Interactions of Destruxin A with Silkworms’ Arginine tRNA Synthetase and Lamin-C Proteins
Abstract
1. Introduction
2. Results
2.1. Interactions of Destruxin A with Three Proteins by BLI and CETSA Analysis
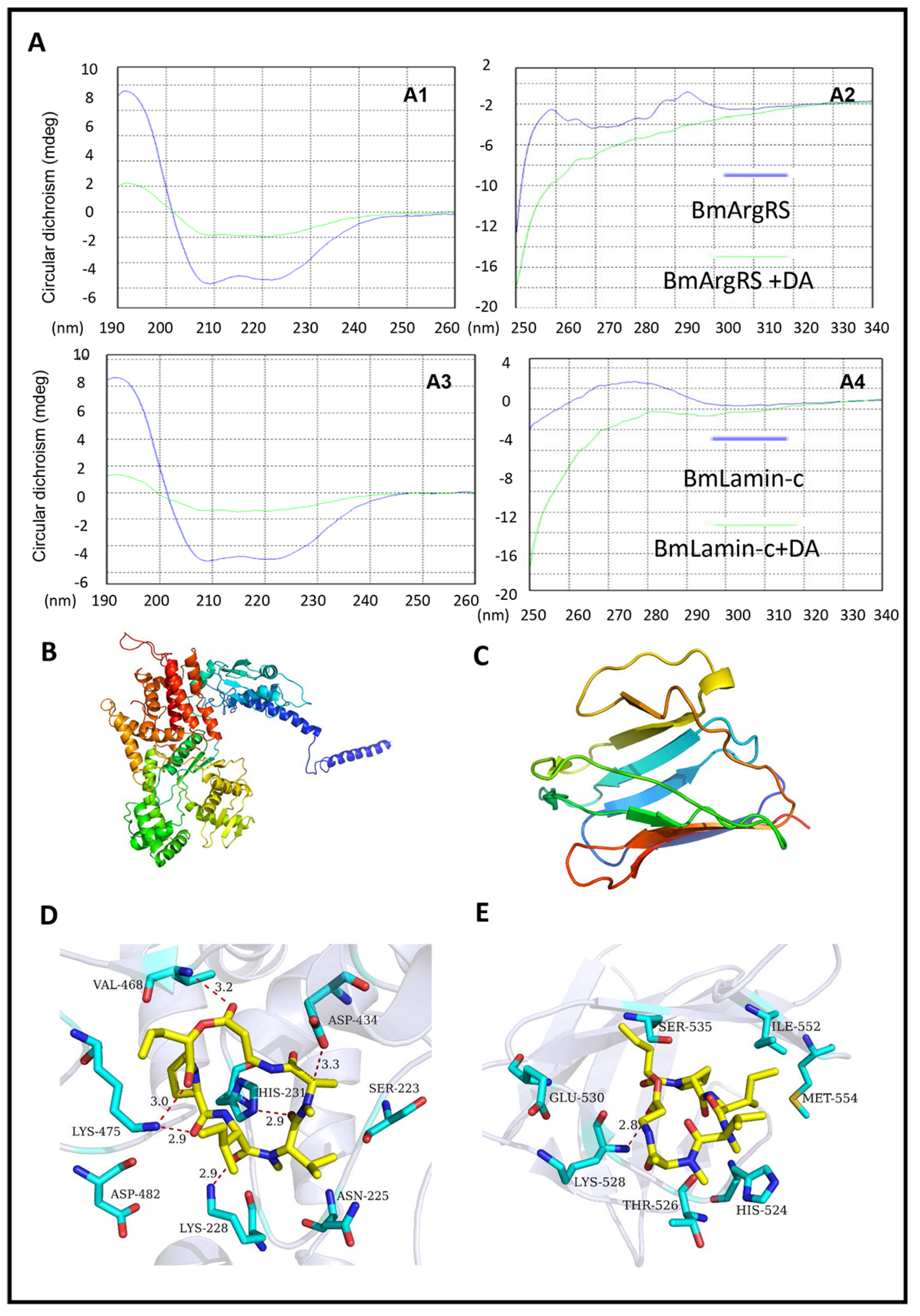
2.2. Key Sites of Interaction of DA with BmArgRS and BmLamin-C
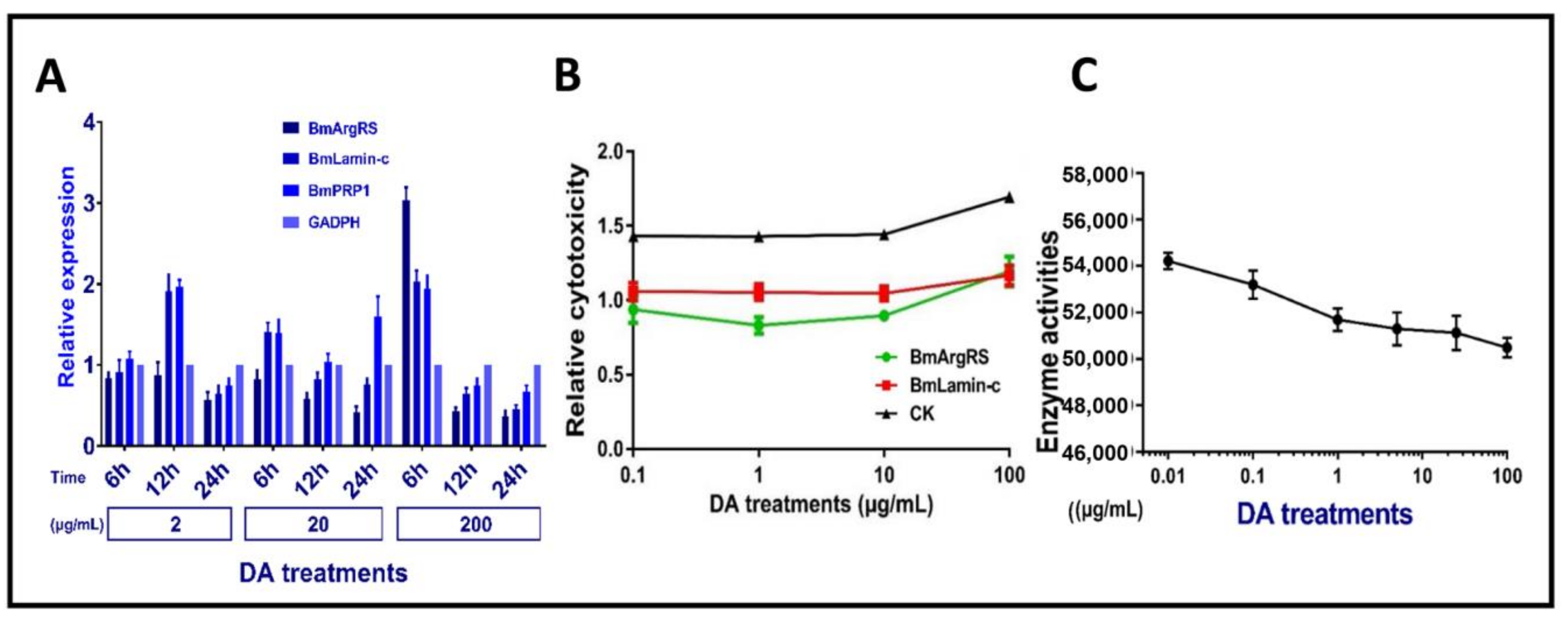
2.3. Gene Expression Levels of Three Proteins in Bm12 Cells
2.4. Changes of DA Cytotoxicity and BmArgRS Enzyme Activity in Bm12 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Destruxin A
4.2. Bio-Layer Interferometry (BLI)
4.3. Cellular Thermal Shift Assay (CETSA)
4.4. RNAi and Toxicity Assessment RNAi
4.5. Survey of Gene Expression
4.6. Homology Modeling and Molecular Docking
4.7. Circular Dichroism Spectrum
Author Contributions
Funding
Conflicts of Interest
References
- Pedras, M.S.C.; Zaharia, L.I.; Ward, D.E. The destruxins: Synthesis, biosynthesis, biotransformation, and biological activity. Phytochemistry 2002, 59, 579–596. [Google Scholar] [CrossRef]
- Liu, B.; Tzeng, Y. Development and applications of destruxins: A review. Biotechnol. Adv. 2012, 30, 1242–1254. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kang, Q.; Lu, Y.; Bai, L.; Wang, C. Unveiling the biosynthetic puzzle of destruxins in Metarhizium species. Proc. Nat. Acad. Sci. USA 2012, 109, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.Q.; Chen, X.R.; Hu, Q.-B. Effects of Destruxin A on Hemocytes Morphology of Bombyx mori. J. Integr. Agric. 2013, 12, 1042–1048. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Q.; Yu, X.; Ren, S. Effects of destruxins on free calcium and hydrogen ions in insect hemocytes. Insect Sci. 2014, 21, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; He, G.; Wang, J.; Hu, Q. The Effects of Destruxin A on Relish and Rel Gene Regulation to the Suspected Immune-Related Genes of Silkworm. Molecules 2016, 22, 41. [Google Scholar] [CrossRef]
- Zuela, N.; Bar, D.Z.; Gruenbaum, Y. Lamins in development, tissue maintenance and stress. EMBO Rep. 2012, 13, 1070–1078. [Google Scholar] [CrossRef]
- Wang, J.; Hu, W.; Hu, Q. BmTudor-sn Is a Binding Protein of Destruxin A in Silkworm Bm12 Cells. Toxins 2019, 11, 67. [Google Scholar] [CrossRef]
- Wang, J.; Wen, Q.; Hu, Q. Effects of Destruxin A on Silkworm’s Immunophilins. Toxins 2019, 11, 349. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, W.; Xiao, M.; Ou, S.; Hu, Q. Destruxin A induces and binds HSPs in Bombyx mori Bm12 cells. J. Agric. Food Chem. 2017, 65, 9849–9853. [Google Scholar] [CrossRef]
- Lomenick, B.; Hao, R.; Jonai, N.; Chin, R.M.; Aghajan, M.; Warburton, S.; Wang, J.; Wu, R.P.; Gomez, F.; Loo, J.A. Target identification using drug affinity responsive target stability (DARTS). Proc. Natl. Acad. Sci. USA 2009, 106, 21984–21989. [Google Scholar] [CrossRef] [PubMed]
- Al-Saaidi, R.; Peter, B. Do lamin A and lamin C have unique roles? Chromosoma 2015, 124, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bence, A.K.; Crooks, P.A. The mechanism of L-canavanine cytotoxicity: Arginyl tRNA synthetase as a novel target for anticancer drug discovery. J. Enzym. Inhib. Med. Chem. 2003, 18, 383. [Google Scholar] [CrossRef] [PubMed]
- Sloan, K.E.; Bohnsack, M.T. Unravelling the Mechanisms of RNA Helicase Regulation. Trends Biochem. Sci. 2018, 43, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Jankowsky, E. RNA helicases at work: Binding and rearranging. Trends Biochem. Sci. 2011, 36, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Xu, F. RNA Helicases and Stress Granules. Chin. J. Biochem. Mol. Biol. 2014, 30, 630–635. [Google Scholar]
- Ivanov, P.; Kedersha, N.; Anderson, P. Stress Granules and Processing Bodies in Translational Control. Cold Spring Harb. Perspect. Biol. 2018, 11, a032813. [Google Scholar] [CrossRef]
- Sree, K.S.; Padmaja, V. Destruxin from Metarhizium anisopliae induces oxidative stress effecting larval mortality of the polyphagous pest Spodoptera litura. J. Appl. Entomol. 2010, 132, 68–78. [Google Scholar] [CrossRef]
- Chhibber-Goel, J.; Joshi, S.; Sharma, A. Aminoacyl tRNA synthetases as potential drug targets of the Panthera pathogen Babesia. Parasit Vectors 2019, 12, 482. [Google Scholar] [CrossRef]
- Kim, E.Y.; Lee, J.G.; Lee, J.M.; Kim, A.; Yoo, H.C.; Kim, K.; Lee, M.; Lee, C.; P., N.; Han, G.; et al. Therapeutic effects of the novel Leucyl-tRNA synthetase inhibitor BC-LI-0186 in non-small cell lung cancer. Ther. Adv. Med Oncol. 2019, 11. [Google Scholar] [CrossRef]
- Buckner, F.S.; Ranade, R.M.; Gillespie, J.R.; Shibata, S.; Hulverson, M.A.; Zhang, Z.; Huang, W.; Choi, R.; Ochida, A.; Akao, Y.; et al. Optimization of Methionyl tRNA-Synthetase Inhibitors for Treatment of Cryptosporidium Infection. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Won, L.S.; Byeong Hoon, C.; Gyu, P.S.; Sunghoon, K. Aminoacyl-tRNA synthetase complexes: Beyond translation. J. Cell Sci. 2004, 117, 3725–3734. [Google Scholar]
- Vey, A.; Dumas, C. Mechanism of action of insecticidal mycotoxins of the group of destruxins. Toxicon 1996, 34, 1096. [Google Scholar] [CrossRef]
- Hu, Q.B.; Ren, S.X.; Wu, J.H.; Chang, J.M.; Musa, P.D. Investigation of destruxin A and B from 80 Metarhizium strains in China, and the optimization of cultural conditions for the strain MaQ10. Toxicon 2006, 48, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Abdiche, Y.; Dan, M.; Pinkerton, A.; Pons, J. Determining kinetics and affinities of protein interactions using a parallel real-time label-free biosensor, the Octet. Anal. Biochem. 2008, 377, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Martinez Molina, D.; Jafari, R.; Ignatushchenko, M.; Seki, T.; Larsson, E.A.; Dan, C.; Sreekumar, L.; Cao, Y.; Nordlund, P. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 2013, 341, 84–87. [Google Scholar] [CrossRef] [PubMed]


| Proteins | DA Con. (μM) | Response (nm) | Kon (1/Ms) 1 | Kdis (1/s) 2 | KD (M) 3 |
|---|---|---|---|---|---|
| BmArgRS | 25 | −0.0006 | 6.68 × 102 | 3.70 × 10−2 | 5.53 × 10−5 |
| 200 | 0.0093 | ||||
| 300 | 0.0192 | ||||
| BmLamin-C | 25 | −0.0015 | 3.61 × 102 | 3.12 × 10−2 | 8.64 × 10−5 |
| 200 | 0.008 | ||||
| 300 | 0.0148 | ||||
| BmPRP1 | 15.6 | 0.0107 | / | / | / |
| 62.5 | −0.0047 | ||||
| 125 | −0.0119 | ||||
| 500 | −0.0422 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Weng, Q.; Yin, F.; Hu, Q. Interactions of Destruxin A with Silkworms’ Arginine tRNA Synthetase and Lamin-C Proteins. Toxins 2020, 12, 137. https://doi.org/10.3390/toxins12020137
Wang J, Weng Q, Yin F, Hu Q. Interactions of Destruxin A with Silkworms’ Arginine tRNA Synthetase and Lamin-C Proteins. Toxins. 2020; 12(2):137. https://doi.org/10.3390/toxins12020137
Chicago/Turabian StyleWang, Jingjing, Qunfang Weng, Fei Yin, and Qiongbo Hu. 2020. "Interactions of Destruxin A with Silkworms’ Arginine tRNA Synthetase and Lamin-C Proteins" Toxins 12, no. 2: 137. https://doi.org/10.3390/toxins12020137
APA StyleWang, J., Weng, Q., Yin, F., & Hu, Q. (2020). Interactions of Destruxin A with Silkworms’ Arginine tRNA Synthetase and Lamin-C Proteins. Toxins, 12(2), 137. https://doi.org/10.3390/toxins12020137





