The Dual α-Amidation System in Scorpion Venom Glands
Abstract
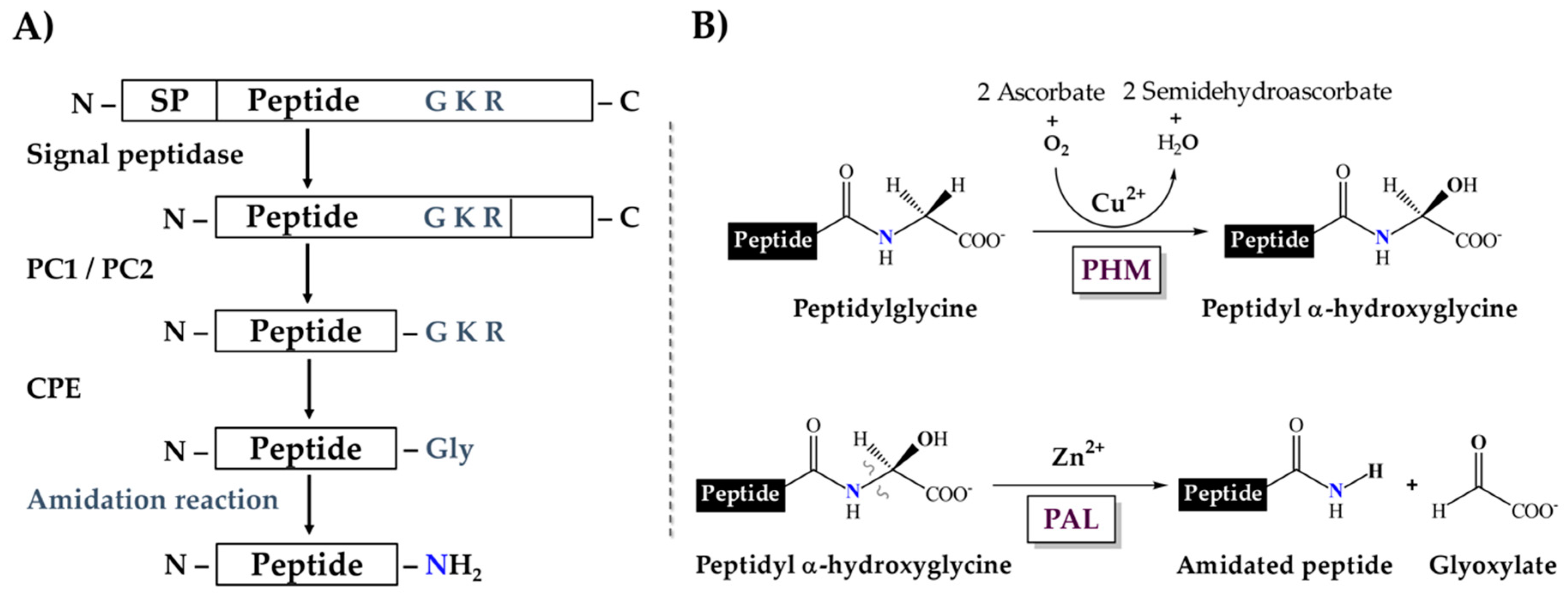
1. Introduction
2. Results
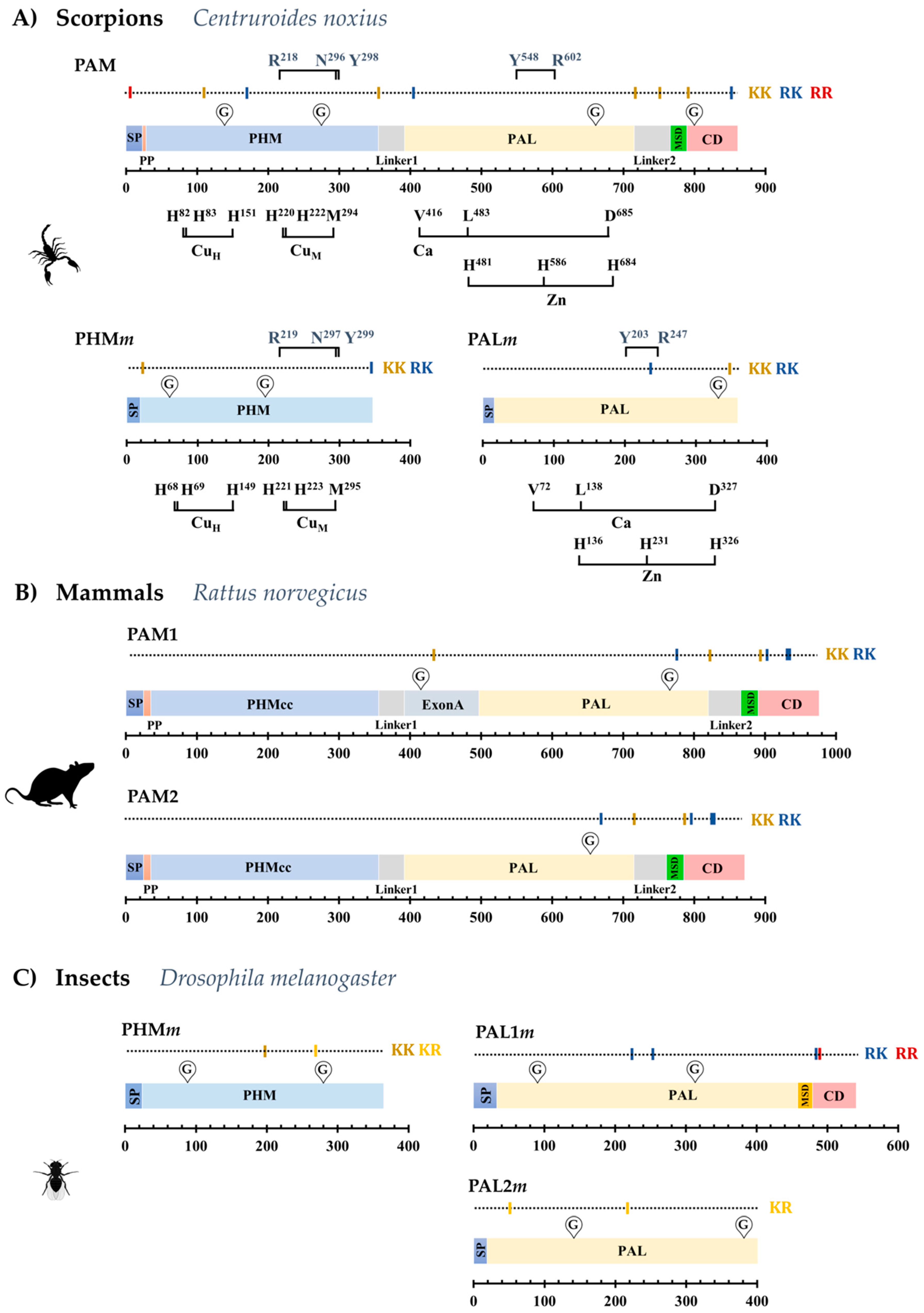
2.1. The Dual Enzymatic System for α-Amidation in the Order Scorpiones
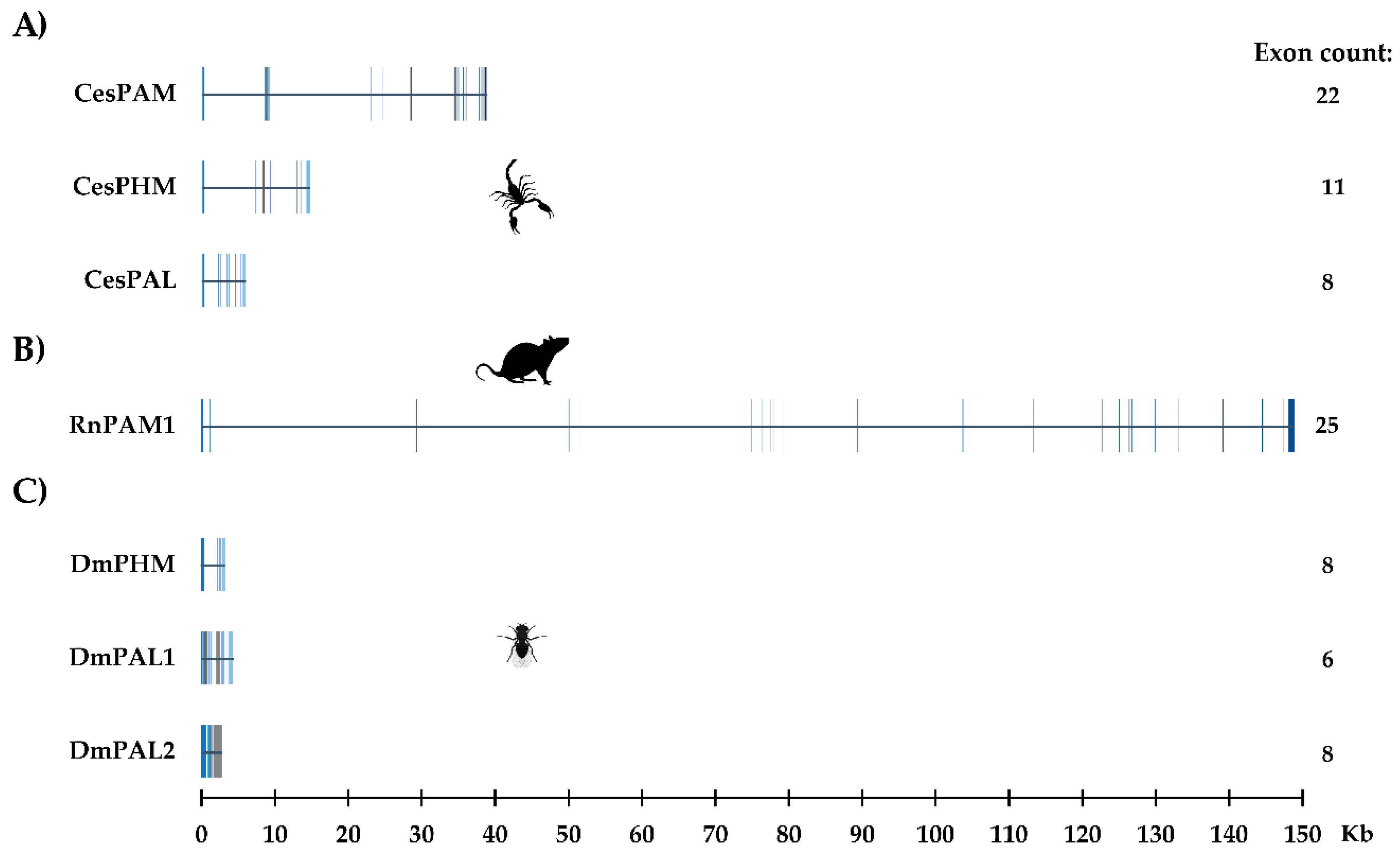
2.2. The PAM-, PHMm- and PALm-Coding Genes
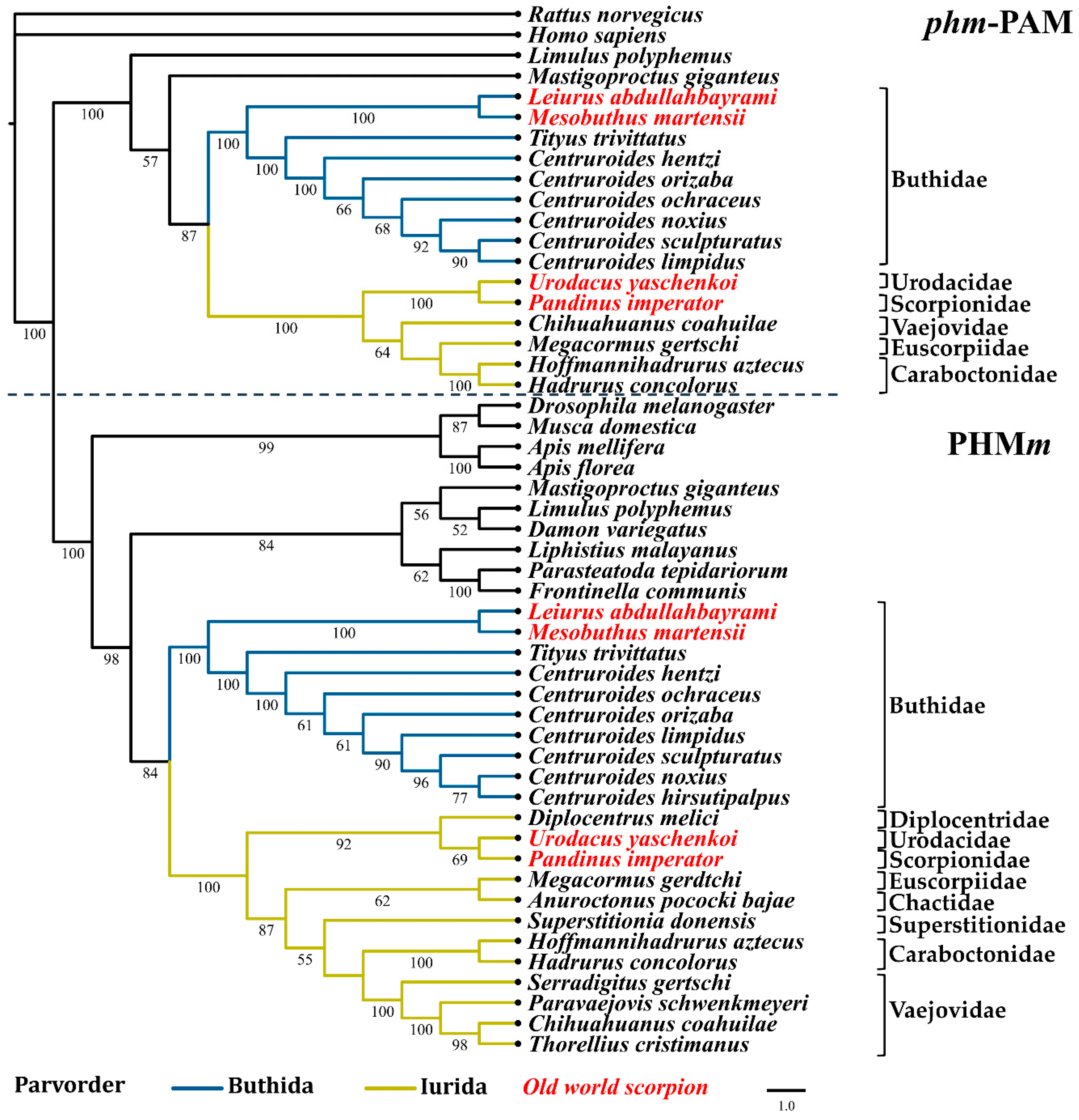
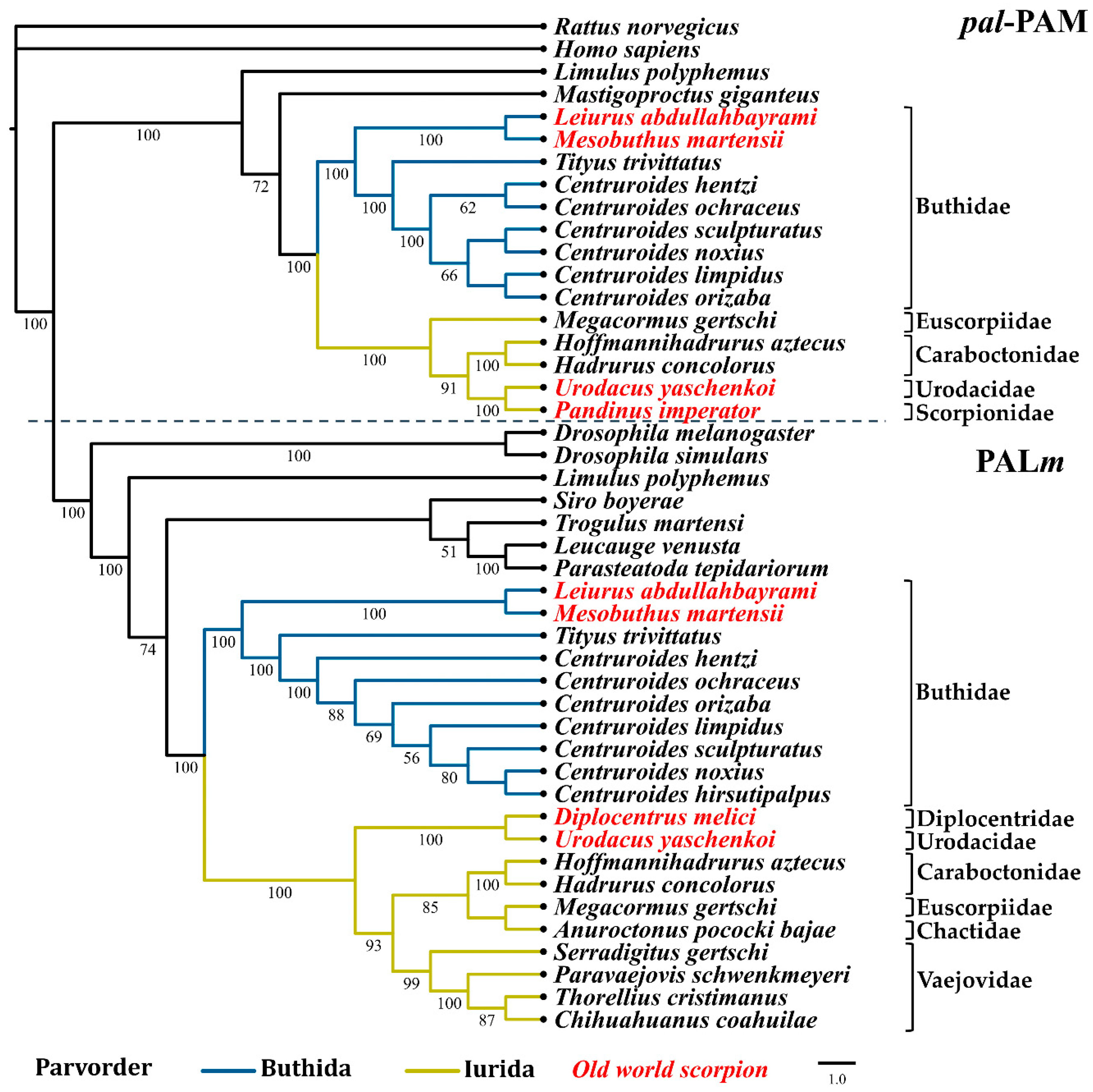
2.3. Phylogenetic Reconstruction of Amidating Enzymes of Arachnids
3. Discussion
4. Materials and Methods
4.1. Sequence Data and Transcriptome Assembly
4.2. Identification and Annotation of Amidating Enzymes in Scorpions and Related Organisms
4.3. Amplification and Cloning of the PAM Sequence from Centruroides noxius
4.4. Multiple Alignments and Phylogeny Reconstruction of PAM, PHM, and PAL
4.5. Genomic Organization of Scorpion PAM, PHM, and PAL
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharma, P.P.; Fernandez, R.; Esposito, L.A.; Gonzalez-Santillan, E.; Monod, L. Phylogenomic resolution of scorpions reveals multilevel discordance with morphological phylogenetic signal. Proc. Biol. Sci. 2015, 282, 20142953. [Google Scholar] [CrossRef] [PubMed]
- Waddington, J.; Rudkin, D.M.; Dunlop, J.A. A new mid-Silurian aquatic scorpion-one step closer to land? Biol. Lett. 2015, 11, 20140815. [Google Scholar] [CrossRef] [PubMed]
- Oldrati, V.; Arrell, M.; Violette, A.; Perret, F.; Sprungli, X.; Wolfender, J.L.; Stocklin, R. Advances in venomics. Mol. Biosyst. 2016, 12, 3530–3543. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, E.; Gurrola, G.B.; Schwartz, E.F.; Possani, L.D. Scorpion venom components as potential candidates for drug development. Toxicon 2015, 93, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Gurrola, G.B.; Zhang, J.; Valdivia, C.R.; SanMartin, M.; Zamudio, F.Z.; Zhang, L.; Possani, L.D.; Valdivia, H.H. Structure-function relationships of peptides forming the calcin family of ryanodine receptor ligands. J. Gen. Physiol. 2016, 147, 375–394. [Google Scholar] [CrossRef]
- Fuller, M.D.; Thompson, C.H.; Zhang, Z.R.; Freeman, C.S.; Schay, E.; Szakacs, G.; Bakos, E.; Sarkadi, B.; McMaster, D.; French, R.J.; et al. State-dependent inhibition of cystic fibrosis transmembrane conductance regulator chloride channels by a novel peptide toxin. J. Biol. Chem. 2007, 282, 37545–37555. [Google Scholar] [CrossRef] [PubMed]
- Gurevitz, M. Mapping of scorpion toxin receptor sites at voltage-gated sodium channels. Toxicon 2012, 60, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Possani, L.D.; Becerril, B.; Delepierre, M.; Tytgat, J. Scorpion toxins specific for Na+-channels. Eur. J. Biochem. 1999, 264, 287–300. [Google Scholar] [CrossRef]
- Rodriguez de la Vega, R.C.; Possani, L.D. Overview of scorpion toxins specific for Na+ channels and related peptides: Biodiversity, structure-function relationships and evolution. Toxicon 2005, 46, 831–844. [Google Scholar] [CrossRef]
- Rodriguez de la Vega, R.C.; Possani, L.D. Current views on scorpion toxins specific for K+-channels. Toxicon 2004, 43, 865–875. [Google Scholar] [CrossRef]
- Chippaux, J.P. Emerging options for the management of scorpion stings. Drug Des. Dev. Ther. 2012, 6, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Chippaux, J.P.; Goyffon, M. Epidemiology of scorpionism: A global appraisal. Acta Trop. 2008, 107, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Jekely, G. Global view of the evolution and diversity of metazoan neuropeptide signaling. Proc. Natl. Acad. Sci. USA 2013, 110, 8702–8707. [Google Scholar] [CrossRef] [PubMed]
- Gutte, B. Peptides: Synthesis, Structures, and Applications, 1st ed.; Academic Press: San Diego, CA, USA, 1995; pp. 288–289. [Google Scholar]
- Merkler, D.J. C-terminal amidated peptides: Production by the in vitro enzymatic amidation of glycine-extended peptides and the importance of the amide to bioactivity. Enz. Microb. Technol. 1994, 16, 450–456. [Google Scholar] [CrossRef]
- Coelho, V.A.; Cremonez, C.M.; Anjolette, F.A.; Aguiar, J.F.; Varanda, W.A.; Arantes, E.C. Functional and structural study comparing the C-terminal amidated beta-neurotoxin Ts1 with its isoform Ts1-G isolated from Tityus serrulatus venom. Toxicon 2014, 83, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, G.; Li, B.; Chi, C.; Wu, X. Cloning, co-expression with an amidating enzyme, and activity of the scorpion toxin BmK ITa1 cDNA in insect cells. Mol. Biotechnol. 2003, 24, 21–26. [Google Scholar] [CrossRef]
- Estrada, G.; Restano-Cassulini, R.; Ortiz, E.; Possani, L.D.; Corzo, G. Addition of positive charges at the C-terminal peptide region of CssII, a mammalian scorpion peptide toxin, improves its affinity for sodium channels Nav1.6. Peptides 2011, 32, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Eipper, B.A.; Stoffers, D.A.; Mains, R.E. The biosynthesis of neuropeptides: Peptide alpha-amidation. Annu. Rev. Neurosci. 1992, 15, 57–85. [Google Scholar] [CrossRef] [PubMed]
- Fricker, L.D. Neuropeptide-processing enzymes: Applications for drug discovery. AAPS J. 2005, 7, E449–E455. [Google Scholar] [CrossRef] [PubMed]
- Eipper, B.A.; Perkins, S.N.; Husten, E.J.; Johnson, R.C.; Keutmann, H.T.; Mains, R.E. Peptidyl-alpha-hydroxyglycine alpha-amidating lyase. Purification, characterization, and expression. J. Biol. Chem. 1991, 266, 7827–7833. [Google Scholar]
- Perkins, S.N.; Husten, E.J.; Eipper, B.A. The 108-kDA peptidylglycine alpha-amidating monooxygenase precursor contains two separable enzymatic activities involved in peptide amidation. Biochem. Biophys. Res. Commun. 1990, 171, 926–932. [Google Scholar] [CrossRef]
- Attenborough, R.M.; Hayward, D.C.; Kitahara, M.V.; Miller, D.J.; Ball, E.E. A “neural” enzyme in nonbilaterian animals and algae: Preneural origins for peptidylglycine alpha-amidating monooxygenase. Mol. Biol. Evol. 2012, 29, 3095–3109. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Park, D.; Vanderzalm, P.J.; Mains, R.E.; Eipper, B.A.; Taghert, P.H. Drosophila uses two distinct neuropeptide amidating enzymes, dPAL1 and dPAL2. J. Neurochem. 2004, 90, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Kolhekar, A.S.; Roberts, M.S.; Jiang, N.; Johnson, R.C.; Mains, R.E.; Eipper, B.A.; Taghert, P.H. Neuropeptide amidation in Drosophila: Separate genes encode the two enzymes catalyzing amidation. J. Neurosci. 1997, 17, 1363–1376. [Google Scholar] [CrossRef] [PubMed]
- Zabriskie, T.M.; Klinge, M.; Szymanski, C.M.; Cheng, H.; Vederas, J.C. Peptide amidation in an invertebrate: Purification, characterization, and inhibition of peptidylglycine alpha-hydroxylating monooxygenase from the heads of honeybees (Apis mellifera). Arch. Insect Biochem. Physiol. 1994, 26, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Eipper, B.A.; Green, C.B.; Campbell, T.A.; Stoffers, D.A.; Keutmann, H.T.; Mains, R.E.; Ouafik, L. Alternative splicing and endoproteolytic processing generate tissue-specific forms of pituitary peptidylglycine alpha-amidating monooxygenase (PAM). J. Biol. Chem. 1992, 267, 4008–4015. [Google Scholar] [PubMed]
- Eipper, B.A.; Park, L.P.; Dickerson, I.M.; Keutmann, H.T.; Thiele, E.A.; Rodriguez, H.; Schofield, P.R.; Mains, R.E. Structure of the precursor to an enzyme mediating COOH-terminal amidation in peptide biosynthesis. Mol. Endocrinol. 1987, 1, 777–790. [Google Scholar] [CrossRef]
- Glauder, J.; Ragg, H.; Rauch, J.; Engels, J.W. Human peptidylglycine alpha-amidating monooxygenase: cDNA, cloning and functional expression of a truncated form in COS cells. Biochem. Biophys. Res. Commun. 1990, 169, 551–558. [Google Scholar] [CrossRef]
- Mizuno, K.; Ohsuye, K.; Wada, Y.; Fuchimura, K.; Tanaka, S.; Matsuo, H. Cloning and sequence of cDNA encoding a peptide C-terminal alpha-amidating enzyme from Xenopus laevis. Biochem. Biophys. Res. Commun. 1987, 148, 546–552. [Google Scholar] [CrossRef]
- Fan, X.; Spijker, S.; Akalal, D.B.; Nagle, G.T. Neuropeptide amidation: Cloning of a bifunctional alpha-amidating enzyme from Aplysia. Brain Res. Mol. Brain Res. 2000, 82, 25–34. [Google Scholar] [CrossRef]
- Spijker, S.; Smit, A.B.; Eipper, B.A.; Malik, A.; Mains, R.E.; Geraerts, W.P. A molluscan peptide alpha-amidating enzyme precursor that generates five distinct enzymes. FASEB J. 1999, 13, 735–748. [Google Scholar] [CrossRef]
- Eipper, B.A.; Milgram, S.L.; Husten, E.J.; Yun, H.Y.; Mains, R.E. Peptidylglycine alpha-amidating monooxygenase: A multifunctional protein with catalytic, processing, and routing domains. Protein Sci. 1993, 2, 489–497. [Google Scholar] [CrossRef]
- Ul-Hasan, S.; Burgess, D.M.; Gajewiak, J.; Li, Q.; Hu, H.; Yandell, M.; Olivera, B.M.; Bandyopadhyay, P.K. Characterization of the peptidylglycine alpha-amidating monooxygenase (PAM) from the venom ducts of neogastropods, Conus bullatus and Conus geographus. Toxicon 2013, 74, 215–224. [Google Scholar] [CrossRef]
- Becerril, B.; Corona, M.; Garcia, C.; Bolivar, F.; Possani, L.D. Cloning of Genes Encoding Scorpion Toxins—An Interpretative Review. J. Tox. Tox. Rev. 1995, 14, 339–357. [Google Scholar] [CrossRef]
- Bougis, P.E.; Rochat, H.; Smith, L.A. Precursors of Androctonus australis scorpion neurotoxins. Structures of precursors, processing outcomes, and expression of a functional recombinant toxin II. J. Biol. Chem. 1989, 264, 19259–19265. [Google Scholar]
- Romero-Gutierrez, T.; Peguero-Sanchez, E.; Cevallos, M.A.; Batista, C.V.F.; Ortiz, E.; Possani, L.D. A Deeper Examination of Thorellius atrox Scorpion Venom Components with Omic Techonologies. Toxins 2017, 9, 3399. [Google Scholar] [CrossRef]
- De Oliveira, U.C.; Nishiyama, M.Y., Jr.; Dos Santos, M.B.V.; Santos-da-Silva, A.P.; Chalkidis, H.M.; Souza-Imberg, A.; Candido, D.M.; Yamanouye, N.; Dorce, V.A.C.; Junqueira-de-Azevedo, I.L.M. Proteomic endorsed transcriptomic profiles of venom glands from Tityus obscurus and T. serrulatus scorpions. PLoS ONE 2018, 13, e0193739. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Quintero-Hernandez, V.; Possani, L.D. Venom proteomic and venomous glands transcriptomic analysis of the Egyptian scorpion Scorpio maurus palmatus (Arachnida: Scorpionidae). Toxicon 2013, 74, 193–207. [Google Scholar] [CrossRef]
- Santibanez-Lopez, C.E.; Gonzalez-Santillan, E.; Monod, L.; Sharma, P.P. Phylogenomics facilitates stable scorpion systematics: Reassessing the relationships of Vaejovidae and a new higher-level classification of Scorpiones (Arachnida). Mol. Phylogenet. Evol. 2019, 135, 22–30. [Google Scholar] [CrossRef]
- Sharma, P.P.; Baker, C.M.; Cosgrove, J.G.; Johnson, J.E.; Oberski, J.T.; Raven, R.J.; Harvey, M.S.; Boyer, S.L.; Giribet, G. A revised dated phylogeny of scorpions: Phylogenomic support for ancient divergence of the temperate Gondwanan family Bothriuridae. Mol. Phylogenet. Evol. 2018, 122, 37–45. [Google Scholar] [CrossRef]
- Ballesteros, J.A.; Sharma, P.P. A Critical Appraisal of the Placement of Xiphosura (Chelicerata) with Account of Known Sources of Phylogenetic Error. Syst. Biol. 2019, 10. [Google Scholar] [CrossRef]
- Leite, D.J.; Baudouin-Gonzalez, L.; Iwasaki-Yokozawa, S.; Lozano-Fernandez, J.; Turetzek, N.; Akiyama-Oda, Y.; Prpic, N.M.; Pisani, D.; Oda, H.; Sharma, P.P.; et al. Homeobox gene duplication and divergence in arachnids. Mol. Biol. Evol. 2018, 35. [Google Scholar] [CrossRef]
- Schwager, E.E.; Sharma, P.P.; Clarke, T.; Leite, D.J.; Wierschin, T.; Pechmann, M.; Akiyama-Oda, Y.; Esposito, L.; Bechsgaard, J.; Bilde, T.; et al. The house spider genome reveals an ancient whole-genome duplication during arachnid evolution. BMC Biol. 2017, 15, 62. [Google Scholar] [CrossRef]
- Katopodis, A.G.; May, S.W. Novel substrates and inhibitors of peptidylglycine alpha-amidating monooxygenase. Biochemistry 1990, 29, 4541–4548. [Google Scholar] [CrossRef]
- Kuhn-Nentwig, L.; Langenegger, N.; Heller, M.; Koua, D.; Nentwig, W. The Dual Prey-Inactivation Strategy of Spiders-In-Depth Venomic Analysis of Cupiennius salei. Toxins 2019, 11, 167. [Google Scholar] [CrossRef]
- Ward, M.J.; Ellsworth, S.A.; Rokyta, D.R. Venom-gland transcriptomics and venom proteomics of the Hentz striped scorpion (Centruroides hentzi; Buthidae) reveal high toxin diversity in a harmless member of a lethal family. Toxicon 2018, 142, 14–29. [Google Scholar] [CrossRef]
- Cid-Uribe, J.I.; Meneses, E.P.; Batista, C.V.F.; Ortiz, E.; Possani, L.D. Dissecting Toxicity: The Venom Gland Transcriptome and the Venom Proteome of the Highly Venomous Scorpion Centruroides limpidus (Karsch, 1879). Toxins 2019, 11, 247. [Google Scholar] [CrossRef]
- Cid-Uribe, J.I.; Santibanez-Lopez, C.E.; Meneses, E.P.; Batista, C.V.F.; Jimenez-Vargas, J.M.; Ortiz, E.; Possani, L.D. The diversity of venom components of the scorpion species Paravaejovis schwenkmeyeri (Scorpiones: Vaejovidae) revealed by transcriptome and proteome analyses. Toxicon 2018, 151, 47–62. [Google Scholar] [CrossRef]
- Luna-Ramirez, K.; Quintero-Hernandez, V.; Juarez-Gonzalez, V.R.; Possani, L.D. Whole Transcriptome of the Venom Gland from Urodacus yaschenkoi Scorpion. PLoS ONE 2015, 10, e0127883. [Google Scholar] [CrossRef]
- Romero-Gutierrez, M.T.; Santibanez-Lopez, C.E.; Jimenez-Vargas, J.M.; Batista, C.V.F.; Ortiz, E.; Possani, L.D. Transcriptomic and Proteomic Analyses Reveal the Diversity of Venom Components from the Vaejovid Scorpion Serradigitus gertschi. Toxins 2018, 10, 359. [Google Scholar] [CrossRef]
- Santibanez-Lopez, C.E.; Cid-Uribe, J.I.; Batista, C.V.; Ortiz, E.; Possani, L.D. Venom Gland Transcriptomic and Proteomic Analyses of the Enigmatic Scorpion Superstitionia donensis (Scorpiones: Superstitioniidae), with Insights on the Evolution of Its Venom Components. Toxins 2016, 8, 367. [Google Scholar] [CrossRef]
- Santibanez-Lopez, C.E.; Cid-Uribe, J.I.; Zamudio, F.Z.; Batista, C.V.F.; Ortiz, E.; Possani, L.D. Venom gland transcriptomic and venom proteomic analyses of the scorpion Megacormus gertschi Diaz-Najera, 1966 (Scorpiones: Euscorpiidae: Megacorminae). Toxicon 2017, 133, 95–109. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Santibanez-Lopez, C.E.; Francke, O.F.; Ureta, C.; Possani, L.D. Scorpions from Mexico: From Species Diversity to Venom Complexity. Toxins 2016, 8, 2. [Google Scholar] [CrossRef]
- Artimo, P.; Jonnalagedda, M.; Arnold, K.; Baratin, D.; Csardi, G.; de Castro, E.; Duvaud, S.; Flegel, V.; Fortier, A.; Gasteiger, E.; et al. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012, 40, W597–W603. [Google Scholar] [CrossRef]
- Geer, L.Y.; Domrachev, M.; Lipman, D.J.; Bryant, S.H. CDART: Protein homology by domain architecture. Genome Res. 2002, 12, 1619–1623. [Google Scholar] [CrossRef]
- Petersen, T.N.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef]
- Kall, L.; Krogh, A.; Sonnhammer, E.L. A combined transmembrane topology and signal peptide prediction method. J. Mol. Biol. 2004, 338, 1027–1036. [Google Scholar] [CrossRef]
- Pineda, S.S.; Chaumeil, P.A.; Kunert, A.; Kaas, Q.; Thang, M.W.C.; Le, L.; Nuhn, M.; Herzig, V.; Saez, N.J.; Cristofori-Armstrong, B.; et al. ArachnoServer 3.0: An online resource for automated discovery, analysis and annotation of spider toxins. Bioinformatics 2018, 34, 1074–1076. [Google Scholar] [CrossRef]
- Moller, S.; Croning, M.D.; Apweiler, R. Evaluation of methods for the prediction of membrane spanning regions. Bioinformatics 2001, 17, 646–653. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Kapustin, Y.; Souvorov, A.; Tatusova, T.; Lipman, D. Splign: Algorithms for computing spliced alignments with identification of paralogs. Biol. Direct. 2008, 3, 20. [Google Scholar] [CrossRef]
| Type | Peptide | Uniprot | Precursor Sequence | C-terminus |
|---|---|---|---|---|
| Sodium toxins | AaH2 | P01484 | MNYLVMISLALLFVTGVESVKDGYIVDDVNCTYFCGRNAYCNEECTKLKGESGYCQWASPYGNACYCYKLPDHVRTKGPGRCHGR | H-NH2 |
| LqhIT2 | Q26292 | MKLLLLLIVSASMLIESLVNADGYIKRRDGCKVACLIGNEGCDKECKAYGGSYGYCWTWGLACWCEGLPDDKTWKSETNTCGGKK | G-NH2 | |
| BmKITa | Q9XY87 | MKLFLLLLISASMLIDGLVNADGYIRGSNGCKVSCLWGNEGCNKECRAYGASYGYCWTWGLACWCQGLPDDKTWKSESNTCGGKK | G-NH2 | |
| Cn2 | P01495 | LLIITACLALIGTVWAKEGYLVDKNTGCKYECLKLGDNDYCLRECKQQYGKGAGGYCYAFACWCTHLYEQAIVWPLPNKRCSGK | S-NH2 | |
| Css4 | P60266 | MNSLLMITACLALVGTVWAKEGYLVNSYTGCKFECFKLGDNDYCLRECRQQYGKGSGGYCYAFGCWCTHLYEQAVVWPLPNKTCNGK | N-NH2 | |
| CsEI | P01491 | MNSLLMITACLVLIGTVWAKDGYLVEKTGCKKTCYKLGENDFCNRECKWKHIGGSYGYCYGFGCYCEGLPDSTQTWPLPNKTCGKK | C-NH2 | |
| CsEv3 | P01494 | MNSLLMITACLFLIGTVWAKEGYLVNKSTGCKYGCLKLGENEGCDKECKAKNQGGSYGYCYAFACWCEGLPESTPTYPLPNKSCGKK | C-NH2 | |
| Ts1 | P15226 | MKGMILFISCLLLIGIVVECKEGYLMDHEGCKLSCFIRPSGYCGRECGIKKGSSGYCAWPACYCYGLPNWVKVWDRATNKCGKK | C-NH2 | |
| Ts3 | P01496 | LVVVCLLTAGTEGKKDGYPVEYDNCAYICWNYDNAYCDKLCKDKKADSGYCYWVHILCYCYGLPDSEPTKTNGKCKSGKK | S-NH2 | |
| Potassium toxins | NTx | P08815 | MKAFYGILIILLFCSMFNLNESTIINVKCTSPKQCSKPCKELYGSSAGAKCMNGKCKCYNNG | N-NH2 |
| BmKTX | Q9NII7 | MKVFFAVLITLFICSMIIGIHGVGINVKCKHSGQCLKPCKDAGMRFGKCINGKCDCTPKG | K-NH2 | |
| CoTx1 | O46028 | MEGIAKITLILLFLFVTMHTFANWNTEAAVCVYRTCDKDCKRRGYRSGKCINNACKCYPYGK | Y-NH2 | |
| OcKTx5 | Q6XLL5 | MNAKFILLLVLTTMMLLPDTKGAEVIRCSGSKQCYGPCKQQTGCTNSKCMNKVCKCYGCG | C-NH2 | |
| OcKTx1 | Q6XLL9 | MNAKFILLLLVVATTMLLPDTQGAEVIKCRTPKDCAGPCRKQTGCPHGKCMNRTCRCNRCG | C-NH2 | |
| Non disulfide bridged peptides | IsCT | Q8MMJ7 | MKTQFAILLVALVLFQMFAQSDAILGKIWEGIKSLFGKRGLSDLDGLDELFDGEISKADRDFLRELMR | F-NH2 |
| BmKb1 | Q718F4 | MEIKYLLTVFLVLLIVSDHCQAFLFSLIPSAISGLISAFKGRRKRDLNGYIDHFKNFRKRDAELEELLSKLPIY | K-NH2 | |
| Hp1090 | P0DJ02 | MKTQFAIFLITLVLFQMFSQSDAIFKAIWSGIKSLFGKRGLSDLDDLDESFDGEVSQADIDFLKELMQ | F-NH2 | |
| IsCT2 | Q8MTX2 | MKTQFAILLVALVLFQMFAQSEAIFGAIWNGIKSLFGRRALNNDLDLDGLDELFDGEISQADVDFLKELMR | F-NH2 | |
| VAMP-2 | E4VP07 | MKSQTFFLLFLVVFLLAITQSEAIFGAIAGLLKNIFGKRSLRDMDTMKYLYDPSLSAADLKTLQKLMENY | F-NH2 |
| Family | Species | PAM | PHM | PAL | PC1 | PC2 | CPE |
|---|---|---|---|---|---|---|---|
| Buthidae | Centruroides sculpturatus | ◼-◼ | ◼ | ◼ | ⬤ | ⬤ | ⬤ |
| Centruroides hentzi | ◼-◼ | ◼ | ◼ | ⬤ | ⬤ | ||
| Centruroides noxius a | ◼-◼ | ◼ | ◼ | ⬤ | ⬤ | ||
| Centruroides limpidus b | ◼-◼ | ◼ | ◼ | ⬤ | ⬤ | ⬤ | |
| Centruroides orizaba | ◼-◼ | ◼ | ◼ | ⬤ | ⬤ | ⬤ | |
| Centruroides ochraceus | ◼-◼ | ◼ | ◼ | ⬤ | ⬤ | ⬤ | |
| Centruroides hirsutipalpus | ✓ | ◼ | ◼ | ⬤ | ⬤ | ||
| Tityus trivittatus | ◼-◼ | ◼ | ◼ | ⬤ | ⬤ | ⬤ | |
| Leiurus abdullahbayrami * | ◼-◼ | ◼ | ◼ | ⬤ | ⬤ | ⬤ | |
| Mesobuthus martensii * | ◼-◼ | ◼ | ◼ | ||||
| Vaejovidae | Thorellius cristimanus | ◼-◼ | ◼ | ◼ | ⬤ | ⬤ | ⬤ |
| Paravaejovis schwenkmeyeri | ✓ | ◼ | ◼ | ⬤ | ⬤ | ||
| Chihuahuanus coahuilae | ◼-◼ | ◼ | ◼ | ⬤ | ⬤ | ⬤ | |
| Serradigitus gertschi | ✓ | ◼ | ◼ | ||||
| Caraboctonidae | Hoffmannihadrurus aztecus | ◼-◼ | ◼ | ◼ | |||
| Hadrurus concolorus | ◼-◼ | ◼ | ◼ | ✓ | ⬤ | ||
| Euscorpiidae | Megacormus gertschi | ◼-◼ | ◼ | ◼ | |||
| Chactidae | Anuroctonus pococki bajae | ✓ | ◼ | ◼ | |||
| Superstitionidae | Superstitionia donensis | ✓ | ◼ | ✓ | ✓ | ||
| Diplocentridae | Diplocentrus melici | ◼-◼ | ◼ | ◼ | ⬤ | ||
| Urodacidae | Urodacus yaschenkoi * | ◼-◼ | ◼ | ◼ | ⬤ | ✓ | ⬤ |
| Scorpionidae | Pandinus imperator * | ◼-◼ | ◼ | ⬤ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Prudencio, G.; Possani, L.D.; Becerril, B.; Ortiz, E. The Dual α-Amidation System in Scorpion Venom Glands. Toxins 2019, 11, 425. https://doi.org/10.3390/toxins11070425
Delgado-Prudencio G, Possani LD, Becerril B, Ortiz E. The Dual α-Amidation System in Scorpion Venom Glands. Toxins. 2019; 11(7):425. https://doi.org/10.3390/toxins11070425
Chicago/Turabian StyleDelgado-Prudencio, Gustavo, Lourival D. Possani, Baltazar Becerril, and Ernesto Ortiz. 2019. "The Dual α-Amidation System in Scorpion Venom Glands" Toxins 11, no. 7: 425. https://doi.org/10.3390/toxins11070425
APA StyleDelgado-Prudencio, G., Possani, L. D., Becerril, B., & Ortiz, E. (2019). The Dual α-Amidation System in Scorpion Venom Glands. Toxins, 11(7), 425. https://doi.org/10.3390/toxins11070425






