Effects of Modification of Light Parameters on the Production of Cryptophycin, Cyanotoxin with Potent Anticancer Activity, in Nostoc sp.
Abstract
:1. Introduction
2. Results
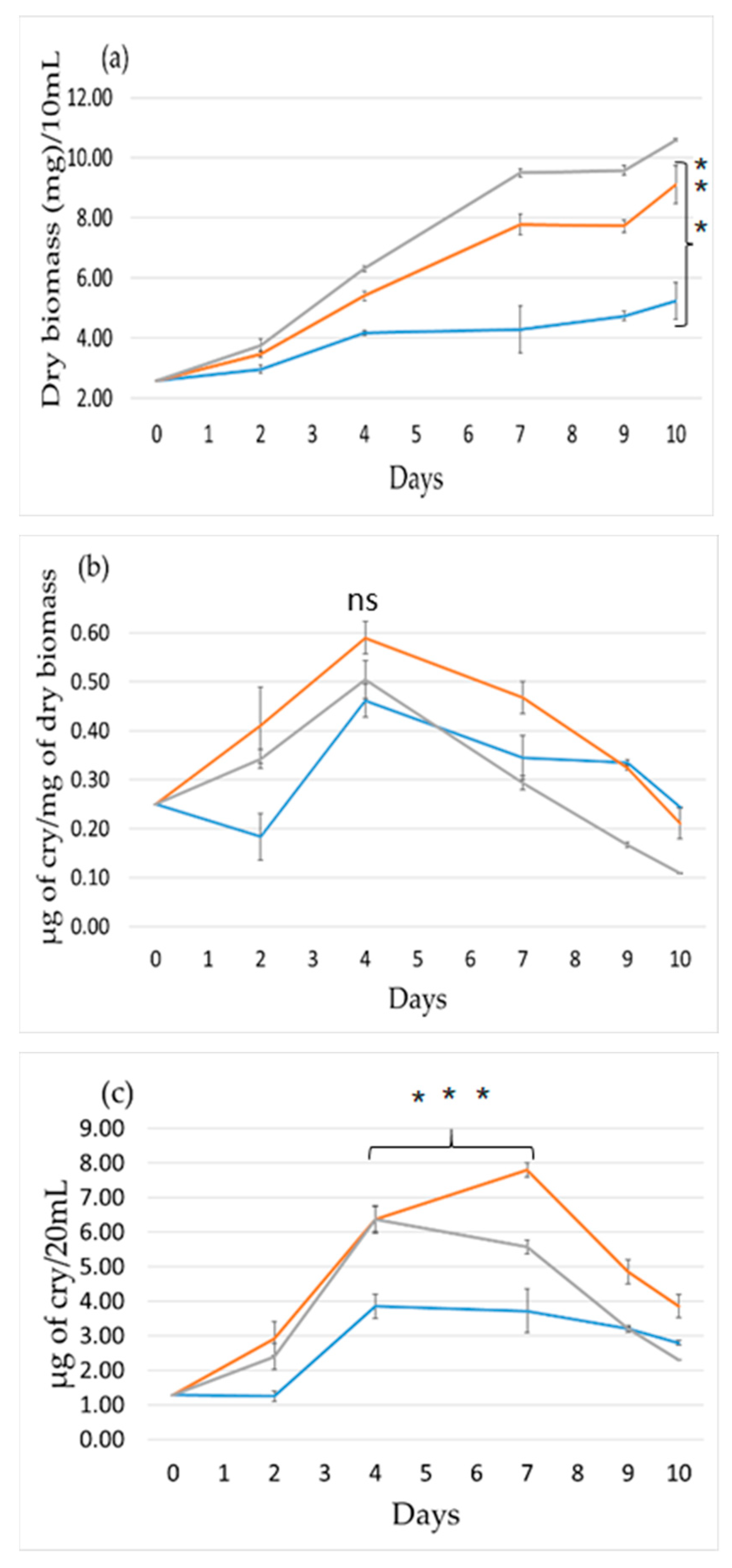
2.1. Effect of Light Intensity
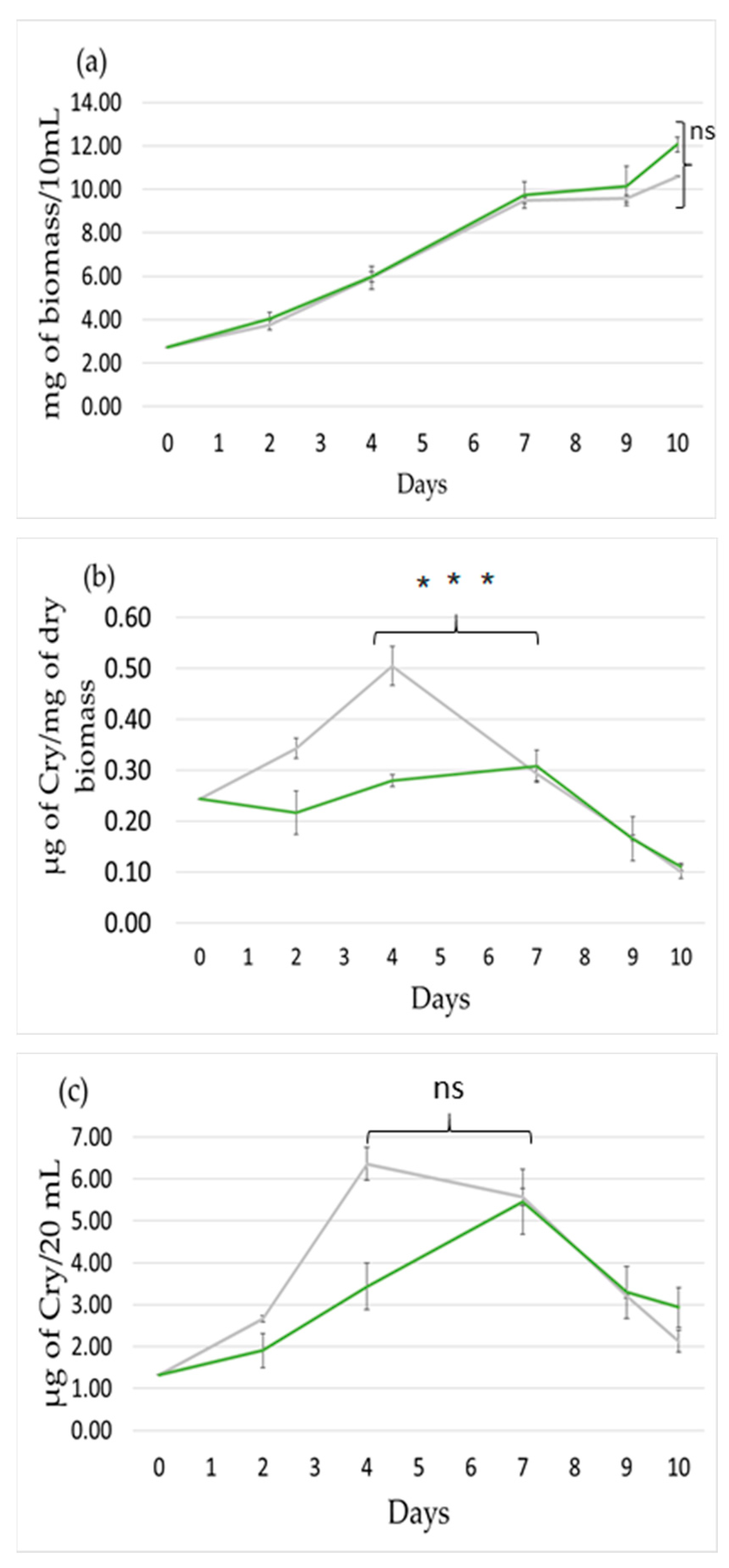
2.2. Effect of Light Wavelength
2.3. Effect Light Photoperiod
3. Discussion
4. Materials and Methods
4.1. Cyanobacterial Strain and Culture Conditions
4.2. Growth Measurements
4.3. Cry Preliminary Quantification
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hitzfeld, B.C.; Höger, S.J.; Dietrich, D.R. Cyanobacterial toxins: Removal during drinking water treatment, and human risk assessment. Environ. Health Perspect. 2000, 108, 113–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheehan, J.; Dunahay, T.; Benemann, J.; Roessler, P. A Look Back at the US Department of Energy’s Aquatic Species Program: Biodiesel from Algae; Close-Out Report; National Renewable Energy Lab: Golden, CO, USA, 1998.
- Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.M. Microalgae for the production of bulk chemicals and biofuels. Biofuels Bioprod. Biorefining 2010, 4, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, M.J.; Wijffels, R.H. Biofuels from microalgae. In Handbook of Microalgal Culture, 2nd ed.; Richmond, A., Hu, Q., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 566–577. [Google Scholar] [CrossRef]
- Hongsthong, A.; Bunnag, B. Overview of Spirulina: Biotechnological, biochemical and molecular biological aspects. In Handbook on Cyanobacteria: Biochemistry, Biotechnology and Applications; Gault, P.M., Marler, H.J., Eds.; Nova Science Publishers: New York, NY, USA, 2009; pp. 51–103. [Google Scholar]
- Vanthoor-Koopmans, M.; Cordoba-Matson, M.V.; Arredondo-Vega, B.O.; Lozano-Ramírez, C.; Garcia-Trejo, J.F.; Rodriguez-Palacio, M.C. Microalgae and cyanobacteria production for feed and food supplements. In Biosystems Engineering: Biofactories for Food Production in the Century XXI.; Guevara-Gonzalez, R., Torres-Pacheco, I., Eds.; Springer: Cham, Switzerland, 2014; pp. 253–275. [Google Scholar] [CrossRef]
- Vaishampayan, A.; Sinha, R.P.; Hader, D.P.; Dey, T.; Gupta, A.K.; Bhan, U.; Rao, A.L. Cyanobacterial biofertilizers in rice agriculture. Bot. Rev. 2001, 67, 453–516. [Google Scholar] [CrossRef]
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front. Microbiol. 2016, 7, 529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borowitzka, M.A. Microalgae as sources of pharmaceuticals and other biologically active compounds. J. Appl. Phycol. 1995, 7, 3–15. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- Rickards, R.W.; Rothschild, J.M.; Willis, A.C.; De Chazal, N.M.; Kirk, J.; Kirk, K.; Saliba, K.J.; Smith, G.D. Calothrixins A and B, novel pentacyclic metabolites from Calothrix cyanobacteria with potent activity against malaria parasites and human cancer cells. Tetrahedron 1999, 55, 13513–13520. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kurosu, M.; Ohyabu, N.; Wang, W.; Fujii, S.; Kitagawa, I. The absolute stereostructure of arenastatin a, a potent cytotoxic depsipeptide from the okinawan marine sponge dysidea arenaria. Chem. Pharm. Bull. 1994, 42, 2196–2198. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, R.E.; Hirsch, C.F.; Sesin, D.F.; Flor, J.E.; Chartrain, M.; Fromtling, R.E.; Harris, G.H.; Salvatore, M.J.; Liesch, J.M.; Yudin, K. Pharmaceuticals from cultured algae. J. Ind. Microbiol. Biotechnol. 1990, 5, 113–123. [Google Scholar] [CrossRef]
- Hirsch, C.F.; Liesch, J.M.; Salvatore, M.J.; Schwartz, R.E.; Sesin, D.F. Antifungal Fermentation Product and Method. U.S. Patent No. 4,946,835, 7 August 1990. [Google Scholar]
- Trimurtulu, G.; Ohtani, I.; Patterson, G.M.L.; Moore, R.E.; Corbett, T.H.; Valeriote, F.A.; Demchik, L. Total structures of cryptophycins, potent antitumor depsipeptides from the blue-green alga Nostoc sp. strain GSV 224. J. Am. Chem. Soc. 1994, 116, 4729–4737. [Google Scholar] [CrossRef]
- Nowruzi, B.; Khavari-Nejad, R.A.; Sivonen, K.; Kazemi, B.; Najafi, F.; Nejadsattari, T. Identification and toxigenic potential of a Nostoc sp. Algae 2012, 27, 303–313. [Google Scholar] [CrossRef]
- Smith, C.D.; Zhang, X.; Mooberry, S.L.; Patterson, G.M.; Moore, R.E. Cryptophycin: A new antimicrotubule agent active against drug-resistant cells. Cancer Res. 1994, 54, 3779–3784. [Google Scholar]
- Smith, C.D.; Zhang, X. Mechanism of Action of Cryptophycin: Interaction with the Vinca alkaloid domain of tubulin. J. Biol. Chem. 1996, 271, 6192–6198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panda, D.; Himes, R.H.; Moore, R.E.; Wilson, L.; Jordan, M.A. Mechanism of action of the unusually potent microtubule inhibitor cryptophycin-1. Biochemistry 1997, 36, 12948–12953. [Google Scholar] [CrossRef] [PubMed]
- Eggen, M.; Georg, G.I. The cryptophycins: Their synthesis and anticancer activity. Med. Res. Rev. 2002, 22, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Weiss, C.; Figueras, E.; Borbely, A.N.; Sewald, N. Cryptophycins: Cytotoxic cyclodepsipeptides with potential for tumor targeting. J. Pept. Sci. 2017, 23, 514–531. [Google Scholar] [CrossRef]
- Moore, R.E.; Corberr, T.H.; Patterson, G.M.L.; Valeriote, F.A. The Search for new antitumor drugs from blue-green algae. Curr. Pharm. Des. 1996, 2, 317–330. [Google Scholar]
- Back, S.; Liang, J. Production of cryptophycin from blue-green algae. J. Young Investig. 2005, 12, 1654–1673. [Google Scholar]
- Biondi, N.; Piccardi, R.; Margheri, M.C.; Rodolfi, L.; Smith, G.D.; Tredici, M.R. Evaluation of Nostoc strain ATCC 53789 as a potential source of natural pesticides. Appl. Environ. Microbiol. 2004, 70, 3313–3320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, H.S.; Lee, C.S.; Srivastava, A.; Oh, H.M.; Ahn, C.Y. Effects of environmental factors on cyanobacterial production of odorous compounds: Geosmin and 2-methylisoborneol. J. Microbiol. Biotechnol. 2017, 27, 1316–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khajepour, F.; Hosseini, S.A.; Nasrabadi, R.G.; Markou, G. Effect of Light Intensity and Photoperiod on Growth and Biochemical Composition of a Local Isolate of Nostoc calcicola. Appl. Biochem. Biotechnol. 2015, 176, 2279–2289. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.F.; Oishi, S. Effects of environmental factors on toxicity of a cyanobacterium (Microcystis aeruginosa) under culture conditions. Appl. Environ. Microbiol. 1985, 49, 1342–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob-Lopes, E.; Scoparo, C.H.G.; Lacerda, L.M.C.F.; Franco, T.T. Effect of light cycles (night/day) on CO2 fixation and biomass production by microalgae in photobioreactors. Chem. Eng. Process. Process. Intensif. 2009, 48, 306–310. [Google Scholar] [CrossRef]
- Zevenboom, W.; Mur, L.R. Growth and photosynthetic response of the cyanobacterium Microcystis aeruginosa in relation to photoperiodicity and irradiance. Arch. Microbiol. 1984, 139, 232–239. [Google Scholar] [CrossRef]
- Ludwig, F.; Medger, A.; Börnick, H.; Opitz, M.; Lang, K.; Göttfert, M.; Röske, I. Identification and expression analyses of putative sesquiterpene synthase genes in Phormidium sp. and prevalence of geoA-like genes in a drinking water reservoir. Appl. Environ. Microbiol. 2007, 73, 6988–6993. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.J. Effect of light with different wavelengths on Nostoc flagelliforme cells in liquid culture. J. Microbiol. Biotechnol. 2013, 23, 534–538. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.Y.; Na Kim, N.; Shin, H.S.; Park, H.G.; Cheon, S.-G.; Kil, G.-S. The effect of various wavelengths of light from light-emitting diodes on the antioxidant system of marine cyanobacteria, Synechococcus sp. Mol. Cell. Toxicol. 2013, 9, 295–302. [Google Scholar] [CrossRef]
- Na Kim, N.; Shin, H.S.; Park, H.G.; Lee, J.; Kil, G.-S.; Choi, C.Y. Profiles of photosynthetic pigment accumulation and expression of photosynthesis-related genes in the marine cyanobacteria Synechococcus sp.: Effects of LED wavelengths. Biotechnol. Bioprocess. Eng. 2014, 19, 250–256. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Aguilera, J.; Niell, F.X. Red and blue light regulation of growth and photosynthetic metabolism in Porphyra umbilicalis (Bangiales, Rhodophyta). Eur. J. Phycol. 1995, 30, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Kirilovsky, D. Modulating energy arriving at photochemical reaction centers: Orange carotenoid protein-related photoprotection and state transitions. Photosynth. Res. 2015, 126, 3–17. [Google Scholar] [CrossRef]
- Luimstra, V.M.; Schuurmans, J.M.; Verschoor, A.M.; Hellingwerf, K.J.; Huisman, J.; Matthijs, H.C.P. Blue light reduces photosynthetic efficiency of cyanobacteria through an imbalance between photosystems I and II. Photosynth. Res. 2018, 138, 177–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaebernick, M.; Neilan, B.A.; Börner, T.; Dittmann, E. Light and the transcriptional response of the microcystin biosynthesis gene cluster. Appl. Environ. Microbiol. 2000, 66, 3387–3392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Westhuizen, A.J.; Eloff, J.N. Effect of temperature and light on the toxicity and growth of the blue-green alga Microcystis aeruginosa (UV-006). Planta 1985, 163, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Sivonen, K. Effects of light, temperature, nitrate, orthophosphate, and bacteria on growth of and hepatotoxin production by Oscillatoria agardhii strains. Appl. Environ. Microbiol. 1990, 56, 2658–2666. [Google Scholar] [CrossRef] [Green Version]
- Rapala, J.; Sivonen, K.; Lyra, C.; Niemelä, S.I. Variation of microcystins, cyanobacterial hepatotoxins, in Anabaena spp. as a function of growth stimuli. Appl. Environ. Microbiol. 1997, 63, 2206–2212. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Sano, T.; Li, R.; Watanabe, M.M.; Liu, Y.; Kaya, K. Microcystin production of Microcystis viridis (cyanobacteria) under different culture conditions. Phycol. Res. 1998, 46, 19–23. [Google Scholar] [CrossRef]
- Kurmayer, R. The toxic cyanobacterium Nostoc sp. strain 152 produces highest amounts of microcystin and nostophycin under stress condtions. J. Phycol. 2011, 47, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Johnson, E.M.; Kumar, K.; Das, D. Physicochemical parameters optimization, and purification of phycobiliproteins from the isolated Nostoc sp. Bioresour. Technol. 2014, 166, 541–547. [Google Scholar] [CrossRef]
- Ma, R.; Lu, F.; Bi, Y.; Hu, Z. Effects of light intensity and quality on phycobiliprotein accumulation in the cyanobacterium Nostoc sphaeroides Kützing. Biotechnol. Lett. 2015, 37, 1663–1669. [Google Scholar] [CrossRef] [Green Version]
- Chmelík, D.; Hrouzek, P.; Fedorko, J.; Vu, D.L.; Urajová, P.; Mareš, J.; Červený, J. Accumulation of cyanobacterial oxadiazine nocuolin A is enhanced by temperature shift during cultivation and is promoted by bacterial co-habitants in the culture. Algal Res. 2019, 44, 101673. [Google Scholar] [CrossRef]
- Zheng, T.; Zhou, M.; Yang, L.; Wang, Y.; Wang, Y.; Meng, Y.; Liu, J.; Zuo, Z. Effects of high light and temperature on Microcystis aeruginosa cell growth and β-cyclocitral emission. Ecotoxicol. Environ. Saf. 2020, 192, 110313. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R.; Stanier, R.Y.; Deruelles, J.; Herdman, M.; Waterbury, J.B. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polyzois, A.; Kirilovsky, D.; Dufat, T.-h.; Michel, S. Effects of Modification of Light Parameters on the Production of Cryptophycin, Cyanotoxin with Potent Anticancer Activity, in Nostoc sp. Toxins 2020, 12, 809. https://doi.org/10.3390/toxins12120809
Polyzois A, Kirilovsky D, Dufat T-h, Michel S. Effects of Modification of Light Parameters on the Production of Cryptophycin, Cyanotoxin with Potent Anticancer Activity, in Nostoc sp. Toxins. 2020; 12(12):809. https://doi.org/10.3390/toxins12120809
Chicago/Turabian StylePolyzois, Alexandros, Diana Kirilovsky, Thi-hanh Dufat, and Sylvie Michel. 2020. "Effects of Modification of Light Parameters on the Production of Cryptophycin, Cyanotoxin with Potent Anticancer Activity, in Nostoc sp." Toxins 12, no. 12: 809. https://doi.org/10.3390/toxins12120809
APA StylePolyzois, A., Kirilovsky, D., Dufat, T.-h., & Michel, S. (2020). Effects of Modification of Light Parameters on the Production of Cryptophycin, Cyanotoxin with Potent Anticancer Activity, in Nostoc sp. Toxins, 12(12), 809. https://doi.org/10.3390/toxins12120809



