The Impacts of Asparagus Extract Fractions on Growth and Fumonisins Biosynthesis in Fusarium Proliferatum
Abstract
1. Introduction
2. Results
2.1. Fumonisins
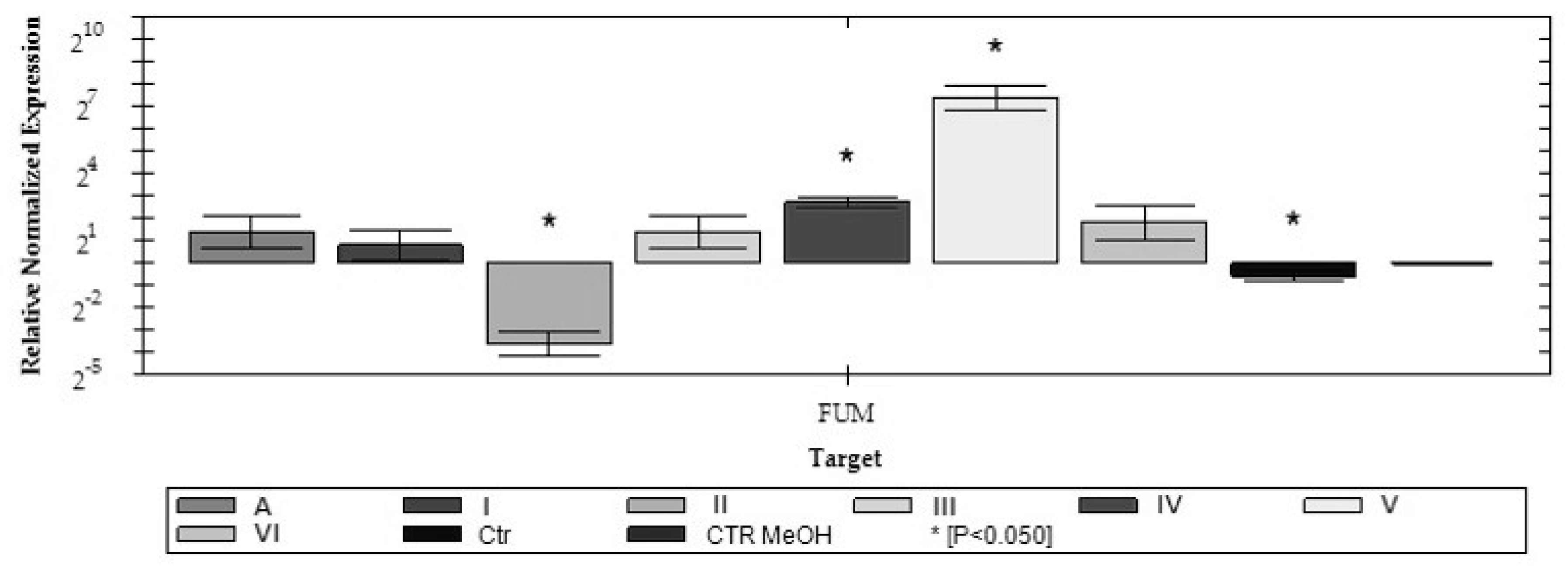
2.2. Analysis of FUM1 Gene Expression
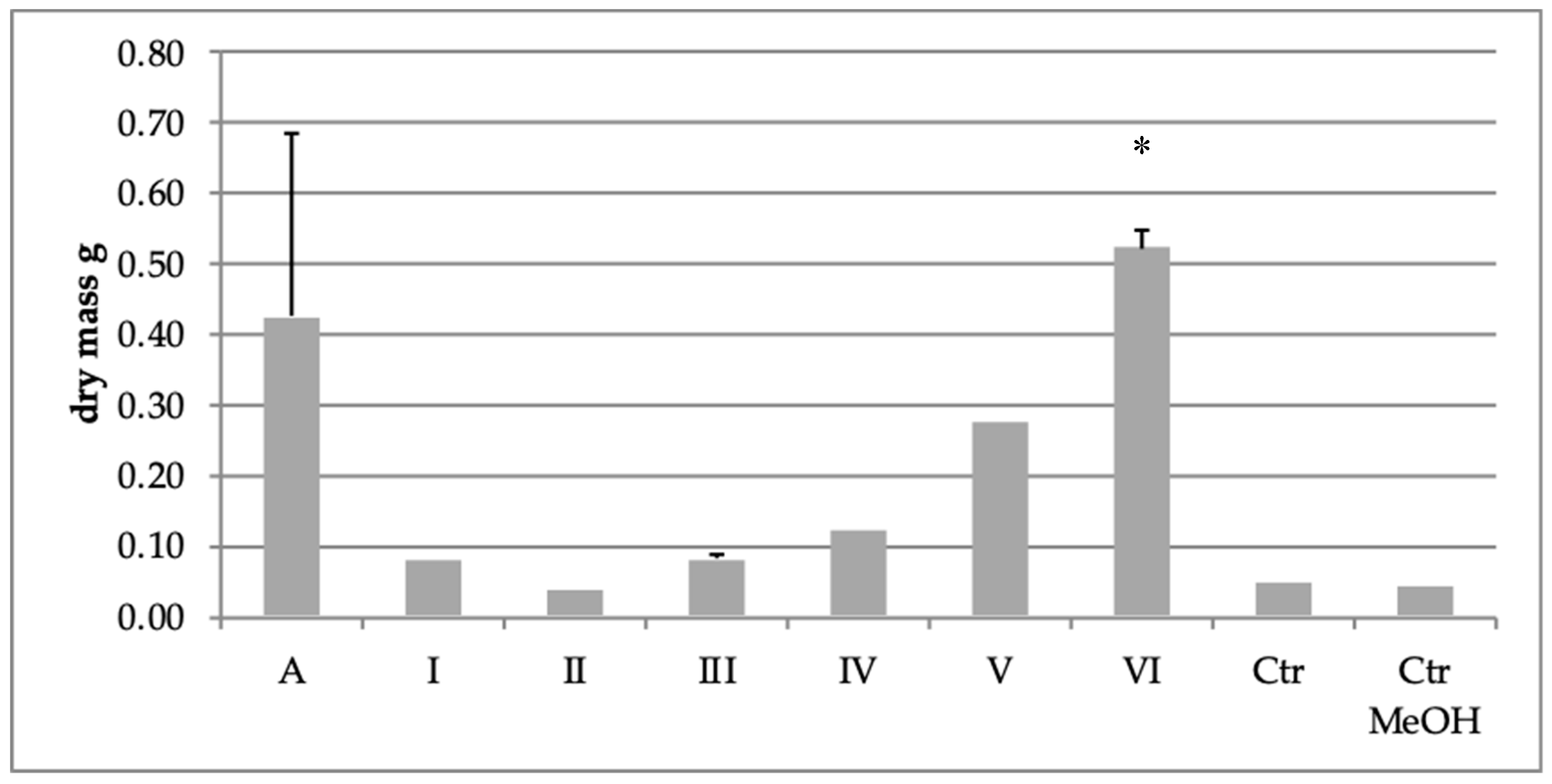
2.3. Effect of Extract Fraction on Fungal Biomass
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Fungal Strain and Culture Conditions
5.2. Extract Preparation
5.3. Fraction Preparation
5.4. Fumonisins Quantification
5.5. Expression Analysis of FUM1 by RT-qPCR
5.6. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Fuentes Alventosa, J.M.; Moreno Rojas, J.M. Bioactive compounds in Asparagus and impact on storage and processing. In Processing and impact on active components in food; Academic Press: Cambridge, MA, USA, 2015; pp. 103–110. [Google Scholar]
- Negi, J.S.; Singh, P.; Joshi, G.P.; Rawat, M.S.; Bisht, V.K. Chemical consituents of Asparagus. Pharmacogn. Rev. 2010, 4, 215–220. [Google Scholar] [PubMed]
- Hafizur, R.M.; Kabir, N.; Chishti, S. Asparagus officinalis extract controls blood glucose by improving insulin secretion and β-cell function in streptozotocin-induced type 2 diabetic rats. Br. J. Nutr. 2012, 108, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Chin, C.-K.; Ho, C.-T.; Ma, W.; Garrison, S.A.; Huang, M.-T. Anti-tumor activity of the crude saponins obtained from Asparagus. Cancer Lett. 1996, 104, 31–36. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Zhao, J.; Zhang, W.; Pang, X. Saponins extracted from by-product of Asparagus officinalis L. suppress tumour cell migration and invasion through targeting Rho GTPase signalling pathway. J. Sci. Food Agric. 2013, 93, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Shimoyamada, M.; Suzuki, M.; Maruyama, M.; Watanabe, K. An Antifungal Saponin from White Asparagus (Asparagus officinalis L) Bottoms. J. Sci. Food Agric. 1996, 72, 430–434. [Google Scholar] [CrossRef]
- Shimoyamada, M.; Suzuki, M.; Sonta, H.; Maruyama, M.; Okubo, K. Antifungal Activity of the Saponin Fraction Obtained from Asparagus officinalis L. and Its Active Principle. Agric. Boil. Chem. 1990, 54, 2553–2557. [Google Scholar] [CrossRef][Green Version]
- Zhu, X.; Zhang, W.; Zhao, J.; Wang, J.; Qu, W. Hypolipidaemic and hepatoprotective effects of ethanolic and aqueous extracts from Asparagus officinalis L. by-products in mice fed a high-fat diet. J. Sci. Food Agric. 2010, 90, 1129–1135. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, W.; Pang, X.; Wang, J.; Zhao, J.; Qu, W. Hypolipidemic Effect of n-Butanol Extract from Asparagus officinalis L. in Mice fed a High-fat Diet. Phytotherapy Res. 2011, 25, 1119–1124. [Google Scholar] [CrossRef]
- Elmer, W.H. The Economically Important Diseases of Asparagus in the United States. Plant Heal. Prog. 2001, 2, 13. [Google Scholar] [CrossRef]
- Pontaroli, A.C.; Camadro, E.L. Increasing resistance to Fusarium crown and root rot in asparagus by gametophyte selection. Euphytica 2001, 122, 343–350. [Google Scholar] [CrossRef]
- Stępień, Ł.; Waśkiewicz, A.; Urbaniak, M. Wildly growing asparagus (Asparagus officinalis L.) hosts pathogenic Fusarium species and accumulates their mycotoxins. Microb. Ecol. 2016, 71, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Vujanovic, V.; Hamel, C.; Yergeau, E.; St-Arnaud, M. Biodiversity and Biogeography of Fusarium Species from Northeastern North American Asparagus Fields Based on Microbiological and Molecular Approaches. Microb. Ecol. 2006, 51, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Elmer, W.H. Management of Fusarium crown and root rot of asparagus. Crop. Prot. 2015, 73, 2–6. [Google Scholar] [CrossRef]
- Lumsangkul, C.; Chiang, H.-I.; Lo, N.-W.; Fan, Y.-K.; Ju, J.-C. Developmental Toxicity of Mycotoxin Fumonisin B1 in Animal Embryogenesis: An Overview. Toxins 2019, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Berkey, R.; Bendigeri, D.; Xiao, S. Sphingolipids and Plant Defense/Disease: The “Death” Connection and Beyond. Front. Plant Sci. 2012, 3, 68. [Google Scholar] [CrossRef]
- Merrill Jr., A. H. Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem. Rev. 2011, 111, 6387–6422. [Google Scholar] [CrossRef]
- Merrill, A.H.; Sullards, M.C.; Wang, E.; Voss, K.A.; Riley, R.T. Sphingolipid metabolism: Roles in signal transduction and disruption by fumonisins. Environ. Heal. Perspect. 2001, 109, 283–289. [Google Scholar]
- Zoeller, M.; Stingl, N.; Krischke, M.; Fekete, A.; Waller, F.; Berger, S.; Mueller, M.J. Lipid Profiling of the Arabidopsis Hypersensitive Response Reveals Specific Lipid Peroxidation and Fragmentation Processes: Biogenesis of Pimelic and Azelaic Acid1[C][W]. Plant Physiol. 2012, 160, 365–378. [Google Scholar] [CrossRef]
- Salehi, F.; Behboudi, H.; Kavoosi, G.; Ardestani, S.K. Oxidative DNA damage induced by ROS-modulating agents with the ability to target DNA: A comparison of the biological characteristics of citrus pectin and apple pectin. Sci. Rep. 2018, 8, 13902. [Google Scholar] [CrossRef]
- Xing, F.; Li, Z.; Sun, A.; Xing, D. Reactive oxygen species promote chloroplast dysfunction and salicylic acid accumulation in fumonisin B1-induced cell death. FEBS Lett. 2013, 587, 2164–2172. [Google Scholar] [CrossRef]
- Stone, J.M.; Heard, J.E.; Asai, T.; Ausubel, F.M. Simulation of Fungal-Mediated Cell Death by Fumonisin B1 and Selection of Fumonisin B1–Resistant (fbr) Arabidopsis Mutants. Plant Cell 2000, 12, 1811–1822. [Google Scholar] [PubMed]
- Waśkiewicz, A.; Irzykowska, L.; Drzewiecka, K.; Bocianowski, J.; Dobosz, B.; Weber, Z.; Karolewski, Z.; Krzyminiewski, R.; Goliński, P. Plant-pathogen interactions during infection process of asparagus with Fusarium spp. Open Life Sci. 2013, 8, 1065–1076. [Google Scholar] [CrossRef]
- Rocheleau, H.; Al-Harthi, R.; Ouellet, T. Degradation of salicylic acid by Fusarium graminearum. Fungal Boil. 2019, 123, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Naumann, T.A.; Vaughan, M.M.; McCormick, S.; Usgaard, T.; Kelly, A.; Ward, T.J. Characterization of a Fusarium graminearum Salicylate Hydroxylase. Front. Microbiol. 2019, 9, 3129. [Google Scholar] [CrossRef]
- Pueppke, S.G.; VanEtten, H.D. Accumulation of pisatin and three additional antifungal pterocarpans in Fusarium solani-infected tissues of Pisum sativum. Physiol. Plant Pathol. 1976, 8, 51–61. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Minic, Z.; Abdoli, A. The phytoalexin camalexin induces fundamental changes in the proteome of Alternaria brassicicola different from those caused by brassinin. Fungal Boil. 2014, 118, 83–93. [Google Scholar] [CrossRef]
- Houillé, B.; Papon, N.; Boudesocque, L.; Bourdeaud, E.; Besseau, S.; Courdavault, V.; Enguehard-Gueiffier, C.; Delanoue, G.; Guérin, L.; Bouchara, J.-P.; et al. Antifungal Activity of Resveratrol Derivatives against Candida Species. J. Nat. Prod. 2014, 77, 1658–1662. [Google Scholar] [CrossRef]
- Stępień, Ł.; Waśkiewicz, A.; Wilman, K. Host extract modulates metabolism and fumonisin biosynthesis by the plant-pathogenic fungus Fusarium proliferatum. Int. J. Food Microbiol. 2015, 193, 74–81. [Google Scholar] [CrossRef]
- Górna, K.; Pawłowicz, I.; Waśkiewicz, A.; Stepien, L. Fusarium proliferatum strains change fumonisin biosynthesis and accumulation when exposed to host plant extracts. Fungal Boil. 2016, 120, 884–893. [Google Scholar] [CrossRef]
- Górna, K.; Perlikowski, D.; Kosmala, A.; Stępień, Ł. Host extracts induce changes in the proteome of plant pathogen Fusarium proliferatum. Fungal Boil. 2017, 121, 676–688. [Google Scholar] [CrossRef]
- Naima, R.; Oumam, M.; Hannache, H.; Sesbou, A.; Charrier, B.; Pizzi, A.; El Bouhtoury, F.C. Comparison of the impact of different extraction methods on polyphenols yields and tannins extracted from Moroccan Acacia mollissima barks. Ind. Crop. Prod. 2015, 70, 245–252. [Google Scholar] [CrossRef]
- Fuentes-Alventosa, J.; Jaramillo-Carmona, S.; Rodríguez-Gutiérrez, G.; Guillén-Bejarano, R.; Jiménez-Araujo, A.; Fernández-Bolaños, J.; Rodríguez-Arcos, R. Preparation of bioactive extracts from asparagus by-product. Food Bioprod. Process. 2013, 91, 74–82. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, J.H.; Yu, I.H.; Gorinstein, S.; Bae, J.H.; Ku, Y.G. Bioactive Compounds, Antioxidant and Binding Activities and Spear Yield of Asparagus officinalis L. Plant Foods Hum. Nutr. 2014, 69, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Jiménez, G.M.; Cortez-Rocha, M.O.; Rosas-Burgos, E.C.; Burgos-Hernández, A.; Plascencia-Jatomea, M.; Cinco-Moroyoqui, F.J. Antifungal activity of plant methanolic extracts against Fusarium verticillioides (Sacc.) Nirenb. and Fumonisin B1 Production. Rev Mex Fitopatol 2007, 25, 134–142. [Google Scholar]
- Thippeswamy, S.; Umesh, A.R. Effect of plant extracts on inhibition of Fusarium verticillioides growth and its toxin fumonisin B1 production. J Agri Sci Tech 2013, 9, 889–900. [Google Scholar]
- Salhi, N.; Saghir, S.A.M.; Terzi, V.; Brahmi, I.; Ghedairi, N.; Bissati, S. Antifungal Activity of Aqueous Extracts of Some Dominant Algerian Medicinal Plants. BioMed. Res. Int. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Riaz, T.; Khan, S.; Javaid, A. Antifungal activity of plant extracts against Fusarium oxysporum- the cause of corn-rot disease of Gladiolus. Mycopath 2008, 6, 13–15. [Google Scholar]
- Rongai, D.; Pulcini, P.; Pesce, B.; Milano, F. Antifungal activity of some botanical extracts on Fusarium oxysporum. Open Life Sci. 2015, 10, 409–416. [Google Scholar] [CrossRef]
- Truong, D.-H.; Nguyen, D.H.; Ta, N.T.A.; Bui, A.V.; Do, T.H.; Nguyen, H.C. Evaluation of the Use of Different Solvents for Phytochemical Constituents, Antioxidants, and In Vitro Anti-Inflammatory Activities of Severinia buxifolia. J. Food Qual. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Mahdi-Pour, B.; Jothy, S.L.; Latha, L.Y.; Chen, Y.; Sasidharan, S. Antioxidant activity of methanol extracts of different parts of Lantana camara. Asian Pac. J. Trop. Biomed. 2012, 12, 960–965. [Google Scholar] [CrossRef]
- Wong, P.; Kitts, D. Studies on the dual antioxidant and antibacterial properties of parsley (Petroselinum crispum) and cilantro (Coriandrum sativum) extracts. Food Chem. 2006, 97, 505–515. [Google Scholar] [CrossRef]
- Da Cruz-Silva, S.C.B.; Santos, K.S.; Matias, R.; Bono, J.A.M.; Ludwig, J. ANTIFUNGAL POTENTIAL OF EXTRACTS AND FRACTIONS OF Randia nitida LEAVES ON SOYBEAN PATHOGENS AND THEIR PHYTOCHEMISTRY. Rev. Caatinga 2016, 29, 594–602. [Google Scholar] [CrossRef]
- Sales, M.D.C.; Costa, H.B.; Fernandes, P.M.B.; Ventura, J.A.; Meira, D.D. Antifungal activity of plant extracts with potential to control plant pathogens in pineapple. Asian Pac. J. Trop. Biomed. 2016, 6, 26–31. [Google Scholar] [CrossRef]
- Pizzolitto, R.P.; Dambolena, J.S.; Zunino, M.P.; Larrauri, M.; Grosso, N.R.; Nepote, V.; Dalcero, A.M.; Zygadlo, J.A. Activity of natural compounds from peanut skins on Fusarium verticillioides growth and fumonisin B1 production. Ind. Crop. Prod. 2013, 47, 286–290. [Google Scholar] [CrossRef]
- López-Errasquín, E.; Vázquez, C.; Jiménez, M.; González-Jaén, M.T.; Escamilla, M.J. Real-Time RT-PCR assay to quantify the expression of fum1 and fum19 genes from the Fumonisin-producing Fusarium verticillioides. J. Microbiol. Methods 2007, 68, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Jurado, M.; Marín, P.; Callejas, C.; Moretti, A.; Vázquez, C.; González-Jaén, M.T. Genetic variability and Fumonisin production by Fusarium proliferatum. Food Microbiol. 2010, 27, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Jurado, M.; Marín, P.; Magan, N.; González-Jaén, M.T. Relationship between Solute and Matric Potential Stress, Temperature, Growth, and FUM1 Gene Expression in Two Fusarium verticillioides Strains from Spain. Appl. Environ. Microbiol. 2008, 74, 2032–2036. [Google Scholar] [CrossRef]
- Rocha, L.D.O.; Reis, G.M.; Da Silva, V.N.; Braghini, R.; Teixeira, M.M.G.; Corrêa, B. Molecular characterization and fumonisin production by Fusarium verticillioides isolated from corn grains of different geographic origins in Brazil. Int. J. Food Microbiol. 2011, 145, 9–21. [Google Scholar] [CrossRef]
- Rocha, L.O.; Barroso, V.M.; Andrade, L.J.; Pereira, G.H.A.; Ferreira-Castro, F.L.; Duarte, A.P.; Michelotto, M.D.; Corrêa, B. FUM Gene Expression Profile and Fumonisin Production by Fusarium verticillioides Inoculated in Bt and Non-Bt Maize. Front. Microbiol. 2016, 6, 41. [Google Scholar] [CrossRef]
- Medina, A.; Schmidt-Heydt, M.; Cárdenas-Chávez, D.L.; Parra, R.; Geisen, R.; Magan, N. Integrating toxin gene expression, growth and fumonisin B1 and B2 production by a strain of Fusarium verticillioides under different environmental factors. J. R. Soc. Interface 2013, 10, 1–12. [Google Scholar] [CrossRef]
- Battilani, P.; Formenti, S.; Ramponi, C.; Rossi, V. Dynamic of water activity in maize hybrids is crucial for fumonisin contamination in kernels. J. Cereal Sci. 2011, 54, 467–472. [Google Scholar] [CrossRef]
- Fanelli, F.; Schmidt-Heydt, M.; Haidukowski, M.; Geisen, R.; Logrieco, A.F.; Mulè, G.; Haidukowski, E.M. Influence of light on growth, fumonisin biosynthesis and FUM1 gene expression by Fusarium proliferatum. Int. J. Food Microbiol. 2012, 153, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, I.; Susca, A.; Mulè, G.; Ritieni, A.; Ferracane, R.; Marocco, A.; Battilani, P. Effects of temperature and water activity on FUM2 and FUM21 gene expression and fumonisin B production in Fusarium verticillioides. Eur. J. Plant Pathol. 2012, 134, 685–695. [Google Scholar] [CrossRef]
- Proctor, R.H.; Brown, D.W.; Plattner, R.D.; Desjardins, A.E. Co-expression of 15 contiguous genes delineates a fumonisin biosynthetic gene cluster in Gibberella moniliformis. Fungal Genet. Boil. 2003, 38, 237–249. [Google Scholar] [CrossRef]
- Brown, D.W.; Cheung, F.; Proctor, R.H.; Butchko, R.A.; Zheng, L.; Lee, Y.; Utterback, T.; Smith, S.; Feldblyum, T.; Glenn, A.E.; et al. Comparative analysis of 87,000 expressed sequence tags from the fumonisin-producing fungus Fusarium verticillioides. Fungal Genet. Boil. 2005, 42, 848–861. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Mochizuki, N.; Nagatomi, Y.; Toriba, A.; Hayakawa, K. Characterization of Fumonisin A-Series by High-Resolution Liquid Chromatography-Orbitrap Mass Spectrometry. Toxins 2014, 6, 2580–2593. [Google Scholar] [CrossRef] [PubMed]
- Kohut, G.; Ádám, A.L.; Fazekas, B.; Hornok, L. N-starvation stress induced FUM gene expression and fumonisin production is mediated via the HOG-type MAPK pathway in Fusarium proliferatum. Int. J. Food Microbiol. 2009, 130, 65–69. [Google Scholar] [CrossRef]
- Rosado-Álvarez, C.; Molinero-Ruiz, L.; Arcos, R.R.; Basallote-Ureba, M. Antifungal activity of asparagus extracts against phytopathogenic Fusarium oxysporum. Sci. Hortic. 2014, 171, 51–57. [Google Scholar] [CrossRef]
- Stępień, Ł.; Koczyk, G.; Waśkiewicz, A. Genetic and phenotypic variation of Fusarium proliferatum isolates from different host species. J Appl Genet 2011, 52, 487–496. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


| 0 | 2h | 24h | 3d | 5d | 7d | 9d | |
|---|---|---|---|---|---|---|---|
| Ctrl + H2O | 1.97 ± 0.52 | 13.88 ± 4.38 | 4.88 ± 0.07 | 0.34 ± 0.07 | 0.33 ± 0.06 | 0.19 ± 0.04 | 0.13 ± 0.04 |
| Ctrl + MeOH | 0.29 ± 0.07 | 4.26 ± 1.19 | 6.48 ± 1.94 | 0.71 ± 0.18 | 0.37 ± 0.08 | 0.12 ± 0.02 | 0.08 ± 0.01 |
| Extract | 15.30 ± 6.29 | 6.67 ± 1.25 | 7.86 ± 2.09 | 1.08 ± 0.27 | 1.31 ± 0.24 | 0.85 ± 0.17 | 1.35 ± 0.26 |
| Fraction I | 3.54 ± 1.08 | 6.23 ± 1.33 | 11.56 ± 1.71 | 2.88 ± 0.72 | 1.48 ± 0.22 | 0.85 ± 0.16 | 0.26 ± 0.02 |
| Fraction II | 4.18 ± 1.32 | 14.34 ± 3.71 | 8.70 ± 1.55 | 1.67 ± 0.46 | 1.02 ± 0.05 | 0.31 ± 0.22 | 0.23 ± 0.04 |
| Fraction III | 0.88 ± 0.21 | 3.07 ± 0.44 | 6.07 ± 1.57 | 1.03 ± 0.30 | 0.72 ± 0.12 | 0.81 ± 0.22 | 0.49 ± 0.11 |
| Fraction IV | 1.98 ± 0.60 | 4.24 ± 1.02 | 3.37 ± 0.89 | n.d. | n.d. | 0.06 ± 0.02 | n.d. |
| Fraction V | 1.01 ± 0.28 | 5.16 ± 1.33 | 4.91 ± 1.42 | 6.38 ± 1.88 | 2.58 ± 0.00 | 1.69 ± 0.35 | 2.30 ± 0.61 |
| Fraction VI | 0.20 ± 0.03 | 5.26 ± 2.32 | 2.64 ± 0.68 | 0.82 ± 0.12 | 1.01 ± 0.24 | 1.19 ± 0.38 | 0.09 ± 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witaszak, N.; Lalak-Kańczugowska, J.; Waśkiewicz, A.; Stępień, Ł. The Impacts of Asparagus Extract Fractions on Growth and Fumonisins Biosynthesis in Fusarium Proliferatum. Toxins 2020, 12, 95. https://doi.org/10.3390/toxins12020095
Witaszak N, Lalak-Kańczugowska J, Waśkiewicz A, Stępień Ł. The Impacts of Asparagus Extract Fractions on Growth and Fumonisins Biosynthesis in Fusarium Proliferatum. Toxins. 2020; 12(2):95. https://doi.org/10.3390/toxins12020095
Chicago/Turabian StyleWitaszak, Natalia, Justyna Lalak-Kańczugowska, Agnieszka Waśkiewicz, and Łukasz Stępień. 2020. "The Impacts of Asparagus Extract Fractions on Growth and Fumonisins Biosynthesis in Fusarium Proliferatum" Toxins 12, no. 2: 95. https://doi.org/10.3390/toxins12020095
APA StyleWitaszak, N., Lalak-Kańczugowska, J., Waśkiewicz, A., & Stępień, Ł. (2020). The Impacts of Asparagus Extract Fractions on Growth and Fumonisins Biosynthesis in Fusarium Proliferatum. Toxins, 12(2), 95. https://doi.org/10.3390/toxins12020095






