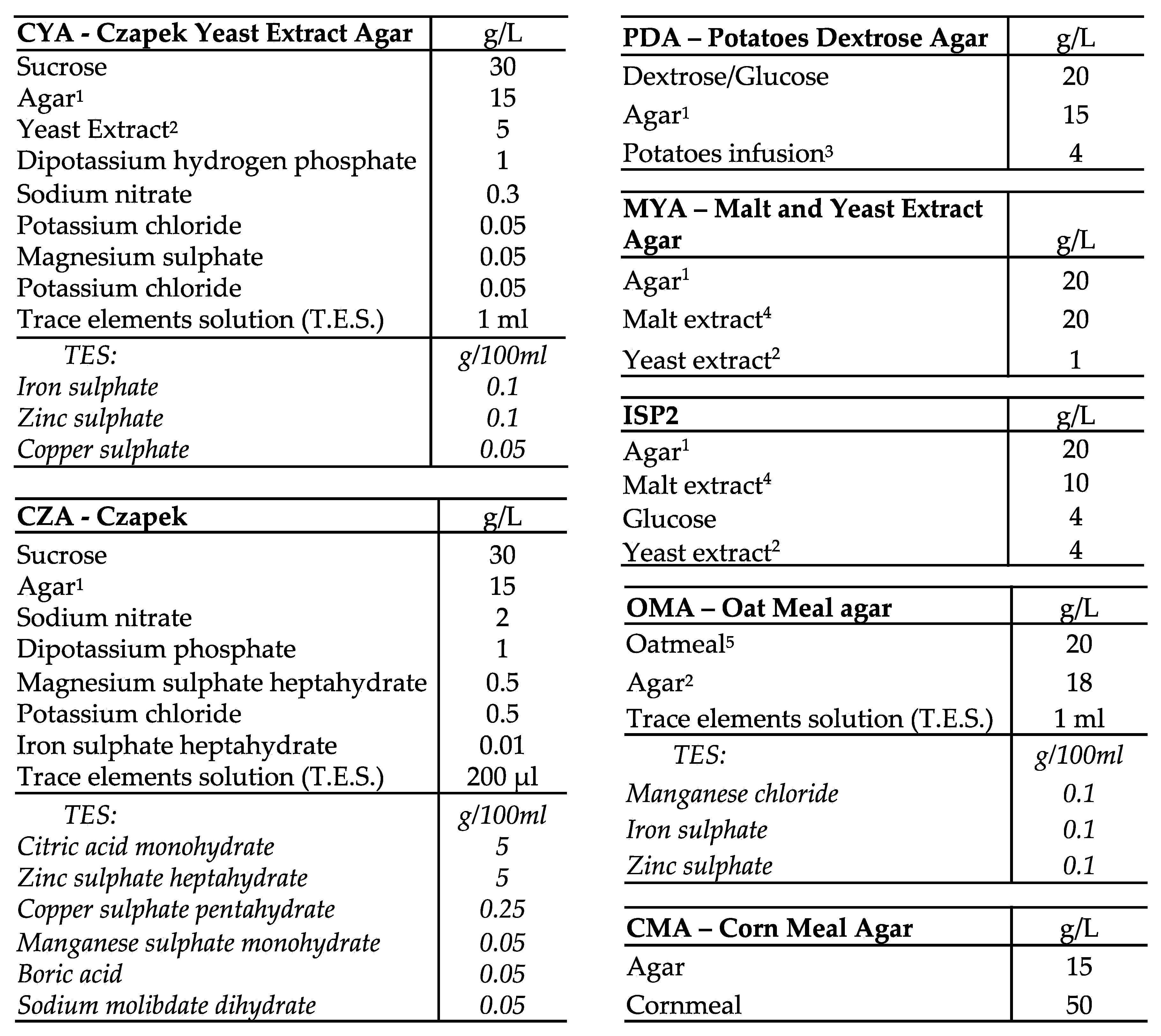
Appendix A.1. Medium Composition
Appendix A.2. Kinetics of Pathogen Growth and Mycotoxin Inhibition in Dual Culture Bioassay with BCAs (Common Media, CM)
References
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium toxins in cereals: Occurrence, legislation, factors promoting the appearance and their management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.S.; Brasel, J.M. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology 2001, 167, 101–134. [Google Scholar] [CrossRef]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- de Ruyck, K.; de Boevre, M.; Huybrechts, I.; de Saeger, S. Dietary mycotoxins, co-exposure, and carcinogenesis in humans: Short review. Mutat. Res. Rev. Mutat. Res. 2015, 766, 32–41. [Google Scholar] [CrossRef]
- Gerez, J.R.; Pinton, P.; Callu, P.; Grosjean, F.; Oswald, I.P.; Bracarense, A.P.F.L. Deoxynivalenol alone or in combination with nivalenol and zearalenone induce systemic histological changes in pigs. Exp. Toxicol. Pathol. 2015, 67, 89–98. [Google Scholar] [CrossRef]
- Sun, L.H.; Lei, M.Y.; Zhang, N.Y.; Gao, X.; Li, C.; Krumm, C.S.; Qi, D.S. Individual and combined cytotoxic effects of aflatoxin B1, zearalenone, deoxynivalenol and fumonisin B1 on BRL 3A rat liver cells. Toxicon 2015, 95, 6–12. [Google Scholar] [CrossRef]
- Jard, G.; Liboz, T.; Mathieu, F.; Guyonvarch, A.; Lebrihi, A. Review of mycotoxin reduction in food and feed: From prevention in the field to detoxification by adsorption or transformation. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2011, 28, 1590–1609. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Torres, A.M.; Palacios, S.A.; Yerkovich, N.; Palazzini, J.M.; Battilani, P.; Leslie, J.F.; Logrieco, A.F.; Chulze, S.N. Fusarium head blight and mycotoxins in wheat: Prevention and control strategies across the food chain. World Mycotoxin J. 2019, 4, 333–355. [Google Scholar] [CrossRef]
- Vallet, M.; Vanbellingen, Q.P.; Fu, T.; le Caer, J.P.; Della-Negra, S.; Touboul, D.; Duncan, K.R.; Nay, B.; Brunelle, A.; Prado, S. An Integrative Approach to Decipher the Chemical Antagonism between the Competing Endophytes Paraconiothyrium variabile and Bacillus subtilis. J. Nat. Prod. 2017, 80, 2863–2873. [Google Scholar] [CrossRef] [PubMed]
- Daguerre, Y.; Siegel, K.; Edel-Hermann, V.; Steinberg, C. Fungal proteins and genes associated with biocontrol mechanisms of soil-borne pathogens: A review. Fungal Biol. Rev. 2014, 28, 97–125. [Google Scholar] [CrossRef]
- Matarese, F.; Sarrocco, S.; Gruber, S.; Seidl-Seiboth, V.; Vannacci, G. Biocontrol of Fusarium head blight: Interactions between Trichoderma and mycotoxigenic Fusarium. Microbiology 2012, 158, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Luo, L.; Long, C. An Characterization of competition for nutrients in the biocontrol of Penicillium italicum by Kloeckera apiculata. Biol. Control 2013, 67, 157–162. [Google Scholar] [CrossRef]
- Celar, F. Competition for ammonium and nitrate forms of nitrogen between some phytopathogenic and antagonistic soil fungi. Biol. Control 2003, 28, 19–24. [Google Scholar] [CrossRef]
- Kim, S.H.; Vujanovic, V. Relationship between mycoparasites lifestyles and biocontrol behaviors against Fusarium spp. and mycotoxins production. Appl. Microbiol. Biotechnol. 2016, 100, 5257–5272. [Google Scholar] [CrossRef] [PubMed]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced Systemic Resistance and Plant Responses to Fungal Biocontrol Agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef]
- Lugtenberg, B.J.J.; Caradus, J.R.; Johnson, L.J. Fungal endophytes for sustainable crop production. FEMS Microbiol. Ecol. 2016, 92, 194. [Google Scholar] [CrossRef]
- Biological Control of Mycotoxigenic Fungi and Their Toxins: An Update for the Pre-Harvest Approach. Available online: https://biblio.ugent.be/publication/8580508/file/8580795 (accessed on 26 February 2020).
- Legrand, F.; Picot, A.; Cobo-Díaz, J.F.; Chen, W.; le Floch, G. Challenges facing the biological control strategies for the management of Fusarium Head Blight of cereals caused by F. graminearum. Biol. Control 2017, 113, 26–38. [Google Scholar] [CrossRef]
- Picot, A.; Barreau, C.; Pinson-Gadais, L.; Caron, D.; Lannou, C.; Richard-Forget, F. Factors of the Fusarium verticillioides-maize environment modulating fumonisin production. Crit. Rev. Microbiol. 2010, 36, 221–231. [Google Scholar] [CrossRef]
- Gardiner, D.M.; Kazan, K.; Manners, J.M. Nutrient profiling reveals potent inducers of trichothecene biosynthesis in Fusarium graminearum. Fungal Genet. Biol. 2009, 46, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Selvaraj, J.N.; Xing, F.; Zhou, L.; Wang, Y.; Song, H.; Tan, X.; Sun, L.; Sangare, L.; Folly, Y.M.E.; et al. Antagonistic action of Bacillus subtilis strain SG6 on Fusarium graminearum. PLoS ONE 2014, 9, 1–11. [Google Scholar] [CrossRef]
- Dal Bello, G.M.; Mónaco, C.I.; Simón, M.R. Biological control of seedling bright of wheat caused by Fusarium graminearum with beneficial rhizosphere microorganisms. World J. Microbiol. Biotechnol. 2002, 18, 627–636. [Google Scholar] [CrossRef]
- Oskay, M. Antifungal and antibacterial compounds from Streptomyces strains. Afr. J. Biotechnol. 2009, 8, 3007–3017. [Google Scholar]
- Igarashi, Y. Screening of Novel Bioactive Compounds from Plant-Associated Actinomycetes. Actinomycetologica 2004, 18, 63–66. [Google Scholar] [CrossRef]
- Lu, D.; Ma, Z.; Xu, X.; Yu, X. Isolation and identification of biocontrol agent Streptomyces rimosus M527 against Fusarium oxysporum f. sp. cucumerinum. J. Basic Microbiol. 2016, 56, 929–933. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Qiao, X.; Li, Z.; Li, F.; Chen, M.; Wang, Y.; Huang, Y.; Cui, H. Antifungal activity of volatile organic compounds from Streptomyces alboflavus TD-1. FEMS Microbiol. Lett. 2013, 341, 45–51. [Google Scholar] [CrossRef]
- Menke, J.; Weber, J.; Broz, K.; Kistler, H.C. Cellular Development Associated with Induced Mycotoxin Synthesis in the Filamentous Fungus Fusarium graminearum. PLoS ONE 2013, 8, e63077. [Google Scholar] [CrossRef]
- Chanda, A.; Roze, L.V.; Kang, S.; Artymovich, K.A.; Hicks, G.R.; Raikhel, N.V.; Calvo, A.M.; Linz, J.E. A key role for vesicles in fungal secondary metabolism. Proc. Natl. Acad. Sci. USA 2009, 106, 19533–19538. [Google Scholar] [CrossRef]
- Inch, S.; Gilbert, J. Scanning electron microscopy observations of the interaction between Trichoderma harzianum and perithecia of Gibberella zeae. Mycologia 2011, 103, 1–9. [Google Scholar] [CrossRef]
- El-Komy, M.H.; Saleh, A.A.; Eranthodi, A.; Molan, Y.Y. Characterization of novel trichoderma asperellum isolates to select effective biocontrol agents against tomato fusarium wilt. Plant Pathol. J. 2015, 31, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Gajera, H.; Domadiya, R.; Patel, S.; Kapopara, M.; Golakiya, B. Molecular mechanism of Trichoderma as bio-control agents against phytopathogen system—A review. Curr. Res. Microbiol. Biotechnol. 2013, 1, 133–142. [Google Scholar]
- Druzhinina, I.S.; Seidl-Seiboth, V.; Herrera-Estrella, A.; Horwitz, B.A.; Kenerley, C.M.; Monte, E.; Mukherjee, P.K.; Zeilinger, S.; Grigoriev, I.V.; Kubicek, C.P. Trichoderma: The genomics of opportunistic success. Nat. Rev. Microbiol. 2011, 9, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Kubicek, C.P.; Herrera-Estrella, A.; Seidl-Seiboth, V.; Martinez, D.A.; Druzhinina, I.S.; Thon, M.; Zeilinger, S.; Casas-Flores, S.; Horwitz, B.A.; Mukherjee, P.K.; et al. Comparative genome sequence analysis underscores mycoparasitism as the ancestral life style of Trichoderma. Genome Biol. 2011, 12, R40. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K. Genomics of Biological Control-Whole Genome Sequencing of Two Mycoparasitic Trichoderma spp. Curr. Sci. 2011, 101, 268. [Google Scholar]
- Tian, Y.; Tan, Y.; Liu, N.; Liao, Y.; Sun, C.; Wang, S.; Wu, A. Functional agents to biologically control Deoxynivalenol contamination in cereal grains. Front. Microbiol. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yang, T.; Friman, V.P.; Xu, Y.; Shen, Q.; Jousset, A. Trophic network architecture of root-associated bacterial communities determines pathogen invasion and plant health. Nat. Commun. 2015, 6, 1–9. [Google Scholar] [CrossRef]
- le Floch, G.; Vallance, J.; Benhamou, N.; Rey, P. Combining the oomycete Pythium oligandrum with two other antagonistic fungi: Root relationships and tomato grey mold biocontrol. Biol. Control 2009, 50, 288–298. [Google Scholar] [CrossRef]
- Cliquet, S.; Tirilly, Y. Development of a defined medium for Pythium oligandrum oospore production. Biocontrol Sci. Technol. 2002, 12, 455–467. [Google Scholar] [CrossRef]
- Hendrix, J.W. Sterol induction of reproduction and stimulation of growth of Pythium and Phytophthora. Science 1964, 144, 1028–1029. [Google Scholar] [CrossRef]
- Ko, W.-H. Sexual reproduction in pythiaceous fungi Chemical stimulation of sexual reproduction in Phytophthora and Pythium. Bot. Bull. Acadamia Singap. 1998, 39, 81–86. [Google Scholar]
- Faure, C.; Rey, T.; Gaulin, E.; Dumas, B. Pythium oligandrum: A necrotrophic mycoparasite of Fusarium graminearum. In Proceedings of the Natural Products and Biocontrol, Perpignan, France, 25–28 September 2018. [Google Scholar]
- Crippin, T.; Renaud, J.B.; Sumarah, M.W.; Miller, J.D. Comparing genotype and chemotype of Fusarium graminearum from cereals in Ontario, Canada. PLoS ONE 2019, 14, e0216735. [Google Scholar] [CrossRef] [PubMed]
- Leslie, J.F.; Summerell, B.A. Fusarium Laboratory Manual; Blackwell: Hoboken, NJ, USA, 2008; ISBN 9780813819198. [Google Scholar]
- Lígia Martins, M.; Marina Martins, H. Influence of water activity, temperature and incubation time on the simultaneous production of deoxynivalenol and zearalenone in corn (Zea mays) by Fusarium graminearum. Food Chem. 2002, 79, 315–318. [Google Scholar] [CrossRef]
- Droce, A.; Sørensen, J.L.; Sondergaard, T.E.; Rasmussen, J.J.; Lysøe, E.; Giese, H. PTR2 peptide transporters in Fusarium graminearum influence secondary metabolite production and sexual development. Fungal Biol. 2017, 121, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Facchini, F.D.A.; Vici, A.C.; Pereira, M.G.; Jorge, J.A.; de Moraes, M.D.L.T. Enhanced lipase production of Fusarium verticillioides by using response surface methodology and wastewater pretreatment application. J. Biochem. Technol. 2015, 6, 996–1002. [Google Scholar]
- Shepherd, M.D.; Kharel, M.K.; Bosserman, M.A.; Rohr, J. Laboratory Maintenance of Streptomyces Species. Curr. Protoc. Microbiol. 2010, 18, 1–8. [Google Scholar] [CrossRef]
- Boudjeko, T.; Tchinda, R.A.M.; Zitouni, M.; Nana, J.A.V.T.; Lerat, S.; Beaulieu, C. Streptomyces cameroonensis sp. nov., a Geldanamycin Producer That Promotes Theobroma cacao Growth. Microbes Environ. 2017, 32, 24–31. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, R.; Ni, M.; Yu, J.; Li, Y.; Yu, C.; Dou, K.; Ren, J.; Chen, J. Identification of a novel fungus, Trichoderma asperellum GDFS1009, and comprehensive evaluation of its biocontrol efficacy. PLoS ONE 2017, 12, e0179957. [Google Scholar] [CrossRef]
- Chutrakul, C.; Alcocer, M.; Bailey, K.; Peberdy, J.F. The Production and Characterisation of Trichotoxin Peptaibols, by Trichoderma asperellum. Chem. Biodivers. 2008, 5, 1694–1706. [Google Scholar] [CrossRef] [PubMed]
- Gerbore, J.; Vallance, J.; Yacoub, A.; Delmotte, F.; Grizard, D.; Regnault-Roger, C.; Rey, P. Characterization of Pythium oligandrum populations that colonize the rhizosphere of vines from the Bordeaux region. FEMS Microbiol. Ecol. 2014, 90, 153–167. [Google Scholar] [CrossRef] [PubMed]
- El-Katatny, M.; Abdelzaher, H.; Shoulkamy, M. Antagonistic actions of Pythium oligandrum and Trichoderma harzianum against phytopathogenic fungi (Fusarium oxysporum and Pythium ultimum var. ultimum). Arch. Phytopathol. Plant Prot. 2006, 39, 289–301. [Google Scholar] [CrossRef]
- Horner, N.R. Molecular Studies of the Oomycete Biocontrol Agent Pythium oligandrum. Ph.D. Thesis, University of Aberdeen, Aberdeen, UK, 2007. [Google Scholar]
- Moreau, S.; Levi, M. Highly Sensitive and Rapid Simultaneous Method for 45 Mycotoxins in Baby Food Samples by Hplc-Ms/Ms Using Fast Polarity Switching (PO-CON1480E); Shimadzu: Tokyo, Japan, 2014. [Google Scholar]
- Reithner, B.; Schuhmacher, R.; Stoppacher, N.; Pucher, M.; Brunner, K.; Zeilinger, S. Signaling via the Trichoderma atroviride mitogen-activated protein kinase Tmk1 differentially affects mycoparasitism and plant protection. Fungal Genet. Biol. 2007, 44, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Bochner, B.R.; Gadzinski, P.; Panomitros, E. Phenotype Microarrays for high-throughput phenotypic testing and assay of gene function. Genome Res. 2001, 11, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Lahaye, M.; Rochas, C. Chemical structure and physico-chemical properties of agar. In International Workshop on Gelidium; Springer: Dordrecht, The Netherlands, 1991; pp. 137–148. [Google Scholar]
- Bridson, E.Y.; Brecker, A. Design and Formulation of Microbial Culture Media. Methods Microbiol. 1970, 3, 229–295. [Google Scholar]
- Filtenborg, O.; Frisvad, J.C.; Thrane, U. The Significance of Yeast Extract Composition on Metabolite Production in Penicillium. Mod. Concepts Penicillium Aspergillus Classif. 1990, 185, 433–441. [Google Scholar]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch—Composition, fine structure and architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Zhu, F.; Cai, Y.-Z.; Ke, J.; Corke, H. Compositions of phenolic compounds, amino acids and reducing sugars in commercial potato varieties and their effects on acrylamide formation. J. Sci. Food Agric. 2010, 90, 2254–2262. [Google Scholar] [CrossRef]
- Robbins, G.S.; Pomeranz, Y. Amino Acid Composition of Malted Cereals and Malt Sprouts. Proc. Annu. Meet. Am. Soc. Brew. Chem. 1971, 29, 15–21. [Google Scholar] [CrossRef]
- Sterna, V.; Zute, S.; Brunava, L. Oat Grain Composition and its Nutrition Benefice. Agric. Agric. Sci. Procedia 2016, 8, 252–256. [Google Scholar] [CrossRef]
- Chwil, S. A study on the effects of foliar feeding under different soil fertilization conditions on the yield structure and quality of common oat (Avena sativa L.). Acta Agrobot. 2014, 67, 109–120. [Google Scholar] [CrossRef][Green Version]
- Lapvetelainen, A.; Aro, T. Protein composition and functionality of high-protein oat flour derived from integrated starch-ethanol process. Cereal Chem. 1994, 71, 133–138. [Google Scholar]

| Selected Media for Dual Culture Bioassay (Pathogen–BCA) | ||||
|---|---|---|---|---|
| Pathogens | F. Graminearum | F. Verticillioides | ||
| BCA | CM | SM | CM | SM |
| Myco | CYA | CZA | PDA | CYA |
| Xeda | CYA | CZA | PDA | MYA |
| Poly | CYA | OMA | PDA | OMA |
| BCAs, Commercial Products | Mycostop | Xedavir | Polyversum |
|---|---|---|---|
| Strain | Streptomyces griseoviridis strain K61 | Trichoderma asperellum strain TV1 | Pythium oligandrum strain M1/ATCC 38472 |
| Family | Actinomycete | Ascomycete | Pythiaceae |
| Kingdom | Bacteria | Fungi | Oomycete |
| Plant/soil of isolation | Sphagnum peat | Root of tomato plant | Sugar beet |
| Locations of isolation | Finland | Italy | Czechoslovakia |
| Recommended Use | General soil treatment | General soil treatment | Barley and wheat aerial treatment |
| Target(s) | Soil-borne pathogen | Soil-borne pathogen | Fusarium graminearum |
| Commercialization company | Lallemand Plant Care® | Xeda International® | DeSangosse® |
| Date of marketing | 2014 | 2013 | 2015 |
| Identified general mechanism of action | Production of antimicrobial compound, mycoparasitism, plant defense induction | Mycoparasitism, plant defense induction | Mycoparasitism, plant defense induction |
| /on mycotoxigenic fungi | /none | /none | /on F. graminearum |
| IS | Polarity | MRM | EC |
|---|---|---|---|
| DON C13 | − | 370.3 > 59.0 | 35 |
| FB1 C13 | + | 756.3 > 356.5 | −47 |
| Mycotoxin | Polarity | MRM Q | EC MRM Q | MRM Q | EC MRM Q | R2 Calibration Curve | IS |
|---|---|---|---|---|---|---|---|
| Fumonisin B1 | + | 722.35 > 334.5 | −43 | 722.35 > 352.0 | −44 | 0.9982 | FB1 C13 |
| Fumonisin B2 | + | 706.2 > 318.4 | −37 | 706.2 > 3336.4 | −44 | 0.9980 | FB1 C13 |
| DON | − | 355.0 > 59.0 | 35 | 355.0 > 265.1 | 35 | 0.9998 | DON C13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellan, L.; Durand, N.; Martinez, V.; Fontana, A.; Schorr-Galindo, S.; Strub, C. Commercial Biocontrol Agents Reveal Contrasting Comportments Against Two Mycotoxigenic Fungi in Cereals: Fusarium Graminearum and Fusarium Verticillioides. Toxins 2020, 12, 152. https://doi.org/10.3390/toxins12030152
Pellan L, Durand N, Martinez V, Fontana A, Schorr-Galindo S, Strub C. Commercial Biocontrol Agents Reveal Contrasting Comportments Against Two Mycotoxigenic Fungi in Cereals: Fusarium Graminearum and Fusarium Verticillioides. Toxins. 2020; 12(3):152. https://doi.org/10.3390/toxins12030152
Chicago/Turabian StylePellan, Lucile, Noël Durand, Véronique Martinez, Angélique Fontana, Sabine Schorr-Galindo, and Caroline Strub. 2020. "Commercial Biocontrol Agents Reveal Contrasting Comportments Against Two Mycotoxigenic Fungi in Cereals: Fusarium Graminearum and Fusarium Verticillioides" Toxins 12, no. 3: 152. https://doi.org/10.3390/toxins12030152
APA StylePellan, L., Durand, N., Martinez, V., Fontana, A., Schorr-Galindo, S., & Strub, C. (2020). Commercial Biocontrol Agents Reveal Contrasting Comportments Against Two Mycotoxigenic Fungi in Cereals: Fusarium Graminearum and Fusarium Verticillioides. Toxins, 12(3), 152. https://doi.org/10.3390/toxins12030152