Fusarium Cyclodepsipeptide Mycotoxins: Chemistry, Biosynthesis, and Occurrence
Abstract
:1. Introduction
2. Chemistry
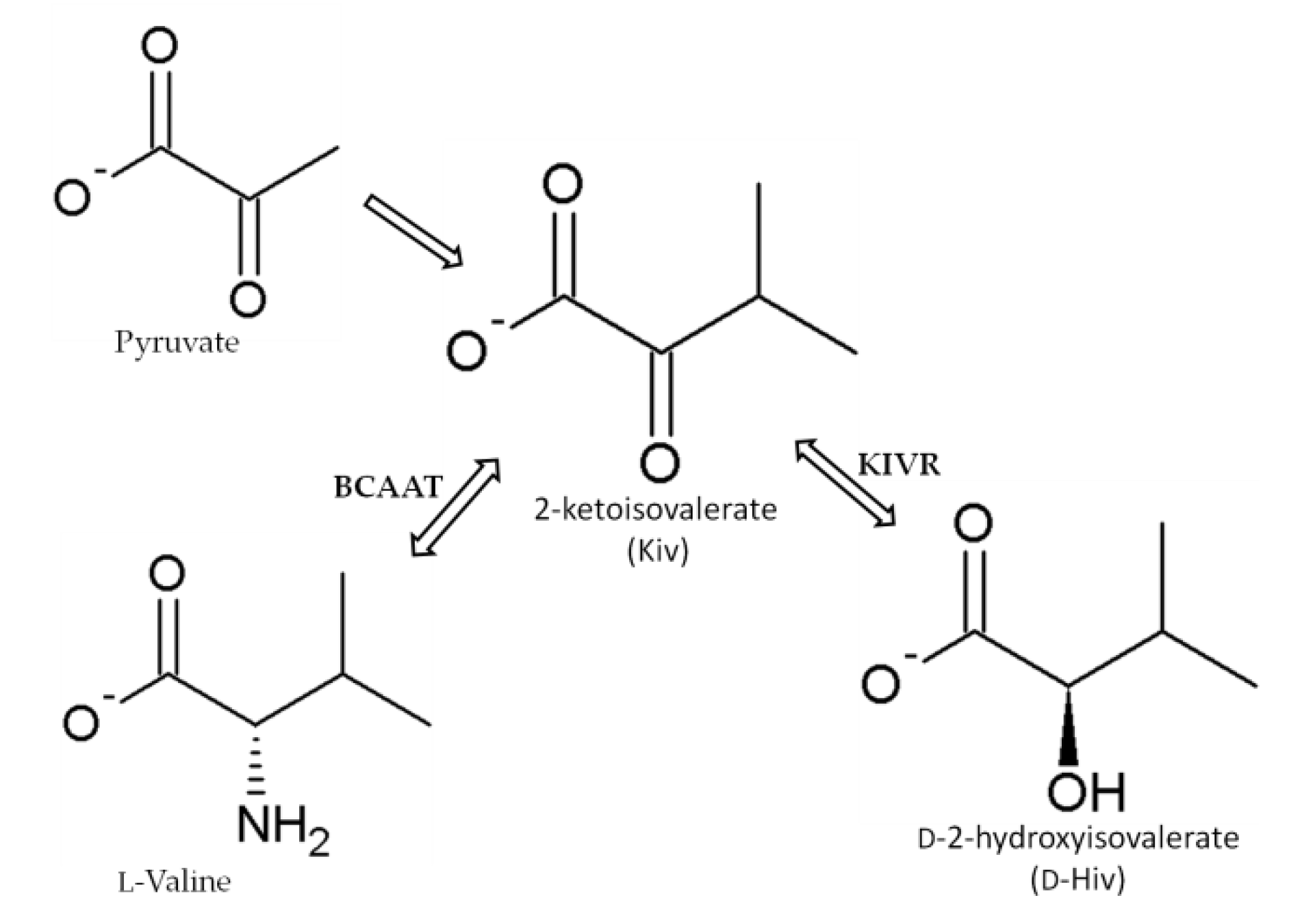
3. Biosynthesis
4. Fusarium Species and Cyclodepsipeptide Mycotoxins in Food and Feed
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jestoi, M.; Rokka, M.; Yli-Mattila, T.; Parikka, P.; Rizzo, A.; Peltonen, K. Presence and Concentrations of the Fusarium-Related Mycotoxins Beauvericin, Enniatins and Moniliformin in Finnish Grain Samples. Food Addit. Contam. 2004, 21, 794–802. [Google Scholar] [CrossRef]
- Urbaniak, M.; Stepien, L.; Uhlig, S. Evidence for Naturally Produced Beauvericins Containing N-Methyl-Tyrosine in Hypocreales Fungi. Toxins 2019, 11, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbaniak, M.; Waskiewicz, A.; Trzebny, A.; Koczyk, G.; Stepien, L. Cyclodepsipeptide Biosynthesis in Hypocreales Fungi and Sequence Divergence of the Non-Ribosomal Peptide Synthase Genes. Pathogens 2020, 9, 552. [Google Scholar] [CrossRef] [PubMed]
- Hornbogen, T.; Glinski, M.; Zocher, R. Biosynthesis of Depsipeptide Mycotoxins in Fusarium. Eur. J. Plant Pathol. 2002, 108, 713–718. [Google Scholar] [CrossRef]
- Logrieco, A.; Rizzo, A.; Ferracane, R.; Ritieni, A. Occurrence of Beauvericin and Enniatins in Wheat Affected by Fusarium avenaceum Head Blight. Appl. Environ. Microbiol. 2002, 68, 82–85. [Google Scholar] [CrossRef] [Green Version]
- Galvez, L.; Urbaniak, M.; Waskiewicz, A.; Stepien, L.; Palmero, D. Fusarium proliferatum—Causal Agent of Garlic Bulb Rot in Spain: Genetic Variability and Mycotoxin Production. Food Microbiol. 2017, 67, 41–48. [Google Scholar] [CrossRef]
- Jajic, I.; Dudas, T.; Krstovic, S.; Krska, R.; Sulyok, M.; Bagi, F.; Savic, Z.; Guljas, D.; Stankov, A. Emerging Fusarium Mycotoxins Fusaproliferin, Beauvericin, Enniatins, and Moniliformin in Serbian Maize. Toxins 2019, 11, 357. [Google Scholar] [CrossRef] [Green Version]
- Stepien, L.; Waskiewicz, A.; Urbaniak, M. Wildly Growing Asparagus (Asparagus officinalis L.) Hosts Pathogenic Fusarium Species and Accumulates Their Mycotoxins. Microb. Ecol. 2016, 71, 927–937. [Google Scholar] [CrossRef] [Green Version]
- Tomczyk, L.; Stepien, L.; Urbaniak, M.; Szablewski, T.; Cegielska-Radziejewska, R.; Stuper-Szablewska, K. Characterisation of the Mycobiota on the Shell Surface of Table Eggs Acquired from Different Egg-Laying Hen Breeding Systems. Toxins 2018, 10, 293. [Google Scholar] [CrossRef] [Green Version]
- Jestoi, M. Emerging Fusarium-Mycotoxins Fusaproliferin, Beauvericin, Enniatins, and Moniliformin: A Review. Crit. Rev. Food Sci. Nutr. 2008, 48, 21–49. [Google Scholar] [CrossRef]
- Bertero, A.; Moretti, A.; Spicer, L.J.; Caloni, F. Fusarium Molds and Mycotoxins: Potential Species-Specific Effects. Toxins 2018, 10, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraeyman, S.; Croubels, S.; Devreese, M.; Antonissen, G. Emerging Fusarium and Alternaria Mycotoxins: Occurrence, Toxicity and Toxicokinetics. Toxins 2017, 9, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belen Serrano, A.; Capriotti, A.L.; Cavaliere, C.; Piovesana, S.; Samperi, R.; Ventura, S.; Lagana, A. Development of a Rapid LC-MS/MS Method for the Determination of Emerging Fusarium Mycotoxins Enniatins and Beauvericin in Human Biological Fluids. Toxins 2015, 7, 3554–3571. [Google Scholar] [CrossRef] [Green Version]
- Perincherry, L.; Lalak-Kanczugowska, J.; Stepien, L. Fusarium-Produced Mycotoxins in Plant-Pathogen Interactions. Toxins 2019, 11, 664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, M.; Zocher, R.; Haese, A. Enniatin Production by Fusarium Strains and Its Effect on Potato Tuber Tissue. Appl. Environ. Microbiol. 1996, 62, 393–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.H.; Wang, F.T.; Chan, W.H. Enniatin B Induces Dosage-Related Apoptosis or Necrosis in Mouse Blastocysts Leading to Deleterious Effects on Embryo Development. Drug Chem. Toxicol. 2020, 1–12. [Google Scholar] [CrossRef]
- Zuzek, M.C.; Grandic, M.; Jakovac Strajn, B.; Frangez, R. Beauvericin Inhibits Neuromuscular Transmission and Skeletal Muscle Contractility in Mouse Hemidiaphragm Preparation. Toxicol. Sci. 2016, 150, 283–291. [Google Scholar] [CrossRef] [Green Version]
- Schoevers, E.J.; Santos, R.R.; Fink-Gremmels, J.; Roelen, B.A. Toxicity of Beauvericin on Porcine Oocyte Maturation and Preimplantation Embryo Development. Reprod. Toxicol. 2016, 65, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Albonico, M.; Schutz, L.F.; Caloni, F.; Cortinovis, C.; Spicer, L.J. In Vitro Effects of the Fusarium Mycotoxins Fumonisin B1 and Beauvericin on Bovine Granulosa Cell Proliferation and Steroid Production. Toxicon 2017, 128, 38–45. [Google Scholar] [CrossRef]
- Kalayou, S.; Ndossi, D.; Frizzell, C.; Groseth, P.K.; Connolly, L.; Sorlie, M.; Verhaegen, S.; Ropstad, E. An Investigation of the Endocrine Disrupting Potential of Enniatin B Using in Vitro Bioassays. Toxicol. Lett. 2015, 233, 84–94. [Google Scholar] [CrossRef]
- Uhlig, S.; Ivanova, L.; Petersen, D.; Kristensen, R. Structural Studies on Minor Enniatins from Fusarium sp. VI 03441: Novel N-Methyl-Threonine Containing Enniatins. Toxicon 2009, 53, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Jow, G.M.; Chou, C.J.; Chen, B.F.; Tsai, J.H. Beauvericin Induces Cytotoxic Effects in Human Acute Lymphoblastic Leukemia Cells through Cytochrome C Release, Caspase 3 Activation: The Causative Role of Calcium. Cancer Lett. 2004, 216, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.F.; Tsai, M.C.; Jow, G.M. Induction of Calcium Influx from Extracellular Fluid by Beauvericin in Human Leukemia Cells. Biochem. Biophys Res. Commun. 2006, 340, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Watjen, W.; Debbab, A.; Hohlfeld, A.; Chovolou, Y.; Kampkotter, A.; Edrada, R.A.; Ebel, R.; Hakiki, A.; Mosaddak, M.; Totzke, F.; et al. Enniatins A1, B and B1 from an Endophytic Strain of Fusarium tricinctum Induce Apoptotic Cell Death in H4IIE Hepatoma Cells Accompanied by Inhibition of ERK Phosphorylation. Mol. Nutr. Food Res. 2009, 53, 431–440. [Google Scholar] [CrossRef]
- Ivanova, L.; Skjerve, E.; Eriksen, G.S.; Uhlig, S. Cytotoxicity of Enniatins A, A1, B, B1, B2 and B3 from Fusarium avenaceum. Toxicon 2006, 47, 868–876. [Google Scholar] [CrossRef]
- Isaka, M.; Yangchum, A.; Sappan, M.; Suvannakad, R.; Srikitikulchai, P. Cyclohexadepsipeptides from Acremonium Sp Bcc 28424. Tetrahedron 2011, 67, 7929–7935. [Google Scholar] [CrossRef]
- Hyun, U.; Lee, D.-H.; Lee, C.; Shin, C.-G. Apoptosis induced by enniatins H and MK1688 isolated from Fusarium oxysporum FB1501. Toxicon 2009, 53, 723–728. [Google Scholar] [CrossRef]
- Kamyar, M.; Rawnduzi, P.; Studenik, C.R.; Kouri, K.; Lemmens-Gruber, R. Investigation of the Electrophysiological Properties of Enniatins. Arch. Biochem. Biophys 2004, 429, 215–223. [Google Scholar] [CrossRef]
- Zhang, L.X.; Yan, K.Z.; Zhang, Y.; Huang, R.; Bian, J.; Zheng, C.S.; Sun, H.X.; Chen, Z.H.; Sun, N.; An, R.; et al. High-Throughput Synergy Screening Identifies Microbial Metabolites as Combination Agents for the Treatment of Fungal Infections. Proc. Natl. Acad. Sci. USA 2007, 104, 4606–4611. [Google Scholar] [CrossRef] [Green Version]
- Bunyapaiboonsri, T.; Vongvilai, P.; Auncharoen, P.; Isaka, M. Cyclohexadepsipeptides from the Filamentous Fungus Acremonium sp. Bcc 2629. Helv. Chim. Acta 2012, 95, 963–972. [Google Scholar] [CrossRef]
- Xu, Y.; Zhan, J.; Wijeratne, E.M.K.; Burns, A.M.; Gunatilaka, A.A.L.; Molnar, I. Cytotoxic and Antihaptotactic Beauvericin Analogues from Precursor-Directed Biosynthesis with the Insect Pathogen Beauveria bassiana ATCC 7159. J. Nat. Prod. 2007, 70, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Dornetshuber, R.; Heffeter, P.; Kamyar, M.R.; Peterbauer, T.; Berger, W.; Lemmens-Gruber, R. Enniatin Exerts P53-Dependent Cytostatic and P53-Independent Cytotoxic Activities against Human Cancer Cells. Chem. Res. Toxicol. 2007, 20, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Nilanonta, C.; Isaka, M.; Kittakoop, P.; Trakulnaleamsai, S.; Tanticharoen, M.; Thebtaranonth, Y. Precursor-Directed Biosynthesis of Beauvericin Analogs by the Insect Pathogenic Fungus Paecilomyces tenuipes BCC 1614. Tetrahedron 2002, 58, 3355–3360. [Google Scholar] [CrossRef]
- Fukuda, T.; Arai, M.; Yamaguchi, Y.; Masuma, R.; Tomoda, H.; Omura, S. New Beauvericins, Potentiators of Antifungal Miconazole Activity, Produced by Beauveria sp. FKI-1366—I. Taxonomy, Fermentation, Isolation and Biological Properties. J. Antibiot. 2004, 57, 110–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Supothina, S.; Isaka, M.; Kirtikara, K.; Tanticharoen, M.; Thebtaranonth, Y. Enniatin Production by the Entomopathogenic Fungus Verticillium hemipterigenum BCC 1449. J. Antibiot. 2004, 57, 732–738. [Google Scholar] [CrossRef] [Green Version]
- Meca, G.; Sospedra, I.; Soriano, J.M.; Ritieni, A.; Moretti, A.; Manes, J. Antibacterial Effect of the Bioactive Compound Beauvericin Produced by Fusarium proliferatum on Solid Medium of Wheat. Toxicon 2010, 56, 349–354. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J.; Zhao, J.; Li, P.; Shan, T.; Wang, J.; Li, X.; Zhou, L. Beauvericin from the Endophytic Fungus, Fusarium redolens, Isolated from Dioscorea zingiberensis and Its Antibacterial Activity. Nat. Prod. Commun. 2010, 5, 811–814. [Google Scholar] [CrossRef] [Green Version]
- Hamill, R.L.; Higgens, C.E.; Boaz, H.E.; Gorman, M. The Structure of Beauvericin, a New Depsipeptide Antibiotic Toxic to Artemia salina. Tetrahedron Lett. 1969, 10, 4255–4258. [Google Scholar] [CrossRef]
- Stepien, L.; Waskiewicz, A. Sequence Divergence of the Enniatin Synthase Gene in Relation to Production of Beauvericin and Enniatins in Fusarium Species. Toxins 2013, 5, 537–555. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, L. Beauvericin, a Bioactive Compound Produced by Fungi: A Short Review. Molecules 2012, 17, 2367–2377. [Google Scholar] [CrossRef]
- Sivanathan, S.; Scherkenbeck, J. Cyclodepsipeptides: A Rich Source of Biologically Active Compounds for Drug Research. Molecules 2014, 19, 12368–12420. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Arai, M.; Tomoda, H.; Omura, S. New Beauvericins, Potentiators of Antifungal Miconazole Activity, Produced by Beauveria sp. FKI-1366—II. Structure Elucidation. J. Antibiot. 2004, 57, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohanka, A.; Capieau, K.; Broberg, A.; Stenlid, J.; Stenstrom, E.; Kenne, L. Enniatins of Fusarium sp. Strain F31 and Their Inhibition of Botrytis cinerea Spore Germination. J. Nat. Prod. 2004, 67, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Song, H.H.; Lee, H.S.; Jeong, J.H.; Park, H.S.; Lee, C. Diversity in Beauvericin and Enniatins H, I, and Mk1688 by Fusarium oxysporum Isolated from Potato. Int. J. Food Microbiol. 2008, 122, 296–301. [Google Scholar] [CrossRef]
- Tomoda, H.; Nishida, H.; Huang, X.H.; Masuma, R.; Kim, Y.K.; Omura, S. New Cyclodepsipeptides, Enniatins D, E and F Produced by Fusarium sp. FO-1305. J. Antibiot. 1992, 45, 1207–1215. [Google Scholar] [CrossRef] [Green Version]
- Blais, L.A.; ApSimon, J.W. Isolation and Characterization of Enniatins from Fusarium avenaceum DAOM 196490. Can. J. Chem. 1992, 70, 1281–1287. [Google Scholar] [CrossRef]
- Visconti, A.; Blais, L.A.; ApSimon, J.W.; Greenhalgh, R.; Miller, J.D. Production of Enniatins by Fusarium acuminatum and Fusarium compactum in Liquid Culture: Isolation and Characterization of Three New Enniatins, B2, B3, and B4. J. Agric. Food Chem. 1992, 40, 1076–1082. [Google Scholar] [CrossRef]
- Bushley, K.E.; Turgeon, B.G. Phylogenomics Reveals Subfamilies of Fungal Nonribosomal Peptide Synthetases and Their Evolutionary Relationships. BMC Evol. Biol. 2010, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Gallo, A.; Ferrara, M.; Perrone, G. Phylogenetic Study of Polyketide Synthases and Nonribosomal Peptide Synthetases Involved in the Biosynthesis of Mycotoxins. Toxins 2013, 5, 717–742. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Orozco, R.; Wijeratne, E.M.; Gunatilaka, A.A.; Stock, S.P.; Molnar, I. Biosynthesis of the Cyclooligomer Depsipeptide Beauvericin, a Virulence Factor of the Entomopathogenic Fungus Beauveria bassiana. Chem. Biol. 2008, 15, 898–907. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Zhuo, Y.; Jia, X.; Liu, J.; Gao, H.; Song, F.; Liu, M.; Zhang, L. Cloning and Characterization of the Gene Cluster Required for Beauvericin Biosynthesis in Fusarium proliferatum. Sci. China Life Sci. 2013, 56, 628–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Orozco, R.; Kithsiri Wijeratne, E.M.; Espinosa-Artiles, P.; Leslie Gunatilaka, A.A.; Patricia Stock, S.; Molnar, I. Biosynthesis of the Cyclooligomer Depsipeptide Bassianolide, an Insecticidal Virulence Factor of Beauveria bassiana. Fungal Genet. Biol. 2009, 46, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Zocher, R.; Keller, U.; Kleinkauf, H. Enniatin Synthetase, a Novel Type of Multifunctional Enzyme Catalyzing Depsipeptide Synthesis in Fusarium oxysporum. Biochemistry 1982, 21, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, V.C.; Mirabelli, V.; Cimmarusti, M.T.; Haidukowski, M.; Leslie, J.F.; Logrieco, A.F.; Caliandro, R.; Fanelli, F.; Mule, G. Enniatin and Beauvericin Biosynthesis in Fusarium Species: Production Profiles and Structural Determinant Prediction. Toxins 2017, 9, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulik, T.; Pszczolkowska, A.; Fordonski, G.; Olszewski, J. Pcr Approach Based on the Esyn1 Gene for the Detection of Potential Enniatin-Producing Fusarium Species. Int. J. Food Microbiol. 2007, 116, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, P.; Simpson, D.; Wilson, A.; Chandler, E.; Thomsett, M. Detection and Differentiation of Trichothecene and Enniatin-Producing Fusarium Species on Small-Grain Cereals. Eur. J. Plant Pathol. 2004, 110, 503–514. [Google Scholar] [CrossRef]
- Stepien, L.; Jestoi, M.; Chelkowski, J. Cyclic Hexadepsipeptides in Wheat Field Samples and Esyn1 Gene Divergence among Enniatin Producing Fusarium avenaceum Strains. World Mycotoxin J. 2013, 6, 399–409. [Google Scholar] [CrossRef]
- Hornbogen, T.; Riechers, S.P.; Prinz, B.; Schultchen, J.; Lang, C.; Schmidt, S.; Mugge, C.; Turkanovic, S.; Sussmuth, R.D.; Tauberger, E.; et al. Functional Characterization of the Recombinant N-Methyltransferase Domain from the Multienzyme Enniatin Synthetase. Chembiochem 2007, 8, 1048–1054. [Google Scholar] [CrossRef]
- Zocher, R.; Keller, U.; Kleinkauf, H. Mechanism of Depsipeptide Formation Catalyzed by Enniatin Synthetase. Biochem. Biophys. Res. Commun. 1983, 110, 292–299. [Google Scholar] [CrossRef]
- Zocher, R.; Keller, U. Thiol Template Peptide Synthesis Systems in Bacteria and Fungi. Adv. Microb. Physiol. 1997, 38, 85–131. [Google Scholar] [CrossRef]
- Feifel, S.C.; Schmiederer, T.; Hornbogen, T.; Berg, H.; Sussmuth, R.D.; Zocher, R. In Vitro Synthesis of New Enniatins: Probing the Alpha-D-Hydroxy Carboxylic Acid Binding Pocket of the Multienzyme Enniatin Synthetase. Chembiochem 2007, 8, 1767–1770. [Google Scholar] [CrossRef] [PubMed]
- Pieper, R.; Kleinkauf, H.; Zocher, R. Enniatin Synthetases from Different Fusaria Exhibiting Distinct Amino Acid Specificities. J. Antibiot. 1992, 45, 1273–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krause, M.; Lindemann, A.; Glinski, M.; Hornbogen, T.; Bonse, G.; Jeschke, P.; Thielking, G.; Gau, W.; Kleinkauf, H.; Zocher, R. Directed Biosynthesis of New Enniatins. J. Antibiot. 2001, 54, 797–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billich, A.; Zocher, R. Constitutive Expression of Enniatin Synthetase During Fermentative Growth of Fusarium scirpi. Appl. Environ. Microbiol. 1988, 54, 2504–2509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haese, A.; Schubert, M.; Herrmann, M.; Zocher, R. Molecular Characterization of the Enniatin Synthetase Gene Encoding a Multifunctional Enzyme Catalysing N-Methyldepsipeptide Formation in Fusarium scirpi. Mol. Microbiol. 1993, 7, 905–914. [Google Scholar] [CrossRef]
- Peeters, H.; Zocher, R.; Kleinkauf, H. Synthesis of Beauvericin by a Multifunctional Enzyme. J. Antibiot. 1988, 41, 352–359. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Wijeratne, E.M.; Espinosa-Artiles, P.; Gunatilaka, A.A.; Molnar, I. Combinatorial Mutasynthesis of Scrambled Beauvericins, Cyclooligomer Depsipeptide Cell Migration Inhibitors from Beauveria bassiana. Chembiochem 2009, 10, 345–354. [Google Scholar] [CrossRef]
- Heider, J.; Mai, X.; Adams, M.W. Characterization of 2-Ketoisovalerate Ferredoxin Oxidoreductase, a New and Reversible Coenzyme a-Dependent Enzyme Involved in Peptide Fermentation by Hyperthermophilic Archaea. J. Bacteriol. 1996, 178, 780–787. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Yoon, D.H.; Oh, J.; Hyun, M.W.; Han, J.G.; Sung, G.H. Calmodulin-Mediated Suppression of 2-Ketoisovalerate Reductase in Beauveria bassiana Beauvericin Biosynthetic Pathway. Environ. Microbiol. 2016, 18, 4136–4143. [Google Scholar] [CrossRef]
- Zhang, T.; Jia, X.; Zhuo, Y.; Liu, M.; Gao, H.; Liu, J.; Zhang, L. Cloning and Characterization of a Novel 2-Ketoisovalerate Reductase from the Beauvericin Producer Fusarium proliferatum LF061. BMC Biotechnol. 2012, 12, 55. [Google Scholar] [CrossRef] [Green Version]
- Covarelli, L.; Beccari, G.; Prodi, A.; Generotti, S.; Etruschi, F.; Meca, G.; Juan, C.; Manes, J. Biosynthesis of Beauvericin and Enniatins in Vitro by Wheat Fusarium Species and Natural Grain Contamination in an Area of Central Italy. Food Microbiol. 2015, 46, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Chełkowski, J.; Ritieni, A.; Wiśniewska, H.; Mulè, G.; Logrieco, A. Occurrence of Toxic Hexadepsipeptides in Preharvest Maize Ear Rot Infected by Fusarium poae in Poland. J. Phytopathol. 2006, 155, 8–12. [Google Scholar] [CrossRef]
- Kulik, T.; Pszczolkowska, A.; Lojko, M. Multilocus Phylogenetics Show High Intraspecific Variability within Fusarium avenaceum. Int. J. Mol. Sci. 2011, 12, 5626–5640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorczyca, A.; Oleksy, A.; Gala-Czekaj, D.; Urbaniak, M.; Laskowska, M.; Waskiewicz, A.; Stepien, L. Fusarium Head Blight Incidence and Mycotoxin Accumulation in Three Durum Wheat Cultivars in Relation to Sowing Date and Density. Sci Nat-Heidelberg. 2018, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arie, T. Fusarium Diseases of Cultivated Plants, Control, Diagnosis, and Molecular and Genetic Studies. J. Pestic. Sci. 2019, 44, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Stanciu, O.; Juan, C.; Miere, D.; Loghin, F.; Manes, J. Presence of Enniatins and Beauvericin in Romanian Wheat Samples: From Raw Material to Products for Direct Human Consumption. Toxins 2017, 9, 189. [Google Scholar] [CrossRef] [Green Version]
- Decleer, M.; Landschoot, S.; De Saeger, S.; Rajkovic, A.; Audenaert, K. Impact of Fungicides and Weather on Cyclodepsipeptide-Producing Fusarium Spp. And Beauvericin and Enniatin Levels in Wheat Grains. J. Sci. Food Agric. 2019, 99, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Logrieco, A.; Moretti, A.; Castella, G.; Kostecki, M.; Golinski, P.; Ritieni, A.; Chelkowski, J. Beauvericin Production by Fusarium Species. Appl. Environ. Microb. 1998, 64, 3084–3088. [Google Scholar] [CrossRef] [Green Version]
- Moretti, A.; Mule, G.; Ritieni, A.; Logrieco, A. Further Data on the Production of Beauvericin, Enniatins and Fusaproliferin and Toxicity to Artemia salina by Fusarium Species of Gibberella fujikuroi Species Complex. Int. J. Food Microbiol. 2007, 118, 158–163. [Google Scholar] [CrossRef]
- Morrison, E.; Kosiak, B.; Ritieni, A.; Aastveit, A.H.; Uhlig, S.; Bernhoft, A. Mycotoxin Production by Fusarium avenaceum Strains Isolated from Norwegian Grain and the Cytotoxicity of Rice Culture Extracts to Porcine Kidney Epithelial Cells. J. Agric. Food Chem. 2002, 50, 3070–3075. [Google Scholar] [CrossRef]
- Fanelli, F.; Ferracane, R.; Ritieni, A.; Logrieco, A.F.; Mule, G. Transcriptional Regulation of Enniatins Production by Fusarium avenaceum. J. Appl. Microbiol. 2014, 116, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Fotso, J.; Leslie, J.F.; Smith, J.S. Production of Beauvericin, Moniliformin, Fusaproliferin, and Fumonisins B(1), B(2), and B(3) by Fifteen Ex-Type Strains of Fusarium Species. Appl. Environ. Microbiol. 2002, 68, 5195–5197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosch, U.; Mirocha, C.J.; Abbas, H.K.; di Menna, M. Toxicity and Toxin Production by Fusarium Isolates from New Zealand. Mycopathologia 1989, 108, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Shephard, G.S.; Sewram, V.; Nieuwoudt, T.W.; Marasas, W.F.; Ritieni, A. Production of the Mycotoxins Fusaproliferin and Beauvericin by South African Isolates in the Fusarium Section Liseola. J. Agric. Food Chem. 1999, 47, 5111–5115. [Google Scholar] [CrossRef]
- Garcia-Cela, E.; Kiaitsi, E.; Medina, A.; Sulyok, M.; Krska, R.; Magan, N. Interacting Environmental Stress Factors Affects Targeted Metabolomic Profiles in Stored Natural Wheat and That Inoculated with F. graminearum. Toxins 2018, 10, 56. [Google Scholar] [CrossRef] [Green Version]
- Leslie, J.F.; Zeller, K.A.; Logrieco, A.; Mule, G.; Moretti, A.; Ritieni, A. Species Diversity of and Toxin Production by Gibberella fujikuroi Species Complex Strains Isolated from Native Prairie Grasses in Kansas. Appl. Environ. Microbiol. 2004, 70, 2254–2262. [Google Scholar] [CrossRef] [Green Version]
- Thrane, U.; Adler, A.; Clasen, P.E.; Galvano, F.; Langseth, W.; Lew, H.; Logrieco, A.; Nielsen, K.F.; Ritieni, A. Diversity in Metabolite Production by Fusarium langsethiae, Fusarium poae, and Fusarium sporotrichioides. Int. J. Food Microbiol. 2004, 95, 257–266. [Google Scholar] [CrossRef]
- Gupta, S.; Krasnoff, S.B.; Underwood, N.L.; Renwick, J.A.; Roberts, D.W. Isolation of Beauvericin as an Insect Toxin from Fusarium semitectum and Fusarium moniliforme Var. subglutinans. Mycopathologia 1991, 115, 185–189. [Google Scholar] [CrossRef]
- Moretti, A.; Mule, G.; Ritieni, A.; Laday, M.; Stubnya, V.; Hornok, L.; Logrieco, A. Cryptic Subspecies and Beauvericin Production by Fusarium subglutinans from Europe. Int. J. Food Microbiol. 2008, 127, 312–315. [Google Scholar] [CrossRef]
- Fumero, M.V.; Villani, A.; Susca, A.; Haidukowski, M.; Cimmarusti, M.T.; Toomajian, C.; Leslie, J.F.; Chulze, S.N.; Moretti, A. Fumonisin and Beauvericin Chemotypes and Genotypes of the Sister Species Fusarium subglutinans and Fusarium temperatum. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef]
- Langseth, W.; Bernhoft, A.; Rundberget, T.; Kosiak, B.; Gareis, M. Mycotoxin Production and Cytotoxicity of Fusarium Strains Isolated from Norwegian Cereals. Mycopathologia 1998, 144, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Altomare, C.; Logrieco, A.; Bottalico, A.; Mule, G.; Moretti, A.; Evidente, A. Production of Type a Trichothecenes and Enniatin B by Fusarium sambucinum Fuckel Sensu Lato. Mycopathologia 1995, 129, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, V.; Randazzo, A.; Meca, G.; Moretti, A.; Cascone, A.; Eriksson, O.; Novellino, E.; Ritieni, A. Production of Enniatins A, A1, B, B1, B4, J1 by Fusarium tricinctum in Solid Corn Culture: Structural Analysis and Effects on Mitochondrial Respiration. Food Chem. 2013, 140, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Bottalico, A.; Logrieco, A.; Ritieni, A.; Moretti, A.; Randazzo, G.; Corda, P. Beauvericin and Fumonisin B1 in Preharvest Fusarium moniliforme Maize Ear Rot in Sardinia. Food Addit. Contam. 1995, 12, 599–607. [Google Scholar] [CrossRef]
- Gromadzka, K.; Blaszczyk, L.; Chelkowski, J.; Waskiewicz, A. Occurrence of Mycotoxigenic Fusarium Species and Competitive Fungi on Preharvest Maize Ear Rot in Poland. Toxins 2019, 11, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oueslati, S.; Meca, G.; Mliki, A.; Ghorbel, A.; Mañes, J. Determination of Fusarium Mycotoxins Enniatins, Beauvericin and Fusaproliferin in Cereals and Derived Products from Tunisia. Food Control 2011, 22, 1373–1377. [Google Scholar] [CrossRef]
- Meca, G.; Zinedine, A.; Blesa, J.; Font, G.; Manes, J. Further Data on the Presence of Fusarium Emerging Mycotoxins Enniatins, Fusaproliferin and Beauvericin in Cereals Available on the Spanish Markets. Food Chem. Toxicol. 2010, 48, 1412–1416. [Google Scholar] [CrossRef]
- Tolosa, J.; Rodriguez-Carrasco, Y.; Ferrer, E.; Manes, J. Identification and Quantification of Enniatins and Beauvericin in Animal Feeds and Their Ingredients by LC-QTRAP/MS/MS. Metabolites 2019, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Mahnine, N.; Meca, G.; Elabidi, A.; Fekhaoui, M.; Saoiabi, A.; Font, G.; Mañes, J.; Zinedine, A. Further Data on the Levels of Emerging Fusarium Mycotoxins Enniatins (A, A1, B, B1), Beauvericin and Fusaproliferin in Breakfast and Infant Cereals from Morocco. Food Chem. 2010, 124, 481–485. [Google Scholar] [CrossRef]
- Juan, C.; Ritieni, A.; Manes, J. Occurrence of Fusarium Mycotoxins in Italian Cereal and Cereal Products from Organic Farming. Food Chem. 2013, 141, 1747–1755. [Google Scholar] [CrossRef]
- Kolawole, O.; Graham, A.; Donaldson, C.; Owens, B.; Abia, W.A.; Meneely, J.; Alcorn, M.J.; Connolly, L.; Elliott, C.T. Low Doses of Mycotoxin Mixtures Below Eu Regulatory Limits Can Negatively Affect the Performance of Broiler Chickens: A Longitudinal Study. Toxins 2020, 12, 433. [Google Scholar] [CrossRef] [PubMed]
- Novak, B.; Rainer, V.; Sulyok, M.; Haltrich, D.; Schatzmayr, G.; Mayer, E. Twenty-Eight Fungal Secondary Metabolites Detected in Pig Feed Samples: Their Occurrence, Relevance and Cytotoxic Effects in Vitro. Toxins 2019, 11, 537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nacher-Mestre, J.; Beltran, E.; Strachan, F.; Dick, J.R.; Perez-Sanchez, J.; Berntssen, M.H.G.; Tocher, D.R. No Transfer of the Non-Regulated Mycotoxins, Beauvericin and Enniatins, from Feeds to Farmed Fish Reared on Plant-Based Diets. Food Chem. 2020, 323, 126773. [Google Scholar] [CrossRef]
- Beccari, G.; Senatore, M.T.; Tini, F.; Sulyok, M.; Covarelli, L. Fungal Community, Fusarium Head Blight Complex and Secondary Metabolites Associated with Malting Barley Grains Harvested in Umbria, Central Italy. Int. J. Food Microbiol. 2018, 273, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Beccari, G.; Colasante, V.; Tini, F.; Senatore, M.T.; Prodi, A.; Sulyok, M.; Covarelli, L. Causal Agents of Fusarium Head Blight of Durum Wheat (Triticum durum Desf.) in Central Italy and Their in Vitro Biosynthesis of Secondary Metabolites. Food Microbiol. 2018, 70, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Fumero, M.V.; Reynoso, M.M.; Chulze, S. Fusarium temperatum and Fusarium subglutinans Isolated from Maize in Argentina. Int. J. Food Microbiol. 2015, 199, 86–92. [Google Scholar] [CrossRef]
- Zinedine, A.; Meca, G.; Mañes, J.; Font, G. Further Data on the Occurrence of Fusarium Emerging Mycotoxins Enniatins (A, A1, B, B1), Fusaproliferin and Beauvericin in Raw Cereals Commercialized in Morocco. Food Control 2011, 22, 1–5. [Google Scholar] [CrossRef]
- Uhlig, S.; Torp, M.; Heier, B.T. Beauvericin and Enniatins A, A1, B and B1 in Norwegian Grain: A Survey. Food Chem. 2006, 94, 193–201. [Google Scholar] [CrossRef]
- De Lourdes Mendes de Souza, M.; Sulyok, M.; Freitas-Silva, O.; Costa, S.S.; Brabet, C.; Machinski Junior, M.; Sekiyama, B.L.; Vargas, E.A.; Krska, R.; Schuhmacher, R. Cooccurrence of Mycotoxins in Maize and Poultry Feeds from Brazil by Liquid Chromatography/Tandem Mass Spectrometry. Sci. World J. 2013, 2013, 427369. [Google Scholar] [CrossRef]
- Jurjevic, Z.; Solfrizzo, M.; Cvjetkovic, B.; De Girolamo, A.; Visconti, A. Occurrence of Beauvericin in Corn from Croatia. Food Technol. Biotechnol. 2002, 40, 91–94. [Google Scholar]
- Sorensen, J.L.; Nielsen, K.F.; Rasmussen, P.H.; Thrane, U. Development of a LC-MS/MS Method for the Analysis of Enniatins and Beauvericin in Whole Fresh and Ensiled Maize. J. Agric. Food Chem. 2008, 56, 10439–10443. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, T.; Suzuki, Y.; Sugita-Konishi, Y.; Ohnishi, T.; Terajima, J. Occurrence of Beauvericin and Enniatins in Wheat Flour and Corn Grits on the Japanese Market, and Their Co-Contamination with Type B Trichothecene Mycotoxins. Food Addit. Contam. Part A 2016, 33, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Srobarova, A.; Moretti, A.; Ferracane, R.; Ritieni, A.; Logrieco, A. Toxigenic Fusarium Species of Liseola Section in Pre-Harvest Maize Ear Rot, and Associated Mycotoxins in Slovakia. Eur. J. Plant Pathol. 2002, 108, 299–306. [Google Scholar] [CrossRef]
- Munkvold, G.; Stahr, H.M.; Logrieco, A.; Moretti, A.; Ritieni, A. Occurrence of Fusaproliferin and Beauvericin in Fusarium-Contaminated Livestock Feed in Iowa. Appl. Environ. Microbiol. 1998, 64, 3923–3926. [Google Scholar] [CrossRef] [Green Version]
- Nazari, F.; Sulyok, M.; Kobarfard, F.; Yazdanpanah, H.; Krska, R. Evaluation of Emerging Fusarium Mycotoxins Beauvericin, Enniatins, Fusaproliferin and Moniliformin in Domestic Rice in Iran. Iran. J. Pharm. Res. 2015, 14, 505–512. [Google Scholar]
- Blesa, J.; Moltó, J.-C.; El Akhdari, S.; Mañes, J.; Zinedine, A. Simultaneous Determination of Fusarium Mycotoxins in Wheat Grain from Morocco by Liquid Chromatography Coupled to Triple Quadrupole Mass Spectrometry. Food Control 2014, 46, 1–5. [Google Scholar] [CrossRef]
- Stanciu, O.; Juan, C.; Miere, D.; Loghin, F.; Manes, J. Occurrence and Co-Occurrence of Fusarium Mycotoxins in Wheat Grains and Wheat Flour from Romania. Food Control 2017, 73, 147–155. [Google Scholar] [CrossRef]





| Compound | MW | MW + NH4+ (18) | MW + Na+ (23) | MW + K+ (39) | Elemental Composition | References |
|---|---|---|---|---|---|---|
| Beauvericin | 783 | 801 | 806 | 822 | C45H57N3O9 | [2,26] |
| Beauvericin A/F/Allobeauvericin A | 797 | 815 | 820 | 836 | C46H59N3O9 | [2,33,42] |
| Beauvericin B/Allobeauvericin B | 811 | 829 | 834 | 850 | C47H61N3O9 | [3,33] |
| Beauvericin C/Allobeauvericin C | 825 | 843 | 848 | 864 | C48H63N3O9 | [2,33] |
| Beauvericin D | 769 | 787 | 792 | 808 | C44H55N3O9 | [2,42] |
| Beauvericin E | 735 | 753 | 758 | 774 | C41H57N3O9 | [3,42] |
| Beauvericin G1 | 769 | 787 | 792 | 808 | C44H55N3O9 | [3,31] |
| Beauvericin G2 | 755 | 773 | 778 | 794 | C43H53N3O9 | [3,31] |
| Beauvericin J | 799 | 817 | 822 | 838 | C45H57N3O10 | [2,26] |
| Beauvericin K | 815 | 833 | 838 | 854 | C45H57N3O11 | [2] |
| Beauvericin L | 831 | 849 | 854 | 870 | C45H57N3O12 | [2] |
| Beauvenniatin A | 735 | 753 | 758 | 774 | C41H57N3O9 | [2,26] |
| Beauvenniatin B | 687 | 705 | 710 | 726 | C37H57N3O9 | [3,26,30] |
| Beauvenniatin G1/G2/G3 | 715 | 733 | 738 | 754 | C39H61N3O9 | [3,30] |
| Beauvenniatin L | 749 | 767 | 772 | 788 | C42H59N3O9 | [2] |
| Enniatin A/F/MK 1688 | 681 | 699 | 704 | 720 | C36H63N3O9 | [25,39,44,45] |
| Enniatin A1/E/I | 667 | 685 | 690 | 706 | C35H61N3O9 | [25,39,44,45] |
| Enniatin A2 | 681 | 699 | 704 | 720 | C35H61N3O9 | [46] |
| Enniatin B | 639 | 657 | 662 | 678 | C33H57N3O9 | [25,39] |
| Enniatin B1/B4/D/H | 653 | 671 | 676 | 692 | C34H59N3O9 | [25,39,44,45,47] |
| Enniatin B2/J2/J3/K1 | 625 | 643 | 648 | 664 | C32H55N3O9 | [25,43] |
| Enniatin B3/J1 | 611 | 629 | 634 | 650 | C31H53N3O9 | [25,43,47] |
| Enniatin P1 | 641 | 659 | 664 | 680 | C33H57N3O10 | [21] |
| Enniatin P2 | 655 | 673 | 678 | 694 | C34H59N3O10 | [21] |
| Fusarium Species | Compound | References |
|---|---|---|
| F. acuminatum | BEA, ENN A, ENN A1, ENN B, ENN B1, ENN B2, ENN B3, ENN B4, ENN P1, ENN P2, BEA C, BEA D, BEA G1, ALLOBEA C | [2,3,5,21,39,47,78] |
| F. acutatum | BEA, mix of ENNs | [79] |
| F. ananatum | BEA, ENN A, ENN B, ENN B1 | [39] |
| F. anthophilum | BEA, ENN A, ENN B, ENN B1 | [39,78] |
| F. arthrosporioides | mix of ENNs | [15] |
| F. avenaceum | BEA, ENN A, ENN A1, ENN B, ENN B1, ENN B2, ENN B3, ENN B4 | [25,39,78,80,81] |
| F. beomiforme | BEA | [78] |
| F. bulbicola | BEA | [79] |
| F. circinatum | BEA | [79,82] |
| F. concentricum | BEA, ENN A, ENN A1, ENN B, ENN B1, BEA A/F, BEA B, BEA C, BEA D, BEA E, BEA G1, BEA G2, BEA J, BEA K, BEA L, BEAE A, BEAE B, BEAE G1/G2/G3, BEAE L, ALLOBEA A, ALLOBEA B, ALLOBEA C | [2,3,39,79,82] |
| F. compactum | ENN A, ENN A1, ENN B, ENN B1, ENN B2 | [47] |
| F. culmorum | mix of ENNs, ENN B | [83] |
| F. denticulatum | BEA | [79] |
| F. dlamini | BEA, ENN A, ENN A1, ENN B1 | [39,78,79] |
| F. equiseti | BEA, ENN A, ENN A1, ENN B, ENN B1 | [39,78] |
| F. fujikuoi | BEA | [79] |
| F. globosum | BEA | [84] |
| F. guttiforme | BEA | [79,82] |
| F. graminearum | ENN A, ENN A1, ENN B, ENN B1 | [85] |
| F. konzum | BEA | [86] |
| F. kyushuense | ENN B, ENN B1 | [87] |
| F. lactis | BEA, ENN A, ENN A1, ENN B, ENN B1 | [39,79] |
| F. langsethiae | BEA, ENN A1, ENN B, ENN B1 | [87] |
| F. lateritium | mix of ENNs | [15] |
| F. longipes | BEA | [78] |
| F. merismoides | mix of ENNs | [15] |
| F. nygamai | BEA, ENN A, ENN A1, ENN B | [39,78,79] |
| F. oxysporum | BEA, BEA A/F, BEA B, BEA C, BEA D, BEA E, BEA G1, BEA G2, BEA J, BEAE A, BEAE B, BEAE L, ALLOBEA A, ALLOBEA B, ALLOBEA C, ENN A1, ENN B, ENN B1, ENN H, ENN I, ENN MK1688 | [2,3,39,44,78] |
| F. poae | BEA, ENN A, ENN A1, ENN B, ENN B1 | [39,71,78,87] |
| F. phyllophilum | BEA | [79] |
| F. proliferatum | BEA, ENN A1, ENN B, ENN B1, BEA A/F, BEA B, BEA C, BEA D, BEA E, BEA G1, BEA G2, BEA J, BEA K, BEAE A, BEAE B, BEAE L, ALLOBEA A, ALLOBEA B, ALLOBEA C | [2,3,39,84] |
| F. pseudoanthophilum | BEA | [82] |
| F. pseudocircinatum | BEA | [79] |
| F. redolens | BEA | [37] |
| F. sacchari | BEA | [79] |
| F. sambucinum | BEA, mix of ENNs | [15,78] |
| F. scirpi | mix of ENNs | [15] |
| F. semitectum | BEA | [88] |
| F. sporotrichioides | BEA, ENN A, ENN B, ENN B1, ENN A1 | [39,71,87] |
| F. subglutinans | BEA, ENN A, ENN B, ENN B1 | [39,88,89,90] |
| F. succisae | BEA | [79] |
| F. temperatum | BEA, ENN A, ENN A1, ENN B, ENN B1 | [39,90] |
| F. torulosum | ENN B | [91,92] |
| F. tricinctum | BEA, ENN A, ENN A1, ENN B, ENN B1, ENN B4, ENN J1 | [5,36,39,93] |
| F. verticillioides | BEA, ENN B, ENN B1, BEA C, BEA D, BEA G1, BEA K, BEAE A, ALLOBEA C | [2,3,39,94] |
| Species | ID Strain | Host | Origin | ENN A | ENN A1 | ENN B | ENN B1 | ENN B2 | ENN B3 | BEA | Analytical Method | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F. acuminatum | KF 3713 | Pea | Poland | 19.62 | 26.92 | 90.89 | 31.49 | NA | NA | 5.31 | HPLC | [39] |
| F. ananatum | KF 3557 | Pineapple | Costa Rica | 6.94 | ND | 8.81 | 27.60 | NA | NA | 27.68 | HPLC | [39] |
| F. avenaceum | KF 3803 | Asparagus | Poland | ND | ≤0.01 | 0.03 | ND | NA | NA | ND | HPLC | [39] |
| 11B14 | Barley | Italy | 10.9 | 193 | 45 | 172 | 55 | 1.58 | NA | LC-MS/MS | [104] | |
| KF 3717 | Pea | Poland | 6.09 | 5.65 | 6.71 | 11.46 | NA | NA | ND | HPLC | [39] | |
| Fa40 | Wheat | Italy | 165.8 | 109.2 | 35.5 | 60.2 | NA | NA | ND | LC-DAD | [71] | |
| KF 1337 | Wheat | Poland | 34.55 | 71.90 | 895.46 | 452.46 | NA | NA | ND | HPLC | [39] | |
| 44 | Wheat | Italy | 7.24 | 34.3 | 6.6 | 17.8 | 0.67 | ≤0.01 | ≤0.01 | LC-MS/MS | [105] | |
| Fa34 | Wheat | Italy | 332.8 | 181.7 | 64.9 | 101.9 | NA | NA | ND | LC-DAD | [71] | |
| KF 3390 | Maize | Poland | 29.12 | 32.40 | 255.08 | 138.15 | NA | NA | ND | HPLC | [39] | |
| F. concentricum | KF 3755 | Pineapple | Costa Rica | 11.40 | 8.69 | 17.33 | 18.17 | NA | NA | 312.2 | HPLC | [39] |
| F. culmorum | KF 3798 | Asparagus | Poland | ND | ND | 0.06 | ND | NA | NA | ND | HPLC | [39] |
| F. equiseti | KF 3563 | Asparagus | Poland | 43.47 | 36.81 | 29.18 | 30.39 | NA | NA | ND | HPLC | [39] |
| KF 3749 | Tomato | Poland | 39.27 | 38.18 | ND | 29.22 | NA | NA | ND | HPLC | [39] | |
| KF 3430 | Banana | Ecuador | 31.17 | 32.15 | 32.98 | 41.22 | NA | NA | ND | HPLC | [39] | |
| Feq16 | Wheat | Italy | ND | ≤0.01 | ≤0.01 | ≤0.01 | NA | NA | ≤0.01 | LC-DAD | [71] | |
| Feq136 | Wheat | Italy | ≤0.01 | 0.02 | ≤0.01 | 0.02 | NA | NA | ND | LC-DAD | [71] | |
| F. fujikuroi | KF 3631 | Rice | Thailand | ND | ND | ND | ND | NA | NA | 428.09 | HPLC | [39] |
| F. globosum | 6646 | Maize | South Africa | NA | NA | NA | NA | NA | NA | 110 | LC-MS | [84] |
| F. lactis | KF 3641 | Pepper | Poland | 30.97 | 26.94 | ND | ND | NA | NA | ND | HPLC | [39] |
| F. nygamai | KF 337 | Pigeon Pea | India | 10.45 | ND | 9.50 | ND | NA | NA | 22.86 | HPLC | [39] |
| F. oxysporum | KF 3567 | Garlic | Poland | ND | 6.42 | 8.25 | 7.28 | NA | NA | 80.03 | HPLC | [39] |
| KF 3805 | Asparagus | Poland | ND | ND | ND | ND | NA | NA | 0.53 | HPLC | [39] | |
| F. poae | Fp26 | Wheat | Italy | ≤0.01 | 0.07 | 0.03 | 0.05 | NA | NA | 3.5 | LC-DAD | [71] |
| 156 | Wheat | Italy | ≤0.01 | 0.03 | 0.03 | ND | ND | ND | 10.5 | LC-MS/MS | [105] | |
| Fp49 | Wheat | Italy | ≤0.01 | 0.1 | 0.05 | 0.04 | NA | NA | 9.4 | LC-DAD | [71] | |
| KF 2576 | Maize | Poland | 34.31 | 26.89 | 28.71 | ND | NA | NA | 37.53 | HPLC | [39] | |
| F. proliferatum | KF 3382 | Pineapple | Hawaii | ND | ND | ND | ND | NA | NA | 3.39 | HPLC | [39] |
| FPG61_CM | Garlic | Spain | NA | NA | NA | NA | NA | NA | 671.80 | HPLC | [6] | |
| KF 3363 | Garlic | Poland | ND | ND | ND | ND | NA | NA | 45.13 | HPLC | [39] | |
| KF 3792 | Asparagus | Poland | ND | 0.39 | 0.13 | 0.06 | NA | NA | 0.41 | HPLC | [39] | |
| KF 3584 | Rice | Thailand | ND | 6.39 | 12.92 | 19.64 | NA | NA | 291.87 | HPLC | [39] | |
| KF 3560 | Rhubarb | Poland | ND | ND | ND | ND | NA | NA | 149.67 | HPLC | [39] | |
| KF 496 | Maize | Italy | ND | 5.48 | 9.61 | 12.89 | NA | NA | ND | HPLC | [39] | |
| F. sambucinum | 179 | Wheat | Italy | ND | ND | ND | ND | ND | ND | 10.1 | LC-MS/MS | [105] |
| F. subglutinans | 1084 | Maize | South Africa | NA | NA | NA | NA | NA | NA | 700 | LC-MS | [84] |
| F. sporotrichioides | KF 3815 | Asparagus | Poland | ND | 0.09 | ND | ND | NA | NA | 0.21 | HPLC | [39] |
| KF 3728 | Pea | Poland | 12.67 | ND | 5.99 | 18.15 | NA | NA | 5.13 | HPLC | [39] | |
| Fsp50 | Wheat | Italy | ND | ≤0.01 | ≤0.01 | 0.02 | NA | NA | 13.7 | LC-DAD | [71] | |
| 194 | Wheat | Italy | ND | ND | ND | ND | ND | ND | 6.89 | LC-MS/MS | [105] | |
| F. temperatum | KF 3321 | Pineapple | Costa Rica | 27.79 | 34.39 | 39.20 | 29.21 | NA | NA | 290.97 | HPLC | [39] |
| RCFT 934 | Maize | Argentina | NA | NA | NA | NA | NA | NA | 1151 | HPLC | [106] | |
| KF 506 | Maize | Poland | ND | ND | 15.17 | 9.88 | NA | NA | 17.47 | HPLC | [39] | |
| F. tricinctum | KF 3795 | Asparagus | Poland | 0.1 | 0.17 | 0.28 | 0.38 | NA | NA | 0.55 | HPLC | [39] |
| 27B14 | Malting barley | Italy | 8.45 | 118 | 39 | 124 | 27 | 0.13 | NA | LC-MS/MS | [104] | |
| 3405 | Wheat | Finland | NA | 94 | 690 | 1200 | NA | NA | 33 | HPLC | [5] | |
| F. verticillioides | KF 393 | Maize | USA | ND | ND | 8.75 | 12.43 | NA | NA | 2.34 | HPLC | [39] |
| Sample | Origin | ENN A | ENN A1 | ENN B | ENN B1 | ENN B4 | BEA | Reference |
|---|---|---|---|---|---|---|---|---|
| Asparagus | Poland | ND | 0.05 | 0.06 | ND | NA | 0.1 | [8] |
| Barley | Italy | ND | ND | ND | ≤0.01 | 0.02 | ≤0.01 | [100] |
| Italy | 0.02 | 0.06 | 0.07 | 0.07 | NA | ≤0.01 | [104] | |
| Finland | 0.95 | 2 | 9.76 | 5.72 | NA | 0.02 | [1] | |
| Morocco | ND | 220 | 49 | 32 | NA | 5 | [107] | |
| Norway | ≤0.01 | 0.04 | 0.49 | 0.17 | NA | ≤0.01 | [108] | |
| Spain | ND | 361.57 | 21.37 | 45.94 | NA | 6.94 | [97] | |
| Tunisia | 33.6 | 149 | 29.2 | 31 | NA | NA | [96] | |
| Maize | Brazil | ≤0.01 | 0.31 | ≤0.01 | ≤0.01 | NA | 0.16 | [109] |
| Croatia | NA | NA | NA | NA | NA | 1.84 | [110] | |
| Denmark | ≤0.01 | ≤0.01 | 0.58 | 0.09 | NA | 0.09 | [111] | |
| Japan | NA | NA | NA | NA | NA | 0.03 | [112] | |
| Morocco | ND | 445 | 100 | 8 | NA | 59 | [107] | |
| Poland | NA | NA | NA | NA | NA | 1.73 | [95] | |
| Serbia | 0.02 | 0.03 | ≤0.01 | 0.02 | NA | 0.14 | [7] | |
| Slovakia | NA | NA | NA | NA | NA | 3 | [113] | |
| Spain | ND | 813.01 | 6.31 | 4.34 | NA | 9.31 | [97] | |
| Tunisia | ND | 29.6 | ND | 17 | NA | NA | [96] | |
| USA | NA | NA | NA | NA | NA | 0.5 | [114] | |
| Oats | Finland | ≤0.01 | ≤0.01 | 0.02 | ≤0.01 | NA | 0.02 | [1] |
| Italy | ND | ≤0.01 | ≤0.01 | ND | 0.05 | ≤0.01 | [100] | |
| Norway | ≤0.01 | ≤0.01 | 0.05 | 0.02 | NA | 0.02 | [108] | |
| Rice | Iran | ND | ≤0.01 | ND | ND | ND | ≤0.01 | [115] |
| Spain | ND | 814.42 | 7.95 | ND | NA | 11.78 | [97] | |
| Rye | Finland | ND | ≤0.01 | 0.05 | ≤0.01 | NA | ND | [1] |
| Italy | ≤0.01 | ND | ≤0.01 | ND | ≤0.01 | ≤0.01 | [100] | |
| Sorghum | Tunisia | 95.6 | 480 | ND | 120.1 | NA | NA | [96] |
| Spelt wheat | Italy | ≤0.01 | ND | ND | ND | ND | ND | [100] |
| Wheat | Finland | 0.49 | 0.94 | 18.3 | 5.1 | NA | ≤0.01 | [1] |
| Italy | ≤0.01 | ≤0.01 | 0.02 | ≤0.01 | 0.04 | ≤0.01 | [100] | |
| Morocco | 0.08 | 0.13 | 2.57 | 0.35 | NA | 0.02 | [116] | |
| Morocco | 34 | 209 | 11 | 19 | NA | 4 | [107] | |
| Norway | ≤0.01 | 0.02 | 0.79 | 0.18 | NA | ≤0.01 | [108] | |
| Poland | 0.27 | 3.6 | 28.52 | 11.8 | NA | 0.02 | [57] | |
| Romania | 0.14 | 0.36 | 0.41 | 0.51 | NA | NA | [117] | |
| Spain | ND | 634.85 | ND | ND | NA | 3.5 | [97] | |
| Tunisia | 75.1 | 177.7 | 180.6 | 58.5 | NA | NA | [96] | |
| UK | 0.04 | 0.17 | 0.13 | 0.30 | NA | NA | [85] | |
| Breakfast cereals | Morocco | 29.7 | 688 | 81.1 | 795 | NA | 5.3 | [99] |
| Spain | ND | 268.54 | ND | ND | NA | 3.12 | [97] | |
| Tunisia | 121.3 | 480 | 295 | 120.1 | NA | NA | [96] | |
| Infant cereals | Morocco | ND | 52 | 5.7 | 14.5 | NA | 10.6 | [99] |
| Pasta | Italy | ≤0.01 | ≤0.01 | 0.11 | ≤0.01 | ≤0.01 | ND | [100] |
| Oat flour | Spain | ND | 388.38 | ND | ND | NA | 4.18 | [97] |
| Wheat flour | Japan | ≤0.01 | 0.03 | 0.63 | 0.09 | NA | ≤0.01 | [112] |
| Corn grits | Japan | ND | ND | ND | ND | NA | 0.03 | [112] |
| Bovine feed | Spain | ND | ≤0.01 | 0.04 | 0.02 | NA | 0.05 | [98] |
| Ovine feed | Spain | ND | ≤0.01 | 0.09 | 0.03 | NA | 0.13 | [98] |
| Caprine feed | Spain | ND | ≤0.01 | 0.02 | ≤0.01 | NA | 0.02 | [98] |
| Horses feed | Spain | ND | ≤0.01 | 0.04 | ≤0.01 | NA | 0.03 | [98] |
| Porcine feed | Finland | 0.31 | 0.55 | 1.51 | 1.85 | NA | 0.41 | [102] |
| Spain | ND | ≤0.01 | 0.06 | 0.02 | NA | ≤0.01 | [98] | |
| Poultry feed | Brazil | ND | ≤0.01 | ≤0.01 | ≤0.01 | NA | 0.02 | [109] |
| Spain | ND | ≤0.01 | 0.05 | 0.02 | NA | 0.02 | [98] | |
| UK | 0.04 | 0.03 | 2.19 | 0.40 | NA | 0.48 | [101] | |
| Rabbits feed | Spain | ND | ≤0.01 | 0.05 | 0.02 | NA | ≤0.01 | [98] |
| Dogs feed | Spain | ND | ≤0.01 | 0.02 | ≤0.01 | NA | 0.04 | [98] |
| Cats feed | Spain | ND | ND | ≤0.01 | ≤0.01 | NA | ND | [98] |
| Fish feed | Scotland/Norway/ Spain | ≤0.01 | ≤0.01 | 0.03 | ≤0.01 | NA | 0.08 | [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbaniak, M.; Waśkiewicz, A.; Stępień, Ł. Fusarium Cyclodepsipeptide Mycotoxins: Chemistry, Biosynthesis, and Occurrence. Toxins 2020, 12, 765. https://doi.org/10.3390/toxins12120765
Urbaniak M, Waśkiewicz A, Stępień Ł. Fusarium Cyclodepsipeptide Mycotoxins: Chemistry, Biosynthesis, and Occurrence. Toxins. 2020; 12(12):765. https://doi.org/10.3390/toxins12120765
Chicago/Turabian StyleUrbaniak, Monika, Agnieszka Waśkiewicz, and Łukasz Stępień. 2020. "Fusarium Cyclodepsipeptide Mycotoxins: Chemistry, Biosynthesis, and Occurrence" Toxins 12, no. 12: 765. https://doi.org/10.3390/toxins12120765
APA StyleUrbaniak, M., Waśkiewicz, A., & Stępień, Ł. (2020). Fusarium Cyclodepsipeptide Mycotoxins: Chemistry, Biosynthesis, and Occurrence. Toxins, 12(12), 765. https://doi.org/10.3390/toxins12120765






