Polymethoxy-1-Alkenes Screening of Chlorella and Spirulina Food Supplements Coupled with In Vivo Toxicity Studies
Abstract
1. Introduction
2. Results
2.1. Identification of Polymethoxy-1-Alkenes in Food Supplements
2.2. Toxicological Screening
2.2.1. Evaluation of Teratogenicity of Lipophilic Fractions in Zebrafish Embryo
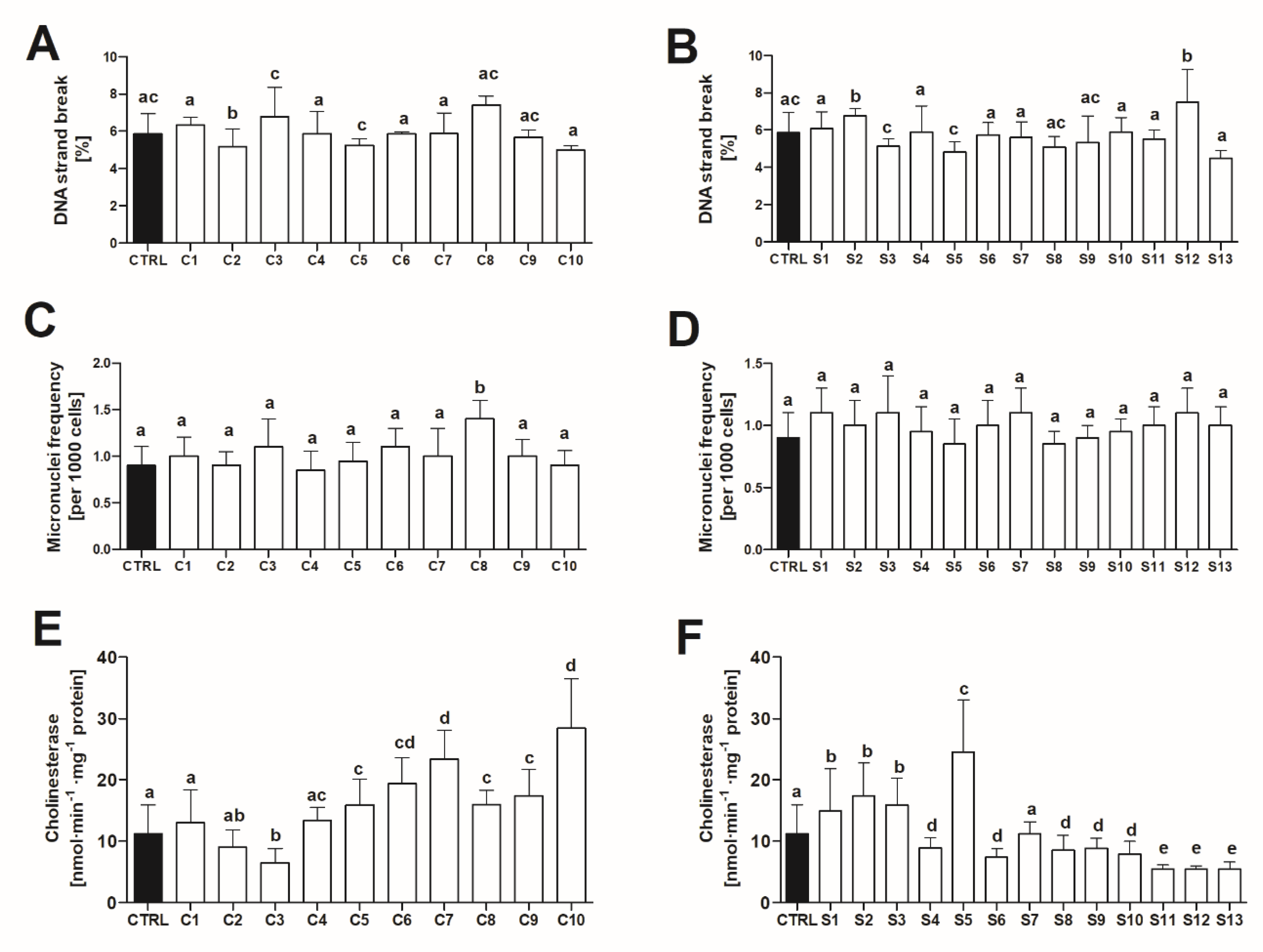
2.2.2. Oxidative Stress in Liver of Adult Zebrafish
2.2.3. Geno and Neurotoxicity Signs in Adult Zebrafish
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Food Supplements
5.2. Sample Extraction
5.3. Mass Spectrometry
5.4. Toxicological Assays
5.4.1. Embryotoxicity Testing in Danio Rerio
5.4.2. Oxidative Stress Assays
5.4.3. Genotoxicity Assays
5.4.4. Neurotoxicity Assay
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- García, J.L.; de Vicente, M.; Galán, B. Microalgae, old sustainable food and fashion nutraceuticals. Microb. Biotechnol. 2017, 10, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Buono, S.; Langellotti, A.L.; Martello, A.; Rinna, F.; Fogliano, V. Functional ingredients from microalgae. Food Funct. 2014, 58, 1669–1685. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2016, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Budzulak, J.; Niedzielski, P.; Klimaszyk, P.; Proch, J.; Kozak, L.; Poniedziałek, B. Essential and toxic elements in commercial microalgal food supplements. J. Appl. Phycol. 2019, 31, 3567–3579. [Google Scholar] [CrossRef]
- Nielsen, C.H.; Balachandran, P.; Christensen, O.; Pugh, N.D.; Tamta, H.; Sufka, K.J.; Wu, X.; Walsted, A.; Schjørring-Thyssen, M.; Enevold, C.; et al. Enhancement of natural killer cell activity in healthy subjects by Immulina®, a Spirulina extract enriched for Braun-type lipoproteins. Planta Med. 2010, 76, 1802–1808. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Lim, Y.; Kim, Y.J.; Kim, J.Y.; Kwon, O. A dietary cholesterol challenge study to assess Chlorella supplementation in maintaining healthy lipid levels in adults: A double-blinded, randomized, placebo-controlled study. Nutr. J. 2016, 15, 54. [Google Scholar] [CrossRef]
- Juszkiewicz, A.; Basta, P.; Petriczko, E.; Machaliński, B.; Trzeciak, J.; Łuczkowska, K.; Skarpańska-Stejnborn, A. An attempt to induce an immunomodulatory effect in rowers with Spirulina extract. J. Int. Soc. Sports Nutr. 2018, 15, 9. [Google Scholar] [CrossRef]
- Papazi, A.; Makridis, P.; Divanach, P. Harvesting Chlorella minutissima using cell coagulants. J. Appl. Phycol. 2010, 22, 349–355. [Google Scholar] [CrossRef]
- Hedegaard, R.; Rokkjær, I.; Sloth, J.J. Total and inorganic arsenic in dietary supplements based on herbs, other botanicals and algae—A possible contributor to inorganic arsenic exposure. Anal. Bioanal. Chem. 2013, 405, 4429–4435. [Google Scholar] [CrossRef]
- Rzymski, P.; Niedzielski, P.; Kaczmarek, N.; Jurczak, T.; Klimaszyk, P. The multidisciplinary approach to safety and toxicity assessment of microalgae-based food supplements following clinical cases of poisoning. Harmful Algae 2015, 46, 34–42. [Google Scholar] [CrossRef]
- Marles, R.J.; Barrett, M.L.; Barnes, J.; Chavez, M.L.; Gardiner, P.; Ko, R.; Mahady, G.B.; Low Dog, T.; Sarma, N.D.; Giancaspro, G.I.; et al. United States Pharmacopeia safety evaluation of Spirulina. Crit. Rev. Food Sci. Nutr. 2011, 51, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Jaśkiewicz, M. Microalgal food supplements from the perspective of Polish consumers: Patterns of use, adverse events, and beneficial effects. J. Appl. Phycol. 2017, 29, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Mynderse, J.S.; Moore, R.E. Isototactic polymethoxy-1-alkenes from the blue-green alga Tolypothrix conglutinate var. Chlorata. Phytochemistry 1979, 18, 1181–1183. [Google Scholar] [CrossRef]
- Mori, Y.; Kohchi, Y.; Noguchi, H.; Suzuki, M.; Carmeli, S.; Moore, R.E.; Patterson, G.M.L. Isotactic polymethoxy-1-alkenes from the terrestrial blue-green alga, Scytonema ocellatum: Structure and synthesis. Tetrahedron 1991, 47, 4889–4904. [Google Scholar] [CrossRef]
- Mori, Y.; Kohchi, Y.; Suzuki, M. Isotactic polymethoxy-1-alkenes from blue-green algae. Synthesis and absolute stereochemistry. J. Org. Chem. 1991, 56, 631–637. [Google Scholar] [CrossRef]
- Banker, R.; Teltsch, B.; Sukenik, A.; Carmeli, S. 7-Epicylindrospermopsin, a toxic minor metabolite of the cyanobacterium Aphanizomenon ovalisporum from Lake Kinneret, Israel. J. Nat. Prod. 2000, 63, 387–389. [Google Scholar] [CrossRef]
- Jaja-Chimedza, A.; Gantar, M.; Gibbs, P.D.; Schmale, M.C.; Berry, J.P. Polymethoxy-1-alkenes from Aphanizomenon ovalisporum inhibit vertebrate development in the zebrafish (Danio rerio) embryo model. Mar. Drugs 2012, 10, 2322–2336. [Google Scholar] [CrossRef]
- Jaja-Chimedza, A.; Saez, C.; Sanchez, K.; Gantar, M.; Berry, J.P. Identification of teratogenic polymethoxy-1-alkenes from Cylindrospermopsis raciborskii, and taxonomically diverse freshwater cyanobacteria and green algae. Harmful Algae 2015, 49, 156–161. [Google Scholar] [CrossRef]
- Rzymski, P.; Evans, D.M.; Murphy, P.J.; Kokociński, M. A study of polymethoxy-1-alkenes in Raphidiopsis (Cylindrospermopsis) raciborskii and Aphanizomenon gracile isolated in Poland. Toxicon 2019, 171, 51–53. [Google Scholar] [CrossRef]
- Kapoor, R.; Mehta, U. Effect of supplementation of blue green alga (Spirulina) on outcome of pregnancy in rats. Plant Foods Hum. Nutr. 1993, 43, 29–35. [Google Scholar] [CrossRef]
- Pankaj, P.P. Efficacy of Spirulina platensis in improvement of the reproductive performance and easing teratogenicity in hyperglycemic albino mice. Indian J. Pharmacol. 2015, 47, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Yusof, J.; Mahdy, Z.A.; Noor, R.M. Use of complementary and alternative medicine in pregnancy and its impact on obstetric outcome. Complement. Therap. Clin. Pract. 2016, 25, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, J.; Noda, K.; Uchikawa, T.; Maruyama, I.; Shimomura, H.; Miyahara, M. Effect of maternal Chlorella supplementation on carotenoid concentration in breast milk at early lactation. Int. J. Food Sci. Nutr. 2014, 65, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Takekoshi, H.; Nakano, M. Chlorella pyrenoidosa supplementation reduces the risk of anemia, proteinuria and edema in pregnant women. Plant Foods Hum. Nutr. 2010, 65, 25–30. [Google Scholar] [CrossRef]
- Sinha, S.; Patro, N.; Patro, I.K. Maternal protein malnutrition: Current and future perspectives of spirulina supplementation in neuroprotection. Front. Neurosci. 2018, 12, 966. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Chitundu, M. Multiple Micronutrient Supplementation Using Spirulina platensis during the First 1000 Days is Positively Associated with Development in Children under Five Years: A Follow up of A Randomized Trial in Zambia. Nutrients 2019, 11, 730. [Google Scholar] [CrossRef] [PubMed]
- Jaja-Chimedza, A.; Sanchez, K.; Gantar, M.; Gibbs, P.; Schmale, M.; Berry, J.P. Carotenoid glycosides from cyanobacteria are teratogenic in the zebrafish (Danio rerio) embryo model. Chemosphere 2017, 174, 478–489. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, J.; Wan, Y.; Giesy, J.P.; Hu, J. Cyanobacteria blooms produce teratogenic retinoic acids. Proc. Natl. Acad. Sci. USA 2012, 109, 9477–9482. [Google Scholar] [CrossRef]
- Taylor, D.A. Botanical supplements: Weeding out the health risks. Environ. Health Perspect. 2004, 112, A750–A753. [Google Scholar] [CrossRef]
- Van Breemen, R.B.; Fong, H.H.; Farnsworth, N.R. Ensuring the safety of botanical dietary supplements. Am. J. Clin. Nutr. 2008, 87, 509S–513S. [Google Scholar] [CrossRef]
- Van Breemen, R.B. Development of safe and effective botanical supplements. J. Med. Chem. 2015, 58, 8360–8372. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Nelson, D.R.; Yi, Z.; Xu, M.; Khraiwesh, B.; Jijakli, K.; Chaiboonchoe, A.; Alzahmi, A.; Al-Khairy, D.; Brynjolfsson, S.; et al. Bioactive compounds from microalgae: Current development and prospects. In Studies in Natural Products Chemistry; Atta ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 54, pp. 199–225. [Google Scholar]
- Singh, S.; Kate, B.N.; Banerjee, U.C. Bioactive Compounds from Cyanobacteria and Microalgae: An Overview. Crit. Rev. Biotechnol. 2005, 25, 73–95. [Google Scholar] [CrossRef] [PubMed]
- Bláha, L.; Pavel, B.; Blahoskav, M. Toxins produced in cyanobacterial water blooms—Toxicity and risks. Interdiscip. Toxicol. 2009, 2, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Poniedziałek, B.; Rzymski, P.; Kokocinski, M.; Karczewski, J. Toxic potencies of metabolite(s) of non-cylindrospermopsin producing Cylindrospermopsis raciborskii isolated from temperate zone in human white cells. Chemosphere 2015, 120, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Poniedziałek, B. In search of environmental role of cylindrospermopsin: A review on global distribution and ecology of its producers. Water Res. 2014, 66, 320–337. [Google Scholar] [CrossRef]
- Satora, P.; Barwińska-Sendra, A.; Duda-Chodak, A.; Wajda, Ł. Strain-dependent production of selected bioactive compounds by Cyanobacteria belonging to the Arthrospira genus. J. Appl. Microbiol. 2015, 119, 736–743. [Google Scholar] [CrossRef]
- Costa, J.A.V.; de Morais, M.G.; Dalcanton, F.; da Cruz Reichert, C.; Durante, A.J. Simultaneous cultivation of Spirulina platensis and the toxigenic cyanobacteria Microcystis aeruginosa. Z. Naturforsch C J. Biosci. 2006, 61, 105–110. [Google Scholar] [CrossRef]
- Jiang, Y. Detection of the hepatotoxic microcystins in 36.660 kinds of cyanobacteria Spirulina food products in China. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 885–894. [Google Scholar] [CrossRef]
- Vichi, S.; Lavorini, P.; Funari, E.; Scardala, S.; Testai, E. Contamination by Microcystis and microcystins of blue-green algae food supplements (BGAS) on the Italian market and possible risk for the exposed population. Food Chem. Toxicol. 2012, 50, 4493–4499. [Google Scholar] [CrossRef]
- Roy-Lachapelle, A.; Solliec, M.; Bouchard, M.F.; Sauve, S. Detection of Cyanotoxins in Algae Dietary Supplements. Toxins 2017, 9, 76. [Google Scholar] [CrossRef]
- Manali, K.M.; Arunraj, R.; Kumar, T.; Ramya, M. Detection of microcystin producing cyanobacteria in Spirulina dietary supplements using multiplex HRM quantitative PCR. J. Appl. Phycol. 2017, 29, 1279–1286. [Google Scholar] [CrossRef]
- Jonas, A.; Buranova, V.; Scholz, S.; Fetter, E.; Novakova, K.; Kohoutek, J.; Hilscherova, K. Retinoid-like activity and teratogenic effects of cyanobacterial exudates. Aquat. Toxicol. 2014, 155, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Truong, L.; Harper, S.; Tanguay, R. Evaluation of Embryotoxicity Using the Zebrafish Model. In Drug Safety Evaluation; Gautier, J.-C., Ed.; Humana Press: New York, NY, USA, 2011; pp. 271–279. [Google Scholar]
- Van der Spiegel, M.; Noordam, M.Y.; van der Fels-Klerx, H.J. Safety of novel protein sources (insects, microalgae, seaweed, duckweed and rapeseed) and legislative aspects for application in food and feed production. Compr. Rev. Food Sci. Food Saf. 2013, 12, 662–678. [Google Scholar] [CrossRef]
- FERA. Occurrence of Polycyclic Aromatic Hydrocarbons in Herbs, Spices, Supplements and Tea Report to Food Standards Agency. 2015. Available online: https://www.food.gov.uk/sites/default/files/media/document/pahs-in-herbs-spices-supplements-and-tea-final-report_0.pdf (accessed on 7 January 2020).
- Billiard, S.M.; Timme-Laragy, A.R.; Wassenberg, D.M.; Cockman, C.; Di Giulio, R.T. The role of the aryl hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons to zebrafish. Toxicol. Sci. 2006, 92, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Heussner, A.H.; Mazija, L.; Fastner, J.; Dietrich, D.R. Toxin content and cytotoxicity of algal dietary supplements. Toxicol. Appl. Pharmacol. 2012, 265, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, M.; Yamamoto, M.; Tanaka, Y.; Kaito, M.; Adachi, Y. Spirulina-associated hepatotoxicity. Am. J. Gastroenterol. 2002, 97, 3212–3213. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.L.; Rodrigues, J.A.; Azevedo, J.; Vasconcelos, V.; Eiras, E.; Campos, M.G. Hepatotoxicity induced by paclitaxel interaction with turmeric in association with a microcystin from a contaminated dietary supplement. Toxicon 2018, 150, 207–211. [Google Scholar] [CrossRef]
- Mazokopakis, E.E.; Starakis, I.K.; Papadomanolaki, M.G.; Mavroeidi, N.G.; Ganotakis, E.S. The hepatoprotective and hypolipidemic effects of Spirulina (Arthrospira platensis) supplementation in a Cretan population with non-alcoholic fatty liver disease: A prospective pilot study. Ann. Gastroenterol. 2014, 27, 387–394. [Google Scholar]
- Gad, A.S.; Khadrawy, Y.A.; El-Nekeety, A.A.; Mohamed, S.R.; Hassan, N.S.; Abdel-Wahhab, M.A. Antioxidant activity and hepatoprotective effects of whey protein and Spirulina in rats. Nutrition 2011, 27, 582–589. [Google Scholar] [CrossRef]
- Soreq, H.; Seidman, S. Acetylcholinesterase—New roles for an old actor. Nat. Rev. Neurosci. 2011, 2, 294–302. [Google Scholar] [CrossRef]
- Mercey, G.; Verdelet, T.; Renou, J.; Kliachyna, M.; Baati, R.; Nachon, F.; Jean, L.; Renard, P.-Y. Reactivators of Acetylcholinesterase Inhibited by Organophosphorus Nerve Agents. Acc. Chem. Res. 2012, 45, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Organization for Economic Cooperation and Development (OECD). OECD Draft Proposal for A New Guideline, 1st Version. In Guideline for the Testing of Chemicals; Fish Embryo Toxicity, FET Test: Paris, France, 2006. [Google Scholar]
- Poniedziałek, B.; Rzymski, P.; Karczewski, J. The role of the enzymatic antioxidant system in cylindrospermopsin-induced toxicity in human lymphocytes. Toxicol. In Vitro 2015, 29, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 139, 292–298. [Google Scholar] [CrossRef]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie/Academic Press Inc.: Weinheim, Germany; New York, NY, USA, 1974; pp. 673–680. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jacoby, W.B. Glutathione-S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [PubMed]
- Olive, P.L. DNA precipitation assay: A rapid and simple method for detecting DNA damage in mammalian cells. Environ. Mol. Mutagen. 1988, 11, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Bester, M.J.; Potgieter, H.C.; Vermaak, W.J.H. Cholate and pH reduce interference by SDS in the determination of DNA with Hoescht. Anal. Biochem. 1994, 223, 299–305. [Google Scholar] [CrossRef]
- Barsiene, J.; Syvokiene, J.; Bjornstad, A. Induction of micronuclei and other nuclear abnormalities in mussels exposed to bisphenol A, diallyl phthalate and tetrabromodiphenyl ether-47. Aquat. Toxicol. 2006, 78, S105–S108. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharm. 1961, 7, 88–95. [Google Scholar] [CrossRef]
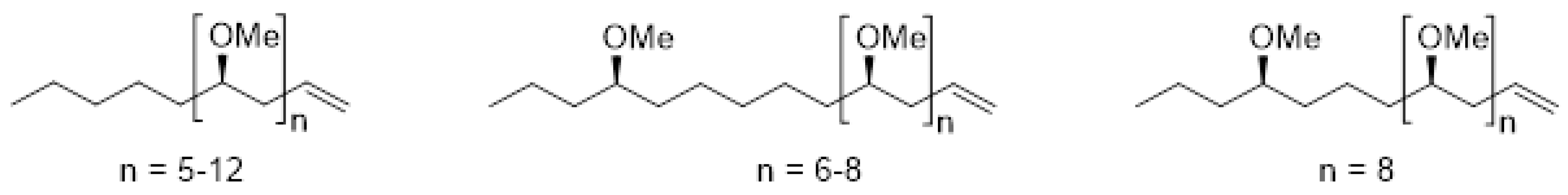

| Sample Code | Species Declared on the Label | Country of Origin | Appearance |
|---|---|---|---|
| Chlorella | |||
| C1 | Chlorella sp. | China | Tablets |
| C2 | Chlorella vulgaris | Taiwan | Tablets |
| C3 | Chlorella pyrenoidosa | Japan | Tablets |
| C4 | Chlorella sp. | India | Tablets |
| C5 | Chlorella sp. | China | Powder |
| C6 | Chlorella sp. | China | Powder |
| C7 | Chlorella pyrenoidosa | China | Powder |
| C8 | Chlorella vulgaris | China | Powder |
| C9 | Chlorella vulgaris | Portugal | Powder |
| C10 | Chlorella vulgaris | China | Tablets |
| Spirulina | |||
| S1 | Spirulina sp. | China | Powder |
| S2 | Spirulina sp. | China | Powder |
| S3 | Spirulina sp. | China | Powder |
| S4 | Spirulina platensis | China | Powder |
| S5 | Spirulina sp. | Taiwan | Powder |
| S6 | Spirulina pacifica | USA | Tablets |
| S7 | Arthrospira platensis | China | Powder |
| S8 | Spirulina platensis | China | Tablets |
| S9 | Spirulina sp. | China | Tablets |
| S10 | Spirulina maxima | China | Tablets |
| S11 | Spirulina sp. | China | Tablets |
| S12 | Spirulina sp. | China | Tablets |
| S13 | Spirulina sp. | India | Powder |
| Developmental Endpoint | Sample | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CTRL | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 | |
| Coagulated eggs | - | - | - | - | - | - | - | - | - | - | - |
| Head malformation | - | - | - | - | - | - | - | - | - | - | - |
| Eyes malformation | - | - | - | - | - | - | - | - | - | - | - |
| Chorda malformation | - | - | - | - | - | - | - | - | - | - | - |
| Tail malformation | - | - | - | - | - | - | - | - | - | - | - |
| Egg yolk malformation | - | - | - | - | - | - | - | - | - | - | - |
| Growth retardation | - | - | - | - | - | - | - | - | - | - | - |
| Spinal curvature | - | - | + | - | - | - | - | - | - | + | - |
| Developmental Endpoint | Sample | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTRL | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 | |
| Coagulated eggs | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Head malformation | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Eyes malformation | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Chorda malformation | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Tail malformation | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Egg yolk malformation | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Growth retardation | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Spinal curvature | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henao, E.; Murphy, P.J.; Falfushynska, H.; Horyn, O.; Evans, D.M.; Klimaszyk, P.; Rzymski, P. Polymethoxy-1-Alkenes Screening of Chlorella and Spirulina Food Supplements Coupled with In Vivo Toxicity Studies. Toxins 2020, 12, 111. https://doi.org/10.3390/toxins12020111
Henao E, Murphy PJ, Falfushynska H, Horyn O, Evans DM, Klimaszyk P, Rzymski P. Polymethoxy-1-Alkenes Screening of Chlorella and Spirulina Food Supplements Coupled with In Vivo Toxicity Studies. Toxins. 2020; 12(2):111. https://doi.org/10.3390/toxins12020111
Chicago/Turabian StyleHenao, Eliana, Patrick J. Murphy, Halina Falfushynska, Oksana Horyn, Daniel M. Evans, Piotr Klimaszyk, and Piotr Rzymski. 2020. "Polymethoxy-1-Alkenes Screening of Chlorella and Spirulina Food Supplements Coupled with In Vivo Toxicity Studies" Toxins 12, no. 2: 111. https://doi.org/10.3390/toxins12020111
APA StyleHenao, E., Murphy, P. J., Falfushynska, H., Horyn, O., Evans, D. M., Klimaszyk, P., & Rzymski, P. (2020). Polymethoxy-1-Alkenes Screening of Chlorella and Spirulina Food Supplements Coupled with In Vivo Toxicity Studies. Toxins, 12(2), 111. https://doi.org/10.3390/toxins12020111








