Stability of Mycotoxins in Individual Stock and Multi-Analyte Standard Solutions
Abstract
1. Introduction
2. Results and Discussion
2.1. Stability of Diluted Individual Stock Standard Solutions
2.1.1. Aflatoxins and Sterigmatocystin
2.1.2. Type A Trichothecenes
2.1.3. Type B Trichothecenes
2.1.4. Fumonisins
2.1.5. Zearalenone and Its Derivatives
2.1.6. Ochratoxin A
2.1.7. Alternaria Toxins
2.1.8. Enniatins A and B, Beauvericin
2.1.9. Moniliformin, Mycophenolic Acid, Citrinin, Citreoveridin, Cyclopiazonic Acid
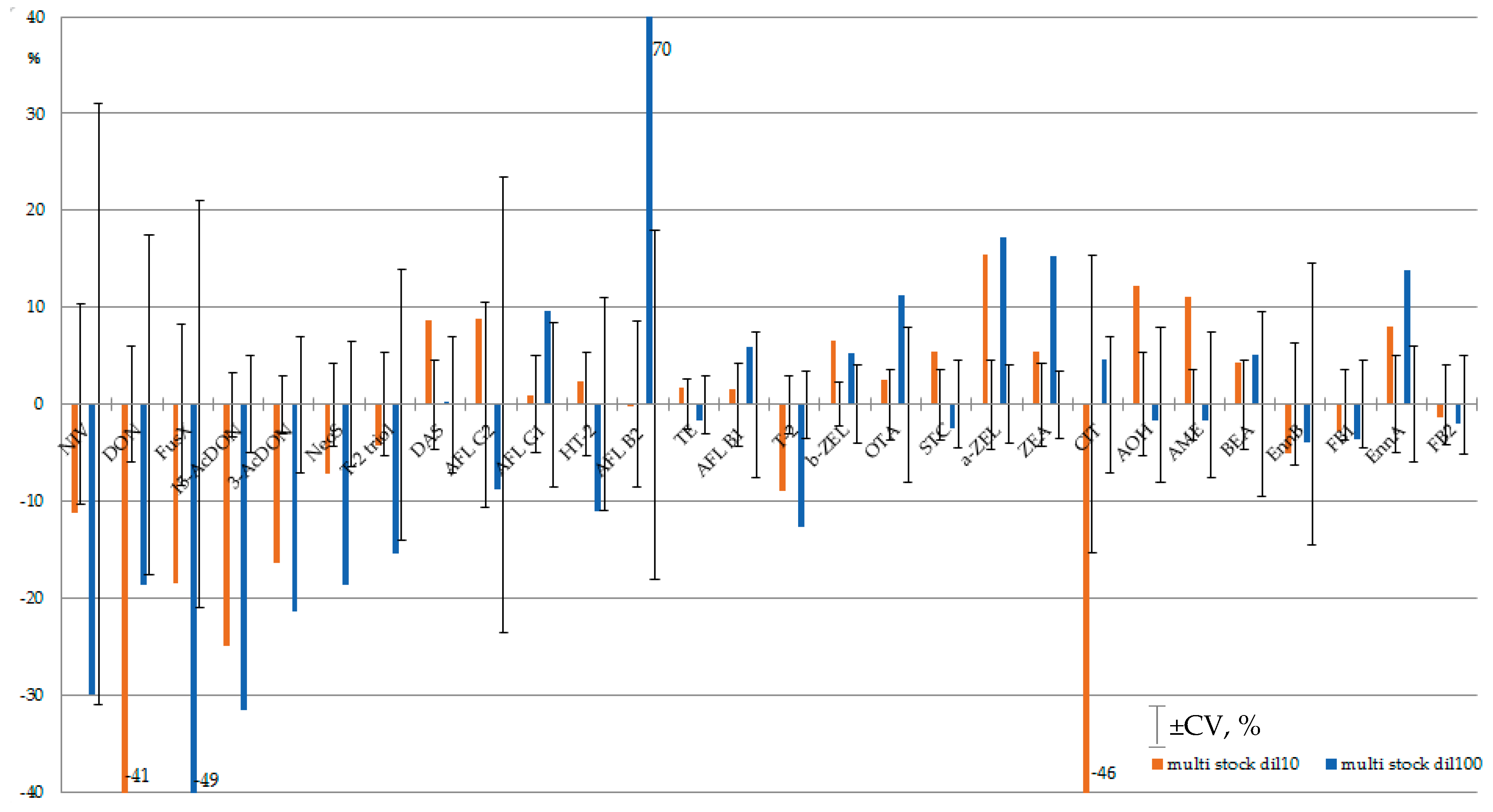
2.2. Multi-mycotoxin Diluted Standard Solutions Stability
3. Materials and Methods
3.1. Standards
3.2. Reagents and Materials
3.3. Stability Experiments
3.3.1. UV Spectrophotometry of Individual Standards
3.3.2. HPLC-MS/MS of Multi-Mycotoxin Standard Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Diao, E.; Li, X.; Zhang, Z.; Ma, W.; Ji, N.; Dong, H. Ultraviolet irradiation detoxification of aflatoxins. Trends Food Sci. Technol. 2015, 42, 64–69. [Google Scholar] [CrossRef]
- Magzoub, R.A.M.; Yassin, A.A.A.; Abdel-Rahim, A.M.; Gubartallah, E.A.; Miskam, M.; Saad, B.; Sabar, S. Photocatalytic detoxification of aflatoxins in Sudanese peanut oil using immobilized titanium dioxide. Food Control 2019, 95, 206–214. [Google Scholar] [CrossRef]
- Milani, J.; Maleki, G. Effects of processing on mycotoxin stability in cereals. J. Sci. Food Agric. 2014, 94, 2372–2375. [Google Scholar] [CrossRef] [PubMed]
- Ben Taheur, F.K.B.; Al Qurashi, Y.M.A.; Ben Salah-Abbes, J.; Chaieb, K. Review: Biotechnology of mycotoxins detoxification using microorganisms and enzymes. Toxicon 2019, 160, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.S.; Brites, C.; Pouca, A.V.; Barbosa, J.; Freitas, A. UHPLC-ToF-MS method for determination of multi-mycotoxins in maize: Development and validation. Current Res. Food Sci. 2019, 1, 1–7. [Google Scholar] [CrossRef]
- Al-Taher, F.C.J.; Zweigenbaum, J.; Lee, H.J.; Jackson, L.; Ryu, D. Detection and quantitation of mycotoxins in infant cereals in the U.S. market by LC-MS/MS using a stable isotope dilution assay. Food Control 2017, 72, 27–35. [Google Scholar] [CrossRef]
- Njumbe Ediage, E.; Van Poucke, C.; De Saeger, S. A multi-analyte LC-MS/MS method for the analysis of 23 mycotoxins in different sorghum varieties: The forgotten sample matrix. Food Chem. 2015, 177, 397–404. [Google Scholar] [CrossRef]
- Wei, D.; Wang, Y.; Jiang, D.; Feng, X.; Li, J.; Wang, M. Survey of Alternaria Toxins and Other Mycotoxins in Dried Fruits in China. Toxins 2017, 9. [Google Scholar] [CrossRef]
- García-Moraleja, A.; Mañes, J.; Ferrer, E. Development of a new method for the simultaneous determination of 21 mycotoxins in coffee beverages by liquid chromatography tandem mass spectrometry. Food Res. Int. 2015, 72, 247–255. [Google Scholar] [CrossRef]
- Andrade, P.D.; Dantas, R.R.; Caldas, E.D. Determination of multi-mycotoxins in cereals and of total fumonisins in maize products using isotope labeled internal standard and liquid chromatography/tandem mass spectrometry with positive ionization. J. Cromatogr. A 2017, 1490, 138–147. [Google Scholar] [CrossRef]
- Widestrand, J.; Pettersson, H. Effect of time, temperature and solvent on the stability of T-2 toxin, HT-2 toxin, deoxynivalenol and nivalenol calibrants. Food Addit.Contam. 2001, 18, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Visconti, A.; Doko, M.B.; Bottalico, C.; Schurer, B.; Boenke, A. Stability of fumonisins (FB1 and FB2) in solution. Food Addit.Contam. 1994, 11, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Sedova, I.B.; Kiseleva, M.G.; Zakharova, L.P.; Tutelyan, V.A. Toxicological and Hygienic Characteristics of Mycotoxin Sterigmatocystin and Methods for Its Determination in Food Products. Hygiene Sanit. 2019, 98(1), 105–117. [Google Scholar] [CrossRef]
- Scientific Opinion on the risk for public and animal health related to the presence of sterigmatocystin in food and feed. EFSA J. 2013, 11(6), 3254. [CrossRef]
- Nesheim, S.; Stack, M.E. Preparation of Mycotoxin Standards. In Mycotoxins Protocols; Trucksess, M.W., Pohland, A.E., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2001; Volume 157, pp. 31–36. [Google Scholar]
- Garcia, M.E.; Blanco, J.L.; Suarez, G. Aflatoxins B, and G1 solubility in standard solutions and stability during cold storage. Mycotoxin Res. 1994, 10, 97–100. [Google Scholar]
- Septien, I.; Cutuli, M.T.; Garcia, M.E.; Suarez, G.; Blanco, J.L. Solubility and stability of sterigmatocystin in different organic solvents. Toxicon 1993, 31, 1337–1340. [Google Scholar] [CrossRef]
- Beaver, R.W. Degradation of Aflatoxins in Common HPLC Solvents. J. High. Resolut. Chromatogr. 1990, 13, 833–835. [Google Scholar] [CrossRef]
- Diaz, G.J.; Cepeda, S.M.; Martos, P.A. Stability of Aflatoxins in Solution. J. AOAC Int. 2012, 95, 1084–1088. [Google Scholar] [CrossRef]
- Arcella, D.; Gergelova, P.; Innocenti, M.L.; Steinkellner, H. Human and animal dietary exposure to T-2 and HT-2 toxin. EFSA J. 2017, 15. [Google Scholar] [CrossRef]
- Schuhmacher-Wolz, U.; Heine, K.; Schneider, K. Report on toxicity data on trichothecene mycotoxins HT-2 and T-2 toxins. CT/EFSA/CONTAM/2010/03. 2010. [Google Scholar] [CrossRef]
- TR CU 015/2011. Customs Union Technical Regulation on Safety of Grain; Moscow, Russia, 2011; p. 37. [Google Scholar]
- Bennet, G.A.; Shotwell, O.L. Criteria for Determining Purity of Fusarium Mycotoxins. J. AOAC Int. 1990, 73, 270–275. [Google Scholar] [CrossRef]
- Schothorst, R.C.; Jekel, A.A.; Van Egmond, H.P.; De Mul, A.; Boon, P.E.; Van Klaveren, J.D. Determination of trichothecenes in duplicate diets of young children by capillary gas chromatography with mass spectrometric detection. Food Addit. Contam. 2005, 22, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Flores-Flores, M.E.; Gonzalez-Penas, E. Development and validation of a high performance liquid chromatographic-mass spectrometry method for the simultaneous quantification of 10 trichothecenes in ultra-high temperature processed cow milk. J. Chromatogr. A 2015, 1419, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.D.; Chu, F.S. Instability of some trichothecenes in methanol. J. AOAC Int. 1986, 69, 902–903. [Google Scholar] [CrossRef]
- Duffy, M.J.; Reid, R.S. Measurement of the Stability of T-2 Toxin in Aqueous Solution. Chem. Res. Toxicol. 1993, 6, 524–529. [Google Scholar] [CrossRef]
- Trusal, L.R. Stability of T-2 Mycotoxin in Aqueous Media. Appl. Environ. Microbiol. 1985, 50, 1311–1312. [Google Scholar] [CrossRef]
- Breidbach, A.; Bouten, K.; Kröger-Negiota, K.; Stroka, J.; Ulberth, F. LC-MS Based Method of Analysis for the Simultaneous Determination of four Mycotoxins in Cereals and Feed. Results of a collaborative study. European Commission. Joint Research Centre. Institute for Reference Material and Measurement, 2013; pp. 1–80. Available online: https://ec.europa.eu/jrc/sites/jrcsh/files/Fusarium%20toxins_2012.pdf. [CrossRef]
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: From simple to complex mycotoxins. Toxins 2011, 3(7), 802–814. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15. [Google Scholar] [CrossRef]
- The Commission of the European Communities. COMMISSION REGULATION (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- Scott, P.M.; Lawrence, G.A.; Telli, A.; Iyengar, J.R. Preparation of Deoxynivalenol (Vomitoxin) from Field-Inoculated Corn. J. AOAC Int. 1984, 67, 32–34. [Google Scholar] [CrossRef]
- Shepherd, M.J.; Gilbert, J. Long-Term Storage Stability of Deoxynivalenol Standard Reference Solutions. J. Agric. Food Chem. 1988, 36, 305–308. [Google Scholar] [CrossRef]
- Visconti, A.; Bottalico, A. Detection of Fusarium Trichothecenes (Nivalenol, Deoxynivalenol, Fusarenone and 3-Acetyldeoxynivalenol) by High-Performance Liquid Chromatography. Chromatographia. 1983, 17, 97–100. [Google Scholar] [CrossRef]
- Kotal, F.; Radova, Z. A Simple Method for Determination of Deoxynivalenol in Cereals and Flours. Czech. J. Food Sci. 2002, 20, 63–68. [Google Scholar] [CrossRef]
- Ok, H.E.; Lee, S.Y.; Chun, H.S. Occurrence and simultaneous determination of nivalenol and deoxynivalenol in rice and bran by HPLC-UV detection and immunoaffinity cleanup. Food Control 2018, 87, 53–59. [Google Scholar] [CrossRef]
- Yang, D.; Geng, Z.M.; Yao, J.B.; Zhang, X.; Zhang, P.P.; Ma, H.X. Simultaneous determination of deoxynivalenol, and 15- and 3-acetyldeoxynivalenol in cereals by HPLC-UV detection. World Mycotoxin J. 2013, 6, 117–125. [Google Scholar] [CrossRef]
- Krska, R.; Schothorst, R.C.; van Egmond, H.P.; Josephs, R.D.; Lepschy, J.; Pettersson, H.; Chan, D.; Berthiller, F.; Schuhmacher, R.; Kandler, W.; et al. Processing and purity assessment of standards for the analysis of type-B trichothecene mycotoxins. Anal. Bioanal. Chem. 2005, 382, 1848–1858. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Sanchis, V.; Ramos, A.J.; Marin, S. Thermal stability and kinetics of degradation of deoxynivalenol, deoxynivalenol conjugates and ochratoxin A during baking of wheat bakery products. Food Chem. 2015, 178, 276–286. [Google Scholar] [CrossRef]
- Vidal, A.; Bendicho, J.; Sanchis, V.; Ramos, A.J.; Marin, S. Stability and kinetics of leaching of deoxynivalenol, deoxynivalenol-3-glucoside and ochratoxin A during boiling of wheat spaghettis. Food Res. Int. 2016, 85, 182–190. [Google Scholar] [CrossRef]
- Jensen, T.; de Boevre, M.; Preußke, N.; de Saeger, S.; Birr, T.; Verreet, J.-A.; Sönnichsen, F.D. Evaluation of High-Resolution Mass Spectrometry for the Quantitative Analysis of Mycotoxins in Complex Feed Matrices. Toxins 2019, 11, 531. [Google Scholar] [CrossRef]
- Lauren, D.R.; Smith, W.A. Stability of the fusarium mycotoxins nivalenol, deoxynivalenol and zearalenone in ground maize under typical cooking environments. Food Add. Contam. 2001, 18, 1011–1016. [Google Scholar] [CrossRef]
- He, J.; Zhou, T.; Young, J.C.; Boland, G.J.; Scott, P.M. Chemical and biological transformations for detoxification of trichothecene mycotoxins in human and animal food chains: A review. Trends Food Sci. Tech. 2010, 21, 67–76. [Google Scholar] [CrossRef]
- Bucheli, T.D.; Wettstein, F.E.; Hartmann, N.; Erbs, M.; Vogelsang, S.; Forrer, H.-R.; Schwarzenbach, R.P. Fusarium Mycotoxins: Overlooked Aquatic Micropollutants? J. Agric. Food Chem. 2008, 56, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on Agriculture, Food, and Human Health and their Management Strategies. Toxins 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Ndube, N.; van der Westhuizen, L.; Shephard, G.S. Determination of fumonisins in maize by HPLC with ultraviolet detection of o-phthaldialdehyde derivatives. Mycotoxin Res. 2009, 25, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Sedova, I.B.; Kiseleva, M.G.; Zakharova, L.P.; Eller, K.I.; Tutel’yan, V.A. Optimization of Conditions for the Determination of Fuminosins B1 and B2 in Corn and Its Products of Its Processing by High-Performance Liquid Chromatography. J. Anal. Chem. 2004, 59, 730–736. [Google Scholar] [CrossRef]
- Chilaka, C.A.; De Boevre, M.; Atanda, O.O.; De Saeger, S. Stability of fumonisin B1, deoxynivalenol, zearalenone, and T-2 toxin during processing of traditional Nigerian beer and spices. Mycotoxin Res. 2018, 34, 229–239. [Google Scholar] [CrossRef]
- Jackson, L.S.; Hlywka, J.J.; Kannaki, R.S.; Bullerman, L.B.; Musser, S.M. Effects of Time, Temperature, and pH on the Stability of Fumonisin B1 in an Aqueous Model System. J. Agric. Food Chem. 1996, 44, 906–912. [Google Scholar] [CrossRef]
- Humpf, H.U.; Voss, K.A. Effects of thermal food processing on the chemical structure and toxicity of fumonisin mycotoxins. Mol. Nutr. Food Res. 2004, 48, 255–269. [Google Scholar] [CrossRef]
- Scientific Opinion on the risks for public health related to the presence of zearalenone in food. EFSA J. 2011, 9(6), 2197. [CrossRef]
- Zinedine, A.; Soriano, J.M.; Molto, J.C.; Manes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chemical Toxicol. 2007, 45(1), 1–18. [Google Scholar] [CrossRef]
- Ryu, D.; Hanna, M.A.; Eskridge, K.M.; Bullerman, L.B. Heat Stability of Zearalenone in an Aqueous Buffered Model System. J. Agric. Food Chem. 2003, 51, 1746–1748. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Xie, S.; Xu, F.; Liu, A.; Wang, Y.; Chen, D.; Pan, Y.; Huang, L.; Peng, D.; Wang, X.; et al. Ochratoxin A: Toxicity, oxidative stress and metabolism. Food Chem Toxicol. 2018, 112, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.E.; Farag, M.M.; Soliman, K.M.; Abdel-Samed, A.K.M.; Naguib, K.M. Evaluation of Methods Used To Determine Ochratoxin A in Coffee Beans. J. Agric. Food Chem. 2007, 55, 9576–9580. [Google Scholar] [CrossRef] [PubMed]
- Vargas, E.A.; Dos Santos, E.A.; Pittet, A. Determination of Ochratoxin A in Green Coffee by Immunoaffinity Column Cleanup and Liquid Chomatography: Collaborative Study. J. AOAC Int. 2005, 88, 773–779. [Google Scholar] [CrossRef]
- Winnie, N.G.; Mankotia, M.; Pantazopolos, P.; Neil, R.J.; Scott, P.M.; Lau, B.P.-Y. Survey of Dry Pasta for Ochratoxin A in Canada. J. Food Prot. 2009, 72, 890–893. [Google Scholar]
- Tam, J.; Pantazopoulos, P.; Scott, P.M.; Moisey, J.; Dabeka, R.W.; Richard, I.D. Application of isotope dilution mass spectrometry: Determination of ochratoxin A in the Canadian Total Diet Study. Food Addit. Contam. Part. A Chem. Anal. Control. Expo. Risk Assess. 2011, 28, 754–761. [Google Scholar] [CrossRef]
- Lippolis, V.; Porricelli, A.C.R.; Cortese, M.; Suman, M.; Zanardi, S.; Pascale, M. Determination of Ochratoxin A in Rye and Rye-Based Products by Fluorescence Polarization Immunoassay. Toxins 2017, 9. [Google Scholar] [CrossRef]
- Igarashi, N.; Nakamura, M.; Watai, M. Liquid Chromatographic Method with Immunoaffinity Column Cleanup for Determination of Ochratoxin A in Barley: Collaborative Study. Mycotoxins. 2008, 58, 97–105. [Google Scholar] [CrossRef][Green Version]
- Liazid, A.; Palma, M.; Brigui, J.; Barroso, C.G. Investigation on Ochratoxin A stability using different extraction techniques. Talanta 2007, 71, 976–980. [Google Scholar] [CrossRef]
- Scientific Opinion on the risks for animal and public health related to the presence ofAlternariatoxins in feed and food. EFSA J. 2011, 9(10), 2407. [CrossRef]
- Fraeyman, S.; Croubels, S.; Devreese, M.; Antonissen, G. Emerging Fusarium and Alternaria Mycotoxins: Occurrence, Toxicity and Toxicokinetics. Toxins 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Zwickel, T.; Klaffke, H.; Richards, K.; Rychlik, M. Development of a high performance liquid chromatography tandem mass spectrometry based analysis for the simultaneous quantification of various Alternaria toxins in wine, vegetable juices and fruit juices. J. Chromatog. A 2016, 1455, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Asam, S.; Konitzer, K.; Schieberle, P.; Rychlik, M. Stable isotope dilution assays of alternariol and alternariol monomethyl ether in beverages. J. Agric. Food Chem. 2009, 57, 5152–5160. [Google Scholar] [CrossRef]
- Scientific Opinion on the risks to human and animal health related to the presence of beauvericin and enniatins in food and feed. EFSA J. 2014, 12(8), 3802. [CrossRef]
- Jestoi, M. Emerging fusarium-mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin: A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 21–49. [Google Scholar] [CrossRef]
- Nielsen, K. Fungal metabolite screening: Database of 474 mycotoxins and fungal metabolites for dereplication by standardised liquid chromatography–UV–mass spectrometry methodology. J. Chromatogr. A 2003, 1002, 111–136. [Google Scholar] [CrossRef]
- Taevernier, L.; Veryser, L.; Vandercruyssen, K.; D’Hondt, M.; Vansteelandt, S.; De Saeger, S.; De Spiegeleer, B. UHPLC-MS/MS method for the determination of the cyclic depsipeptide mycotoxins beauvericin and enniatins in in vitro transdermal experiments. J. Pharm. Biomed. Anal. 2014, 100, 50–57. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Risks to human and animal health related to the presence of moniliformin in food and feed. EFSA J. 2018, 16. [Google Scholar] [CrossRef]
- Scientific Opinion on the risks for public and animal health related to the presence of citrinin in food and feed. EFSA J. 2012, 10. [CrossRef]
- COMMISSION REGULATION (EU) 2019/1901 as regards maximum levels of citrinin in food supplements based on rice fermented with red yeast Monascus purpureus. 2019, 4.
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging Mycotoxins: Beyond Traditionally Determined Food Contaminants. J. Agric. Food Chem. 2017, 65(33), 7052–7070. [Google Scholar] [CrossRef] [PubMed]
- da Rocha, M.W.; Resck, I.S.; Caldas, E.D. Purification and full characterization of citreoviridin produced by Penicillium citreonigrum in Yeast Extract Sucrose (YES) medium. Food Addit. Contam. Part. A. Chem. Anal. Control. Expo. Risk. Assess 2014, 32, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Peromingo, B.; Rodriguez, M.; Nunez, F.; Silva, A.; Rodriguez, A. Sensitive determination of cyclopiazonic acid in dry-cured ham using a QuEChERS method and UHPLC-MS/MS. Food Chem. 2018, 263, 275–282. [Google Scholar] [CrossRef]
- Reinhard, H.; Zimmerli, B. Reversed-phase liquid chromatographic behavior of the mycotoxins citrinin and ochratoxin A. J. Chromatogr. A 1999, 862, 147–159. [Google Scholar] [CrossRef]
- Filek, G.; Lindner, W. Determination of the mycotoxin moniliformin in cereals by high performance liquid chromatography and fluorescence detection. J. Chromatogr. A 1996, 732, 291–298. [Google Scholar] [CrossRef]
- Lim, C.W.; Lai, K.Y.; Yeo, J.F.; Tai, S.H.; Chan, S.H. Quantitative assessment of moniliformin in cereals via alternative precipitation pathways, aided by LC-LIT-MS and LC-Q-TOF-MS. Food Chem. 2015, 174, 372–379. [Google Scholar] [CrossRef]
- Avula, B.; Cohen, P.A.; Wang, Y.H.; Sagi, S.; Feng, W.; Wang, M.; Zweigenbaum, J.; Shuangcheng, M.; Khan, I.A. Chemical profiling and quantification of monacolins and citrinin in red yeast rice commercial raw materials and dietary supplements using liquid chromatography-accurate QToF mass spectrometry: Chemometrics application. J. Pharm. Biomed. Anal. 2014, 100, 243–253. [Google Scholar] [CrossRef]
- Xu, B.; Wang, Q.; Lee, J.; Jia, X.; Sung, C.-K. HPLC analysis of citrinin in red yeast rice. Food Sci. Biotechnol. 2003, 12, 376–380. [Google Scholar]
- Monbaliu, S.; Van Poucke, C.; Van Peteghem, C.; Van Poucke, K.; Heungens, K.; De Saeger, S. Development of a multi-mycotoxin liquid chromatography/tandem mass spectrometry method for sweet pepper analysis. Rapid Commun. Mass Spectrom. 2009, 23, 3–11. [Google Scholar] [CrossRef]
- Slobodchikova, I.; Vuckovic, D. Liquid chromatography-high resolution mass spectrometry method for monitoring of 17 mycotoxins in human plasma for exposure studies. J. Chromatogr. A 2018, 1548, 51–63. [Google Scholar] [CrossRef]
- Sulyok, M.; Berthiller, F.; Krska, R.; Schuhmacher, R. Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Commun. Mass Spectrom. 2006, 20, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.S.; Zeng, J.; Mylott, W.; Arnold, M.; Waltrip, J.; Iacono, L.; Mariannino, T.; Stouffer, B. Liquid chromatography and tandem mass spectrometry for the quantitative determination of ixabepilone (BMS-247550, Ixempra) in human plasma: Method validation, overcoming curve splitting issues and eliminating chromatographic interferences from degradants. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2010, 878, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Fabregat-Cabello, N.; Zomer, P.; Sancho, J.V.; Roig-Navarro, A.F.; Mol, H.G.J. Comparison of approaches to deal with matrix effects in LC-MS/MS based determinations of mycotoxins in food and feed. World Mycotoxin J. 2016, 9, 149–161. [Google Scholar] [CrossRef]
- Furey, A.; Moriarty, M.; Bane, V.; Kinsella, B.; Lehane, M. Ion suppression; a critical review on causes, evaluation, prevention and applications. Talanta 2013, 115, 104–122. [Google Scholar] [CrossRef] [PubMed]

| Mycotoxin | Solvent | Conc., μg/mL | λmax/λref., nm | A (Average, n = 3) | ||
|---|---|---|---|---|---|---|
| Fresh | 10 Months | 14 Months | ||||
| AFL B1 | MeOH | 1 | 360/400 | 0.07 | 0.07 | 0.07 |
| AFL B2 | MeOH | 1 | 360/400 | 0.08 | 0.08 | 0.08 |
| AFL G1 | MeOH | 1 | 365/280 | 0.05 | 0.05 | 0.05 |
| AFL G2 | MeOH | 1 | 365/280 | 0.05 | 0.05 | 0.05 |
| STC | MeOH | 21.5 | 325/280 | 0.86 | 0.83 | 0.85 |
| Mycotoxin | Solvent | Conc., μg/mL | λmax/λref., nm | A (Average, n = 3) | CV, % | ||
|---|---|---|---|---|---|---|---|
| Fresh | 10 Months | 14 Months | |||||
| T-2 | MeOH | 50 | 203/250 | 0.72 | 0.99 (+38%) | 1.01 (+40%) | 17.9 |
| HT-2 | MeOH | 20 | 202/220 | 0.32 | 0.49 (+53%) | 1.20 (+275%) | 69.7 |
| T-2 triol | ACN | 200 | 199/250 | 2.85 | 2.74 (−4%) | 2.86 (+0,4%) | 2.4 |
| NeoS | MeOH | 200 | 208/250 | 1.94 | 2.00 (+3%) | 2.06 (+6%) | 3.0 |
| DAS | MeOH | 50 | 203/300 | 0.93 | 1.05 (+13%) | 1.07 (+15%) | 7.5 |
| Mycotoxin | Solvent | Conc., μg/mL | λmax/λref., nm | A (Average, n = 3) | CV, % | ||
|---|---|---|---|---|---|---|---|
| Fresh | 10 Months | 14 Months | |||||
| NIV | MeOH | 20 | 217/300 | 2.36 | 2.41(+2%) | 2.42(+3%) | 1.3 |
| ACN | 50 | 217/300 | 0.28 | 0.31(+11%) | 0.35(+25%) | 11.2 | |
| DON | MeOH | 50 | 218/260 | 0.76 | 0.82(+8%) | 0.81(+7%) | 4.0 |
| ACN | 50 | 218/260 | 0.99 | 4.17 | 2.52 | 62.1 | |
| 3-AcDON | MeOH | 50 | 217/300 | 2.85 | 2.74(−4%) | 2.86(+0.4%) | 2.4 |
| ACN | 50 | 217/300 | 1.05 | 1.07(+2%) | 0.95(−10%) | 6.3 | |
| 15-AcDON | MeOH | 50 | 221/300 | 1.08 | 1.13(+5%) | 1.16(+7%) | 3.6 |
| ACN | 50 | 219/300 | 1.04 | 1.05(+1%) | 1.07(+3%) | 1.5 | |
| FusX | MeOH | 50 | 216/300 | 1.22 | 1.23(+1%) | 1.25(+2%) | 1.2 |
| ACN | 50 | 218/300 | 1.09 | 1.11(+2%) | -- | 1.3 | |
| Mycotoxin | Solvent | Conc., μg/mL | λmax/λref., nm | A (Average, n = 3) | CV, % | ||
|---|---|---|---|---|---|---|---|
| Fresh | 10 Months | 14 Months | |||||
| ZEA | ACN | 5 | 235/400 | 0.95 | 0.94 | 0.97 | |
| 273/400 | 0.40 | 0.40 | 0.41 | 1.4 | |||
| 314/400 | 0.18 | 0.18 | 0.19 | ||||
| α-ZEL | ACN | 50 | 235/380 | 2.75 | 2.54 (−8%) | 2.28 (−17%) | |
| 272/380 | 1.29 | 1.15 (−11%) | 1.03 (−20%) | 11.3 | |||
| 315/380 | 0.59 | 0.51 (−14%) | 0.47 (−20%) | ||||
| β-ZEL | ACN | 50 | 239/380 | 2.97 | 2.64 (−11%) | 2.06 (−30%) | |
| 274/380 | 1.34 | 1.15 (−15%) | 0.88 (−34%) | 20.6 | |||
| 315/380 | 0.58 | 0.49 (−16%) | 0.36 (−38%) | ||||
| α-ZAL | ACN | 50 | 218/350 | 1.20 | 1.13 (−6%) | 0.95 (−21%) | |
| 264/350 | 0.59 | 0.48 (−19%) | 0.48 (−19%) | 13.3 | |||
| 302/350 | 0.24 | 0.19 (−21%) | 0.18 (−25%) | ||||
| β-ZAL | ACN | 50 | 218/350 | 1.89 | 1.94 (+3%) | 1.71 (−10%) | |
| 261/350 | 0.87 | 0.72 (−17%) | 0.71 (−18%) | 11.7 | |||
| 301/350 | 0.35 | 0.28 (−20%) | 0.27(−23%) | ||||
| Mycotoxin | Solvent | Conc., μg/mL | λmax/λref., nm | A (Average, n = 3) | CV, % | ||
|---|---|---|---|---|---|---|---|
| Fresh | 10 Months | 14 Months | |||||
| AOH | MeOH | 20 | 256/380 | 3.23 | 3.21 | 3.19 | |
| 300/380 | 0.81 | 0.81 | 0.82 | 0.7 | |||
| 340/380 | 0.82 | 0.81 | 0.82 | ||||
| AME | ACN | 10 | 256/380 | 2.71 | 2.73 | 2.77 | |
| 300/380 | 0.57 | 0.57 | 0.60 | 3.0 | |||
| 340/380 | 0.62 | 0.62 | 0.64 | ||||
| ALT | MeOH | 20 | 241/380 | 2.89 | in 4 months*: 2.90 | n.a. | |
| 279/380 | 1.02 | 1.03 | n.a. | 0.7 | |||
| 320/380 | 0.57 | 0.57 | n.a | ||||
| TE | MeOH | 20 | 206/350 | 2.13 | 2.29 | 2.27 | |
| 282/350 | 1.32 | 1.32 | 1.35 | 1.3 | |||
| Mycotoxin | Solvent | Conc., μg/mL | λmax/λref., nm | A (Average, n = 3) | CV, % | ||
|---|---|---|---|---|---|---|---|
| Fresh | 10 month | 14 month | |||||
| EnnA | MeOH | 20 | 206/300 | 1.03 | 1.01 | 1.03 | 1.1 |
| EnnB | MeOH | 20 | 207/300 | 0.73 | 0.74 | 0.72 | 1.4 |
| BEA | MeOH | 20 | 206/300 | 1.94 | 1.97 | 1.92 | 1.3 |
| Mycotoxin | Solvent | Conc., μg/mL | λmax/λref., nm | A (Average, n = 3) | CV, % | ||
|---|---|---|---|---|---|---|---|
| Fresh | 10 Months | 14 Months | |||||
| MO | MeOH | 5 | 227/245 | 0.60 | 0.66 (+10%) | 0.68 (+13%) | 6.4 |
| 259/245 | 0.03 | 0.01 | 0.02 | ||||
| CIT | MeOH | 20 | 211/280 | 2.28 | 2.37 (+4%) | 2.40 (+5%) | |
| 253/280 | 0.74 | 0.72 (−3%) | 0.73 (−1%) | 1.4 | |||
| 318/280 | 0.33 | 0.32 (−3%) | 0.33 | ||||
| MPA | ACN | 250 –storage * 2.5—UV-spec. | 214/350 | 2.45 | in 4 months**: 2.44 | n.a. | |
| 249/350 | 0.4 | 0.4 | n.a. | 0 | |||
| 303/350 | 0.13 | 0.13 | n.a | ||||
| CTV | MeOH | 20 | 233/500 | 0.70 | in 4 months: 0.68 | n.a. | |
| 286/500 | 1.18 | 1.17 | n.a. | 0.6 | |||
| 385/500 | 1.66 | 1.57 | n.a. | ||||
| CPA | MeOH | 20 | 222/350 | 2.68 | in 4 months: 2.75 | n.a. | |
| 281/350 | 1.43 | 1.44 | n.a. | 0.5 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiseleva, M.; Chalyy, Z.; Sedova, I.; Aksenov, I. Stability of Mycotoxins in Individual Stock and Multi-Analyte Standard Solutions. Toxins 2020, 12, 94. https://doi.org/10.3390/toxins12020094
Kiseleva M, Chalyy Z, Sedova I, Aksenov I. Stability of Mycotoxins in Individual Stock and Multi-Analyte Standard Solutions. Toxins. 2020; 12(2):94. https://doi.org/10.3390/toxins12020094
Chicago/Turabian StyleKiseleva, Mariya, Zakhar Chalyy, Irina Sedova, and Ilya Aksenov. 2020. "Stability of Mycotoxins in Individual Stock and Multi-Analyte Standard Solutions" Toxins 12, no. 2: 94. https://doi.org/10.3390/toxins12020094
APA StyleKiseleva, M., Chalyy, Z., Sedova, I., & Aksenov, I. (2020). Stability of Mycotoxins in Individual Stock and Multi-Analyte Standard Solutions. Toxins, 12(2), 94. https://doi.org/10.3390/toxins12020094





