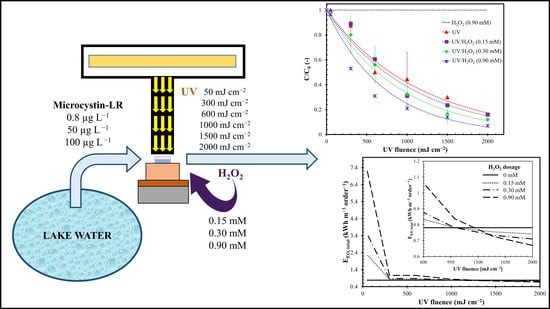
Kinetics of Microcystin-LR Removal in a Real Lake Water by UV/H2O2 Treatment and Analysis of Specific Energy Consumption
Abstract
:1. Introduction
2. Results and Discussion
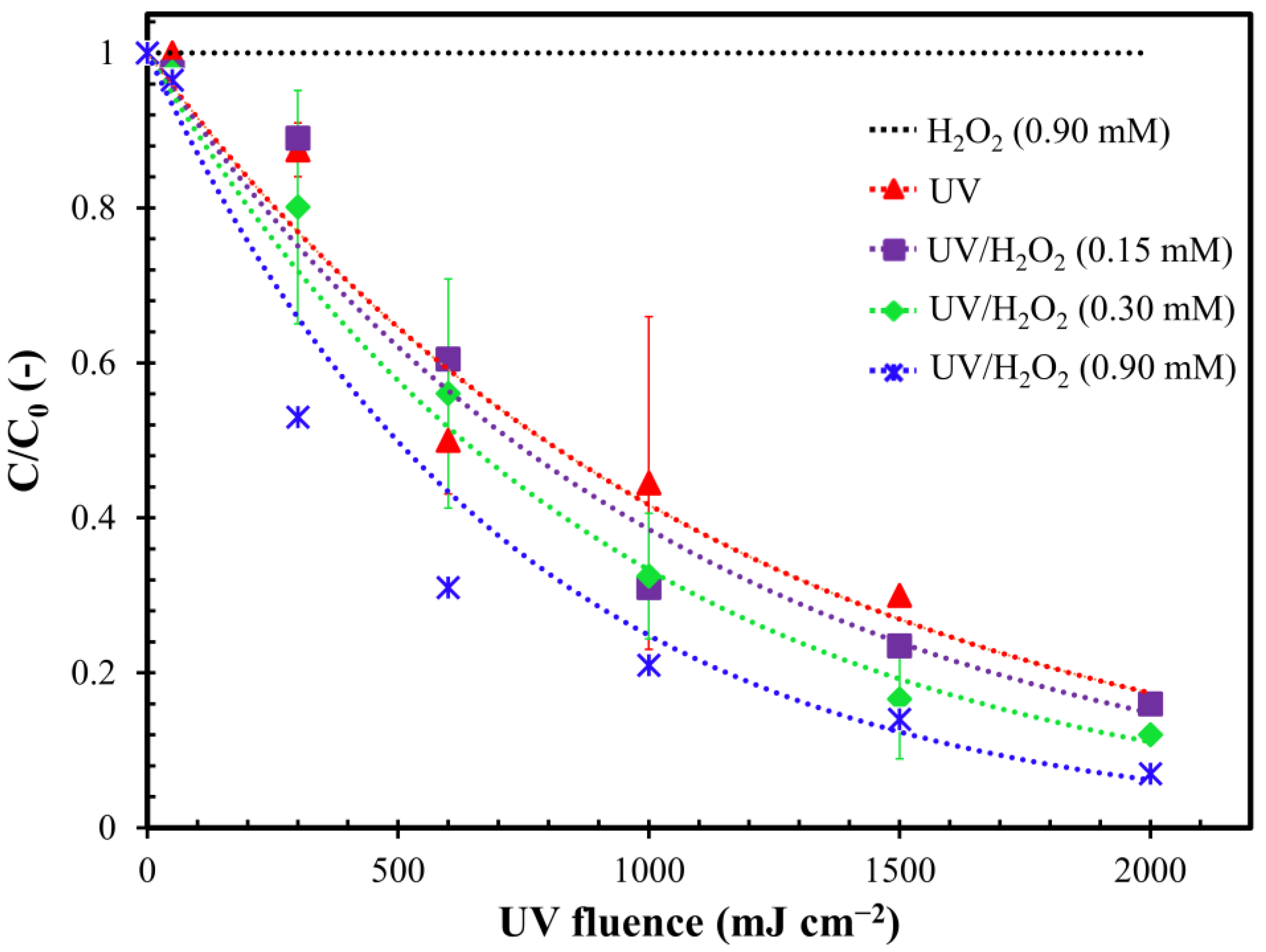
2.1. Effect of the Oxidant
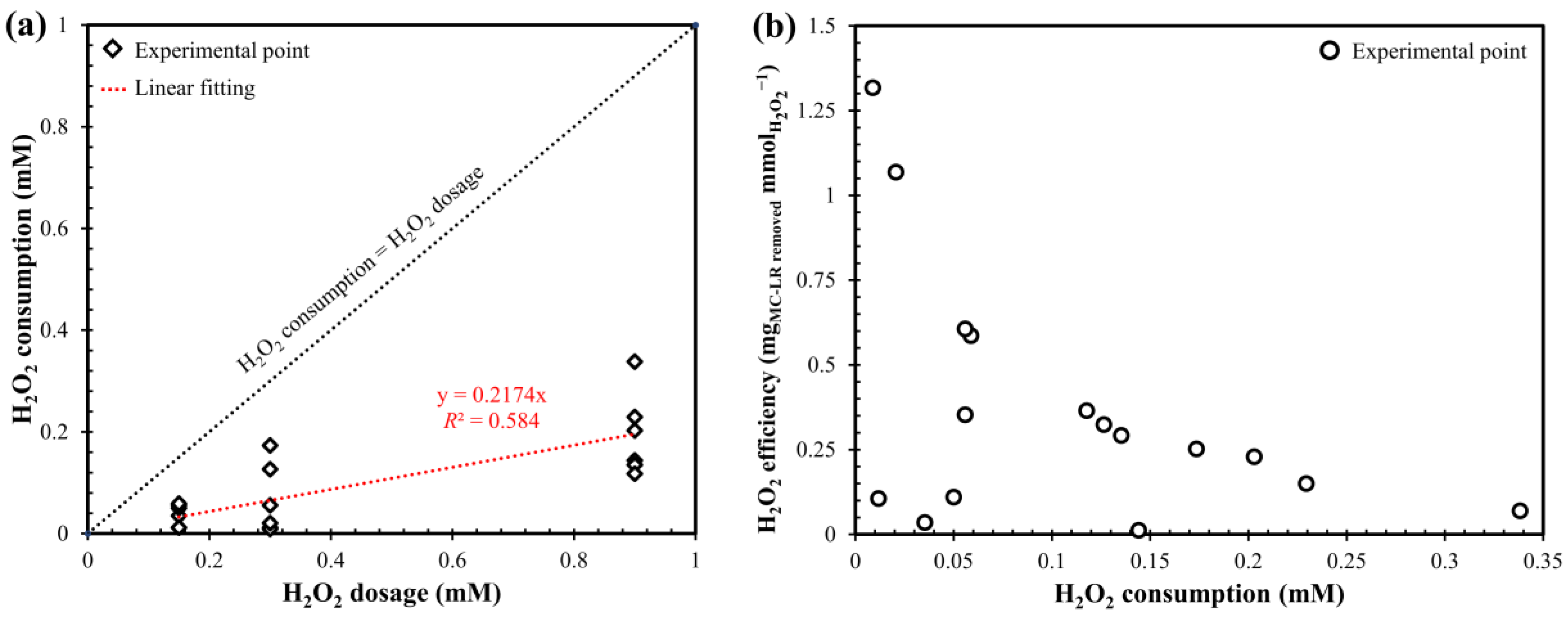
2.2. Influence of H2O2 Dosage
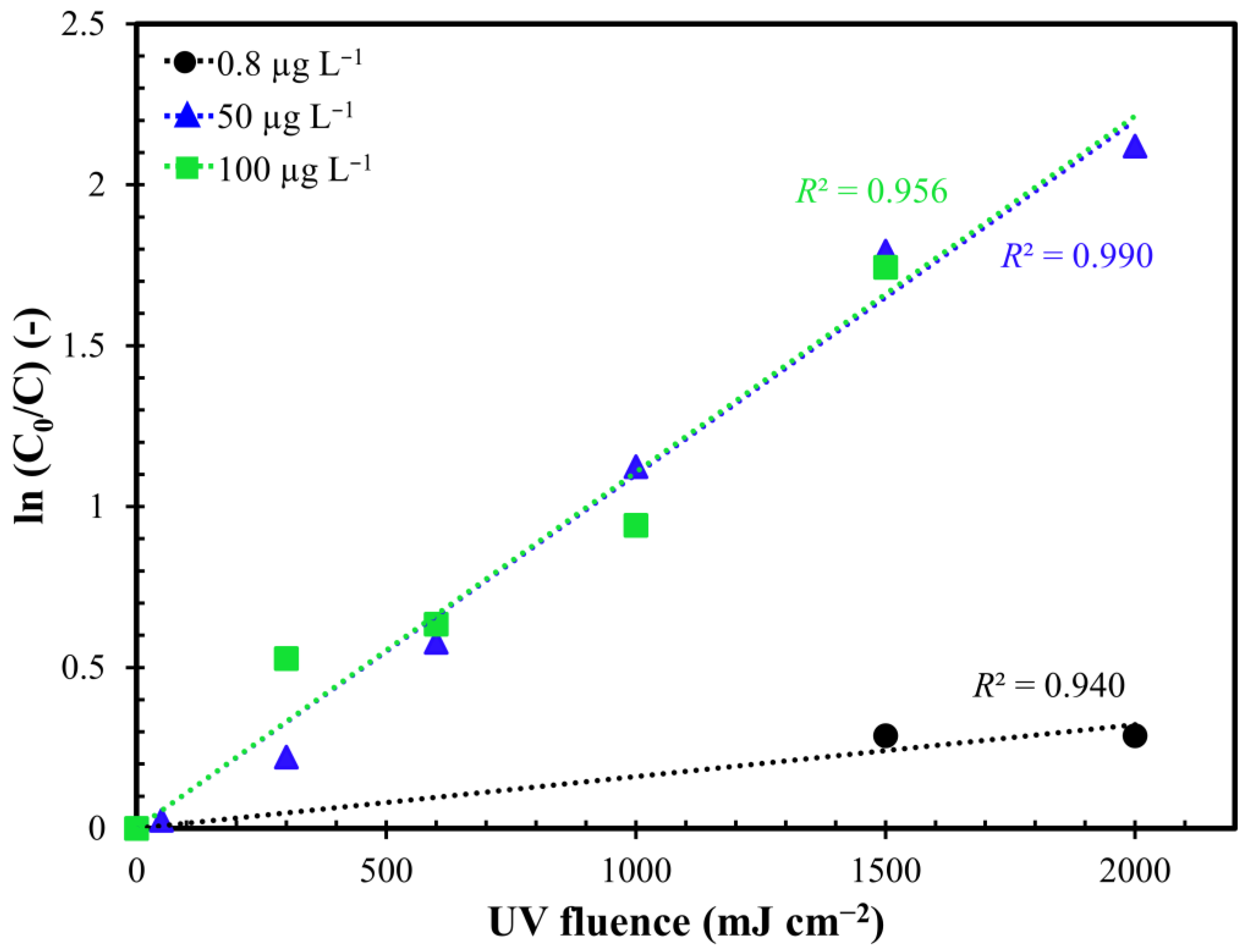
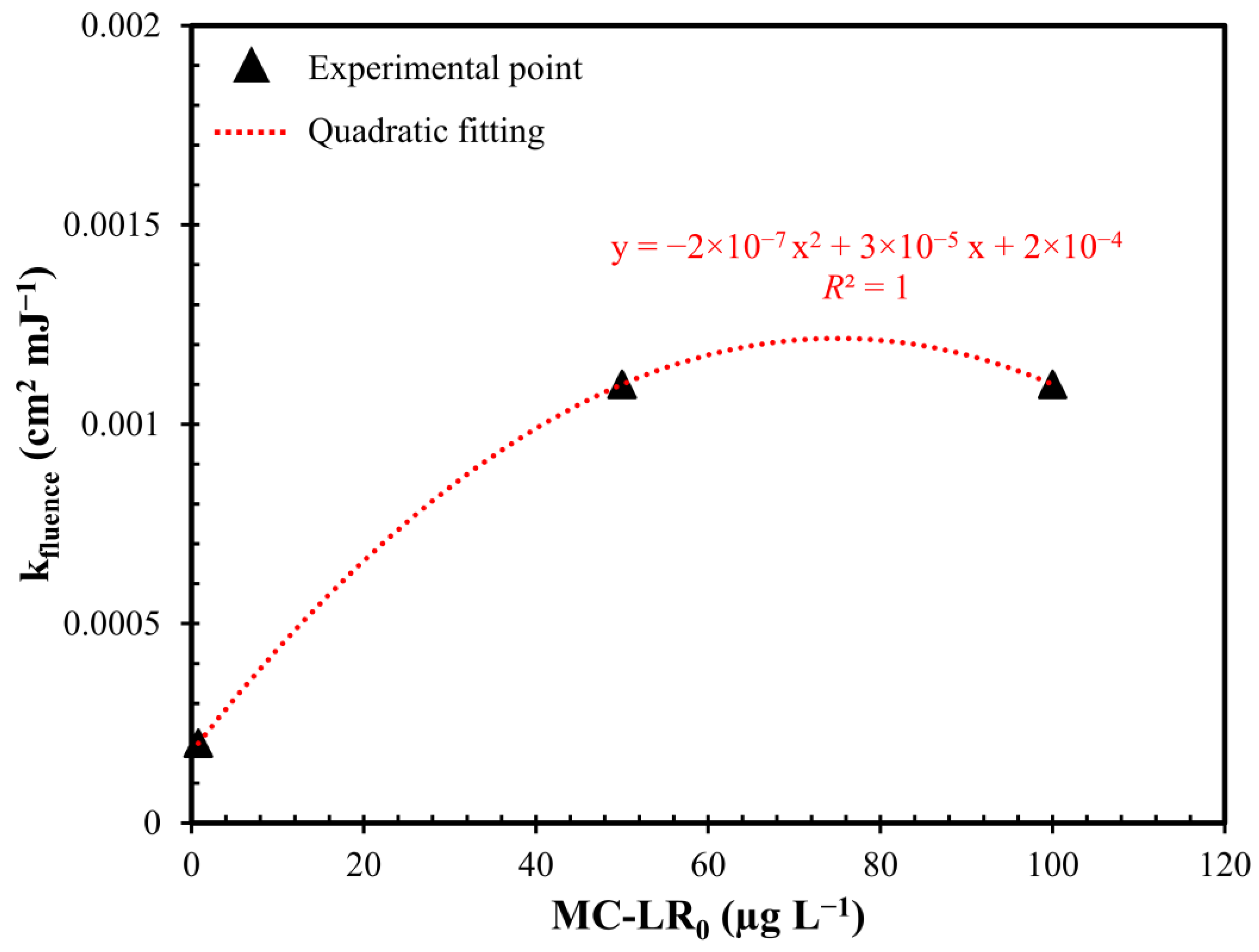
2.3. Influence of Initial MC-LR Concentration
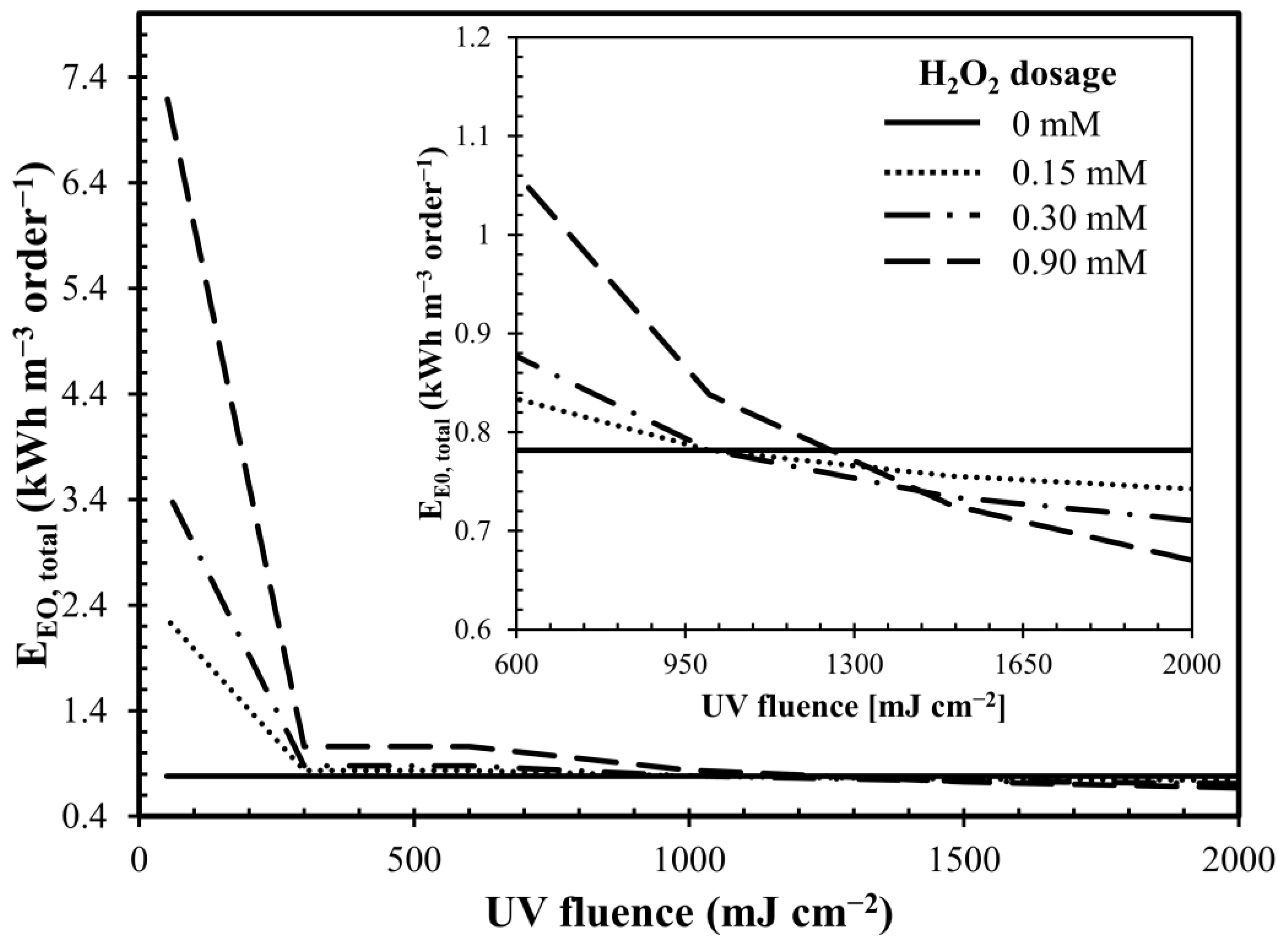
2.4. Energy Consumption
3. Conclusions
4. Materials and Methods
4.1. Water Preparation
4.2. The Lab-Scale System
4.3. Experimental Set-Up and Analytical Methods
- H2O2 alone;
- UV alone;
- UV/H2O2 combination.
4.4. MC-LR Degradation
4.5. Hydrogen Peroxide Consumption
4.6. Energy Consumption
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AOPs | Advanced oxidation processes |
| DW | Drinking water |
| DWTP | Drinking water treatment plant |
| HLT | Half-life time |
| IARC | International Agency for Research on Cancer |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| MC-LR | Microcystin-LR |
| UV | Ultraviolet |
| WHO | World Health Organization |
Appendix A
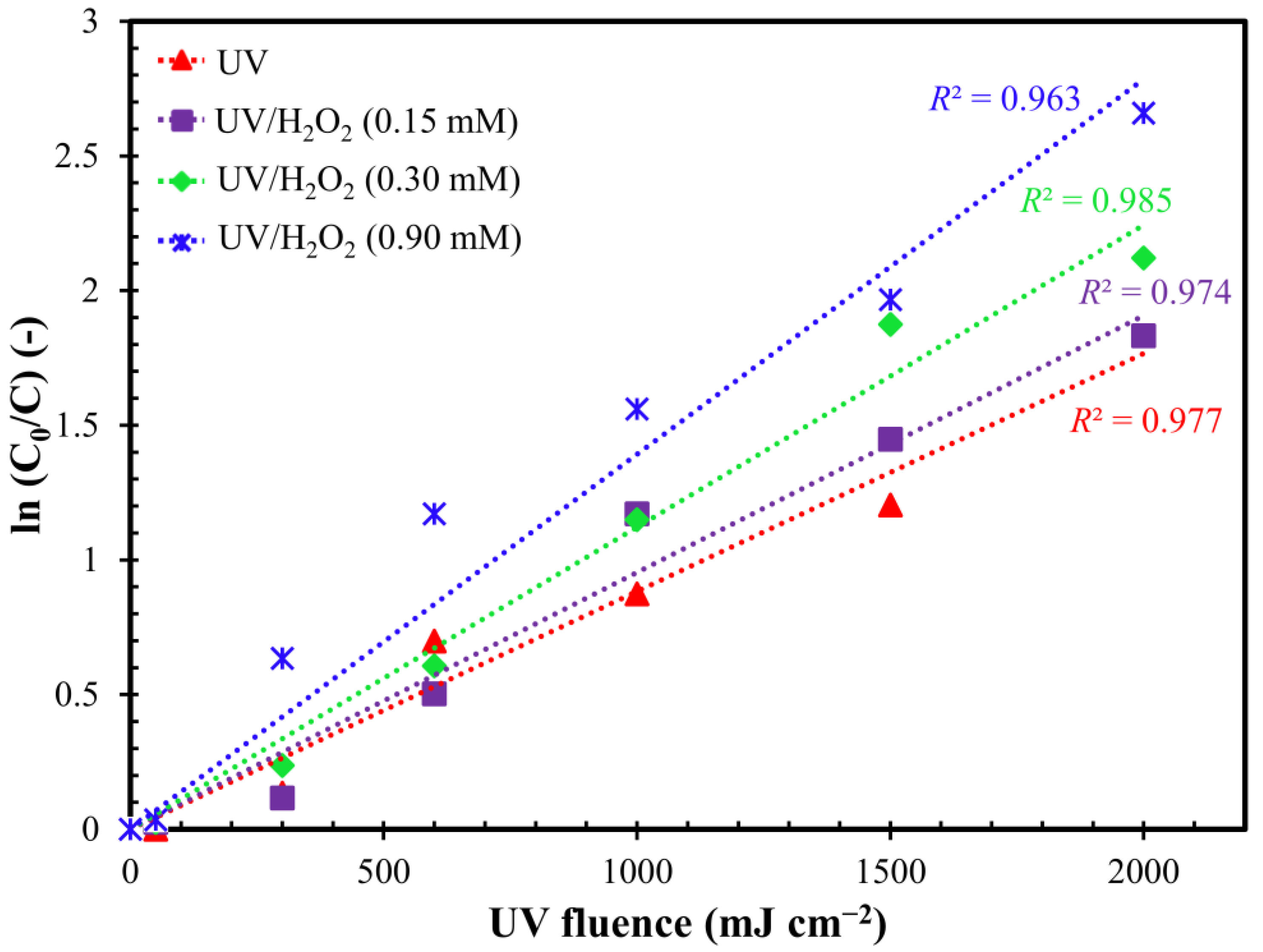


| UV | UV/H2O2 (0.15 mM) | UV/H2O2 (0.30 mM) | UV/H2O2 (0.90 mM) | |
|---|---|---|---|---|
| R2 (-) | 0.977 | 0.974 | 0.985 | 0.963 |
| kfluence (cm2 mJ) | 0.0009 | 0.001 | 0.0011 | 0.0014 |
| ktime (min−1) | 0.0108 | 0.0120 | 0.0132 | 0.0168 |
| HLT (min) | 64.2 | 57.8 | 52.5 | 41.3 |
| n (-) | 12 | 7 | 16 | 7 |
| UV/H2O2 (0.8 µg L−1) | UV/H2O2 (50 µg L−1) | UV/H2O2 (100 µg L−1) | |
|---|---|---|---|
| R2 (-) | 0.940 | 0.990 | 0.956 |
| kfluence (cm2 mJ) | 0.0002 | 0.0011 | 0.0011 |
| ktime (min−1) | 0.0024 | 0.0132 | 0.0132 |
| HLT (min) | 288.8 | 52.5 | 52.5 |
| n (-) | 3 | 16 | 5 |
| Parameter | Unit of Measure | Average Value |
|---|---|---|
| pH | - | 7.5 |
| Dissolved oxygen | mg L−1 | 9.5–9.7 |
| Turbidity | NTU | 2–3 |
| Absorbance UV at 254 nm | 1 cm−1 | 0.010–0.020 |
| Suspended solids | mg L−1 | 0–1 |
| Conductivity at 20 °C | µS cm−1 | 260–270 |
| Alkalinity | mg HCO3− L−1 | 120–130 |
| Bacteria colony count at 22 °C | CFU mL−1 | 80–90 |
| Total coliforms at 37 °C | MPN 100 mL−1 | 65–75 |
| Enterococcus | MPN 100 mL−1 | 2–4 |
| Escherichia coli | MPN 100 mL−1 | 8–10 |
| Pseudomonas aeruginosa | CFU 250 mL−1 | 2–4 |
| Clostridium perfringens | CFU 100 mL−1 | 1–3 |
| Cyanobacterial algae | cells L−1 | 1,700,000–2,000,000 |
| Total algae | cells L−1 | 3,500,000–3,600,000 |
References
- Sorlini, S.; Collivignarelli, M.C.; Carnevale Miino, M. Technologies for the control of emerging contaminants in drinking water treatment plants. Environ. Eng. Manag. J. 2019, 18, 2203–2216. [Google Scholar]
- WHO. WHO Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; World Health Organization: Geneve, Switzerland, 2017; pp. 1–631. [Google Scholar]
- Leblanc, P.; Merkley, N.; Thomas, K.; Lewis, N.I.; Békri, K.; Renaud, S.L.; Pick, F.R.; McCarron, P.; Miles, C.O.; Quilliam, M.A. Isolation and Characterization of [D-Leu1]microcystin-LY from Microcystis aeruginosa CPCC-464. Toxins 2020, 12, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorlini, S.; Collivignarelli, M.C.; Abba, A. Control Measures for Cyanobacteria and Cyanotoxins in Drinking Water. Environ. Eng. Manag. J. 2018, 17, 2455–2463. [Google Scholar] [CrossRef]
- Kim, S.; KimiD, S.; Mehrotra, R.; Sharma, A. Predicting cyanobacteria occurrence using climatological and environmental controls. Water Res. 2020, 175, 115639. [Google Scholar] [CrossRef] [PubMed]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans|Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins; International Agency for Research on Cancer: Lyon, France, 2010; Volume 94, pp. 1–464. [Google Scholar]
- Greer, B.; Meneely, J.P.; Elliott, C.T. Uptake and accumulation of Microcystin-LR based on exposure through drinking water: An animal model assessing the human health risk. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Chen, L.; Wen, C.; Zhang, X.; Feng, X.; Yang, F. MicroRNA expression profiling involved in MC-LR-induced hepatotoxicity using high-throughput sequencing analysis. J. Toxicol. Environ. Heal. Part A 2018, 81, 89–97. [Google Scholar] [CrossRef]
- Sun, Y.T.; Zheng, Q.; Huang, P.; Guo, Z.; Xu, L.H. Microcystin-lr induces protein phosphatase 2a alteration in a human liver cell line. Environ. Toxicol. 2013, 29, 1236–1244. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, H.; Chen, K. Relationship between microcystin in drinking water and colorectal cancer. Biomed. Environ. Sci. 2002, 15, 166–171. [Google Scholar]
- Wen, C.; Zheng, S.; Yang, Y.; Li, X.; Chen, J.; Wang, X.; Feng, X.; Yang, F. Effects of microcystins-LR on genotoxic responses in human intestinal epithelial cells (NCM460). J. Toxicol. Environ. Heal. Part A 2019, 82, 1113–1119. [Google Scholar] [CrossRef]
- Yang, F.; Cao, L.; Massey, I.Y.; Yang, F. The lethal effects and determinants of microcystin-LR on heart: A mini review. Toxin Rev. 2020, 1–10. [Google Scholar] [CrossRef]
- Xiang, L.; Li, Y.W.; Wang, Z.R.; Liu, B.L.; Zhao, H.M.; Li, H.; Cai, Q.Y.; Mo, C.H.; Li, Q. Bioaccumulation and Phytotoxicity and Human Health Risk from Microcystin-LR under Various Treatments: A Pot Study. Toxins 2020, 12, 523. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.K.C.; Chia, M.A.; Arruda-Neto, J.D.D.T.; Tornisielo, V.L.; Vilca, F.Z.; Bittencourt-Oliveira, M.D.C. Microcystin-LR bioaccumulation and depuration kinetics in lettuce and arugula: Human health risk assessment. Sci. Total Environ. 2016, 566, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- EC Proposal for a Directive of the European Parliament and of the Council on the Quality of Water Intended for Human Consumption (Recast). Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:8c5065b2-074f-11e8-b8f5-01aa75ed71a1.0016.02/DOC_2&format=PDF (accessed on 1 December 2020).
- IMH Interministerial Decree. Scheme for the introduction. In Annex I, Part B, of the Legislative Decree 2 February 2001, n. 31, of the “Microcystin-LR” Parameter and its Parameter Value (in Italian); Italian Ministry of Health: Rome, Italy, 2012. [Google Scholar]
- Arnich, N. FRANCE: Regulation, Risk Management, Risk Assessment and Research on Cyanobacteria and Cyanotoxins. In Current Approaches to Cyanotoxin Risk Assessment, Risk Management and Regulations in Different Countries; Chorus, I., Ed.; Federal Environment Agency (Umweltbundesamt): Dessau-Roßlau, Germany, 2012; pp. 63–70. [Google Scholar]
- Government of France. Order of 11 January 2007 Relating to the Quality Limits and References for Raw Water and Water Intended for Human Consumption Mentioned in Articles R. 1321-2, R. 1321-3, R. 1321-7 and R. 1321-38 of Public Health Code; Government of France: Paris, France, 2007. (In French)
- Government of Canada. Guidelines for Canadian Drinking Water Quality; Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada; Government of Canada: Ottawa, ON, Canada, 2020; pp. 1–28.
- Government of Canada. Guidelines for Canadian Recreational Water Quality—Cyanobacteria and their Toxins; Government of Canada: Ottawa, ON, Canada, 2020.
- Australian Government. Australian Drinking Water Guidelines; Australian Government: Canberra, Australia, 2011; pp. 1–1172.
- Luo, Z.; Li, P.; Cai, D.; Chen, Q.; Qin, P.; Tan, T.; Cao, H. Comparison of performances of corn fiber plastic composites made from different parts of corn stalk. Ind. Crop. Prod. 2017, 95, 521–527. [Google Scholar] [CrossRef]
- Czyżewska, W.; Piontek, M. The Efficiency of Microstrainers Filtration in the Process of Removing Phytoplankton with Special Consideration of Cyanobacteria. Toxins 2019, 11, 285. [Google Scholar] [CrossRef] [Green Version]
- Lürling, M.; Kang, L.; Mucci, M.; Van Oosterhout, F.; Noyma, N.P.; Miranda, M.; Huszar, V.L.; Waajen, G.; Marinho, M.M. Coagulation and precipitation of cyanobacterial blooms. Ecol. Eng. 2020, 158, 106032. [Google Scholar] [CrossRef]
- Lama, S.; Muylaert, K.; Karki, T.B.; Foubert, I.; Henderson, R.K.; Vandamme, D. Flocculation properties of several microalgae and a cyanobacterium species during ferric chloride, chitosan and alkaline flocculation. Bioresour. Technol. 2016, 220, 464–470. [Google Scholar] [CrossRef] [Green Version]
- Jeong, B.; Oh, M.S.; Park, H.M.; Park, C.; Kim, E.J.; Hong, S.W. Elimination of microcystin-LR and residual Mn species using permanganate and powdered activated carbon: Oxidation products and pathways. Water Res. 2017, 114, 189–199. [Google Scholar] [CrossRef]
- Drogui, P.; Daghrir, R.; Simard, M.-C.; Sauvageau, C.; Blais, J.F. Removal of microcystin-LR from spiked water using either activated carbon or anthracite as filter material. Environ. Technol. 2011, 33, 381–391. [Google Scholar] [CrossRef]
- Mashile, P.P.; Mpupa, A.; Nomngongo, P.N. Adsorptive removal of microcystin-LR from surface and wastewater using tyre-based powdered activated carbon: Kinetics and isotherms. Toxicon 2018, 145, 25–31. [Google Scholar] [CrossRef]
- Villars, K.; Huang, Y.; Lenhart, J.J. Removal of the Cyanotoxin Microcystin-LR from Drinking Water Using Granular Activated Carbon. Environ. Eng. Sci. 2020, 37, 585–595. [Google Scholar] [CrossRef]
- Moon, B.R.; Kim, T.K.; Kim, M.K.; Choi, J.; Zoh, K.D. Degradation mechanisms of Microcystin-LR during UV-B photolysis and UV/H2O2 processes: Byproducts and pathways. Chemosphere 2017, 185, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Park, J.A.; Yang, B.; Jang, M.; Kim, J.H.; Kim, S.B.; Park, H.D.; Park, H.M.; Lee, S.H.; Choi, J.W. Oxidation and molecular properties of microcystin-LR, microcystin-RR and anatoxin-a using UV-light-emitting diodes at 255 nm in combination with H2O2. Chem. Eng. J. 2019, 366, 423–432. [Google Scholar] [CrossRef]
- Park, J.A.; Yang, B.; Kim, J.H.; Choi, J.W.; Park, H.D.; Lee, S.H. Removal of microcystin-LR using UV-assisted advanced oxidation processes and optimization of photo-Fenton-like process for treating Nak-Dong River water, South Korea. Chem. Eng. J. 2018, 348, 125–134. [Google Scholar] [CrossRef]
- Zhu, G.; Lu, X.; Yang, Z. Characteristics of UV-MicroO 3 Reactor and Its Application to Microcystins Degradation during Surface Water Treatment. J. Chem. 2015, 2015, 1–9. [Google Scholar]
- Sorlini, S.; Biasibetti, M.; Collivignarelli, M.C.; Crotti, B.M. Reducing the chlorine dioxide demand in final disinfection of drinking water treatment plants using activated carbon. Environ. Technol. 2015, 36, 1499–1509. [Google Scholar] [CrossRef]
- Sorlini, S.; Collivignarelli, M.C.; Canato, M. Effectiveness in chlorite removal by two activated carbons under different working conditions: A laboratory study. J. Water Supply Res. Technol. 2015, 64, 450–461. [Google Scholar] [CrossRef]
- Sorlini, S.; Biasibetti, M.; Gialdini, F.; Collivignarelli, M.C. How can drinking water treatments influence chlorine dioxide consumption and by-product formation in final disinfection? Water Sci. Technol. Water Supply 2016, 16, 333–346. [Google Scholar] [CrossRef]
- Li, W.Y.; Liu, Y.; Sun, X.L.; Wang, F.; Qian, L.; Xu, C.; Zhang, J.P. Photocatalytic degradation of MC-LR in water by the UV/TiO2/H2O2 process. Water Supply 2015, 16, 34–43. [Google Scholar] [CrossRef]
- He, X.; Pelaez, M.; Westrick, J.A.; O’Shea, K.E.; Hiskia, A.; Triantis, T.; Kaloudis, T.; Stefan, M.I.; De La Cruz, A.A.; Dionysiou, D.D. Efficient removal of microcystin-LR by UV-C/H2O2 in synthetic and natural water samples. Water Res. 2012, 46, 1501–1510. [Google Scholar] [CrossRef]
- Wang, X.; Utsumi, M.; Yang, Y.; Li, D.; Zhao, Y.; Zhang, Z.; Feng, C.; Sugiura, N.; Cheng, J.J. Degradation of microcystin-LR by highly efficient AgBr/Ag3PO4/TiO2 heterojunction photocatalyst under simulated solar light irradiation. Appl. Surf. Sci. 2015, 325, 1–12. [Google Scholar] [CrossRef]
- Liu, J.; Ye, J.S.; Ou, H.; Lin, J. Effectiveness and intermediates of microcystin-LR degradation by UV/H2O2 via 265 nm ultraviolet light-emitting diodes. Environ. Sci. Pollut. Res. 2017, 24, 4676–4684. [Google Scholar] [CrossRef] [PubMed]
- Collivignarelli, M.C.; Pedrazzani, R.; Sorlini, S.; Abbà, A.; Bertanza, G. H2O2 Based Oxidation Processes for the Treatment of Real High Strength Aqueous Wastes. Sustainability 2017, 9, 244. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Gao, N.Y.; Deng, Y.; Yao, J.J.; Zhang, K.J.; Li, H.J.; Yin, D.D.; Ou, H.S.; Guo, J.W. Experimental and model comparisons of H2O2 assisted UV photodegradation of Microcystin-LR in simulated drinking water. J. Zhejiang Univ. A 2009, 10, 1660–1669. [Google Scholar] [CrossRef]
- Vlad, S.; Anderson, W.B.; Peldszus, S.; Huck, P.M. Removal of the cyanotoxin anatoxin-a by drinking water treatment processes: A review. J. Water Heal. 2014, 12, 601–617. [Google Scholar] [CrossRef] [PubMed]
- Loaiza-González, J.M.; Salazar, M.C.L.; Rubio-Clemente, A.; Rodriguez, D.C.; Peñuela, G.; Salazar, C.L.; Rodríguez, D.C.; Peñuela, G.A. Efficiency of the removal of microcystin-LR by UV-radiation and hydrogen peroxide. Revista Facultad de Ingeniería Universidad de Antioquia 2019, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Penru, Y.; Guastalli, A.R.; Esplugas, S.; Baig, S. Application of UV and UV/H2O2 to seawater: Disinfection and natural organic matter removal. J. Photochem. Photobiol. A Chem. 2012, 233, 40–45. [Google Scholar] [CrossRef]
- Keen, O.; Bolton, J.; Litter, M.; Bircher, K.; Oppenländer, T. Standard reporting of Electrical Energy per Order (EEO) for UV/H2O2 reactors (IUPAC Technical Report). Pure Appl. Chem. 2018, 90, 1487–1499. [Google Scholar] [CrossRef] [Green Version]
- Schneider, M.; Bláha, L. Advanced oxidation processes for the removal of cyanobacterial toxins from drinking water. Environ. Sci. Eur. 2020, 32, 1–24. [Google Scholar] [CrossRef]
- Grützmacher, G.; Böttcher, G.; Chorus, I.; Bartel, H. Removal of microcystins by slow sand filtration. Environ. Toxicol. 2002, 17, 386–394. [Google Scholar] [CrossRef]
- Jeon, Y.; Li, L.; Calvillo, J.; Ryu, H.; Domingo, J.W.S.; Choi, O.; Brown, J.; Seo, Y. Impact of algal organic matter on the performance, cyanotoxin removal, and biofilms of biologically-active filtration systems. Water Res. 2020, 184, 116120. [Google Scholar] [CrossRef]
- Klassen, N.V.; Marchington, D.; McGowan, H.C. H2O2 Determination by the I3- Method and by KMnO4 Titration. Anal. Chem. 1994, 66, 2921–2925. [Google Scholar] [CrossRef]
- Rahn, R.O.; Bolton, J.; Stefan, M.I. The Iodide/Iodate Actinometer in UV Disinfection: Determination of the Fluence Rate Distribution in UV Reactors. Photochem. Photobiol. 2006, 82, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Andrews, S.; Stefan, M.I.; Bolton, J.R. Optimal methods for quenching H2O2 residuals prior to UFC testing. Water Res. 2003, 37, 3697–3703. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Miino, M.C.; Arab, H.; Bestetti, M.; Franz, S. Decolorization and biodegradability of a real pharmaceutical wastewater treated by H2O2-assisted photoelectrocatalysis on TiO2 meshes. J. Hazard. Mater. 2020, 387, 121668. [Google Scholar] [CrossRef] [PubMed]
- Malpass, G.R.P.; Miwa, D.; Mortari, D.; Machado, S.; Motheo, A. Decolorisation of real textile waste using electrochemical techniques: Effect of the chloride concentration. Water Res. 2007, 41, 2969–2977. [Google Scholar] [CrossRef] [PubMed]
- Farkas, J.; Náfrádi, M.; Hlogyik, T.; Pravda, B.C.; Gajda-Schrantz, K.; Hernádi, K.; Alapi, T. Comparison of advanced oxidation processes in the decomposition of diuron and monuron–efficiency, intermediates, electrical energy per order and the effect of various matrices. Environ. Sci. Water Res. Technol. 2018, 4, 1345–1360. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Yang, Y.; Huang, C.-H.; Zhao, L.; Sun, P. Kinetics and modeling of sulfonamide antibiotic degradation in wastewater and human urine by UV/H2O2 and UV/PDS. Water Res. 2016, 103, 283–292. [Google Scholar] [CrossRef]
- Yao, H.; Sun, P.; Minakata, D.; Crittenden, J.C.; Huang, C.-H. Kinetics and Modeling of Degradation of Ionophore Antibiotics by UV and UV/H2O2. Environ. Sci. Technol. 2013, 47, 4581–4589. [Google Scholar] [CrossRef]
- Sun, P.; Tyree, C.; Huang, C.-H. Inactivation of Escherichia coli, Bacteriophage MS2, and Bacillus Spores under UV/H2O2 and UV/Peroxydisulfate Advanced Disinfection Conditions. Environ. Sci. Technol. 2016, 50, 4448–4458. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorlini, S.; Collivignarelli, C.; Carnevale Miino, M.; Caccamo, F.M.; Collivignarelli, M.C. Kinetics of Microcystin-LR Removal in a Real Lake Water by UV/H2O2 Treatment and Analysis of Specific Energy Consumption. Toxins 2020, 12, 810. https://doi.org/10.3390/toxins12120810
Sorlini S, Collivignarelli C, Carnevale Miino M, Caccamo FM, Collivignarelli MC. Kinetics of Microcystin-LR Removal in a Real Lake Water by UV/H2O2 Treatment and Analysis of Specific Energy Consumption. Toxins. 2020; 12(12):810. https://doi.org/10.3390/toxins12120810
Chicago/Turabian StyleSorlini, Sabrina, Carlo Collivignarelli, Marco Carnevale Miino, Francesca Maria Caccamo, and Maria Cristina Collivignarelli. 2020. "Kinetics of Microcystin-LR Removal in a Real Lake Water by UV/H2O2 Treatment and Analysis of Specific Energy Consumption" Toxins 12, no. 12: 810. https://doi.org/10.3390/toxins12120810
APA StyleSorlini, S., Collivignarelli, C., Carnevale Miino, M., Caccamo, F. M., & Collivignarelli, M. C. (2020). Kinetics of Microcystin-LR Removal in a Real Lake Water by UV/H2O2 Treatment and Analysis of Specific Energy Consumption. Toxins, 12(12), 810. https://doi.org/10.3390/toxins12120810