A Novel Bradykinin-Related Peptide, RVA-Thr6-BK, from the Skin Secretion of the Hejiang Frog; Ordorrana hejiangensis: Effects of Mammalian Isolated Smooth Muscle
Abstract
:1. Introduction
2. Results
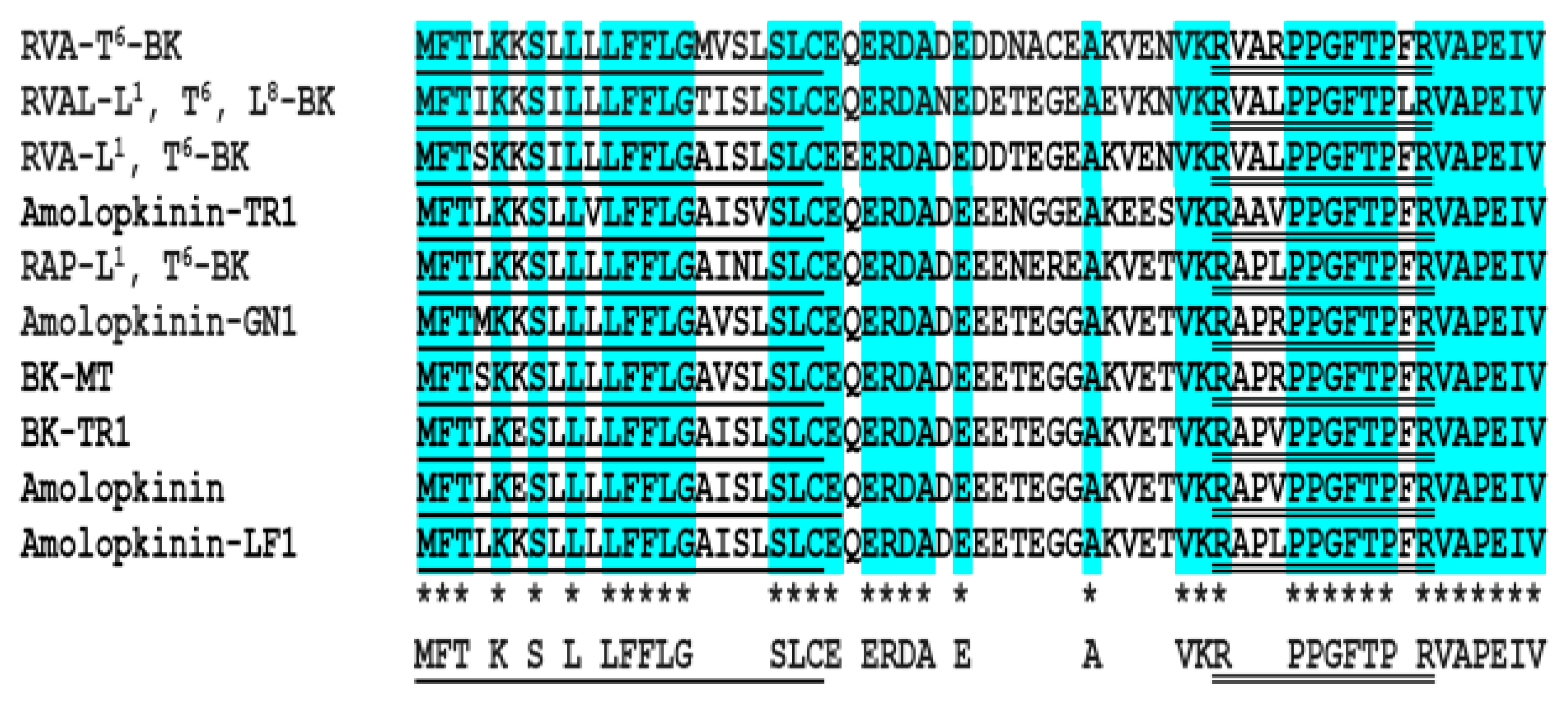
2.1. Molecular Cloning of Biosynthetic Precursor-Encoding cDNA and Structural Characterization of RVA-Thr6-BK from RP-HPLC Fractions of Skin Secretion
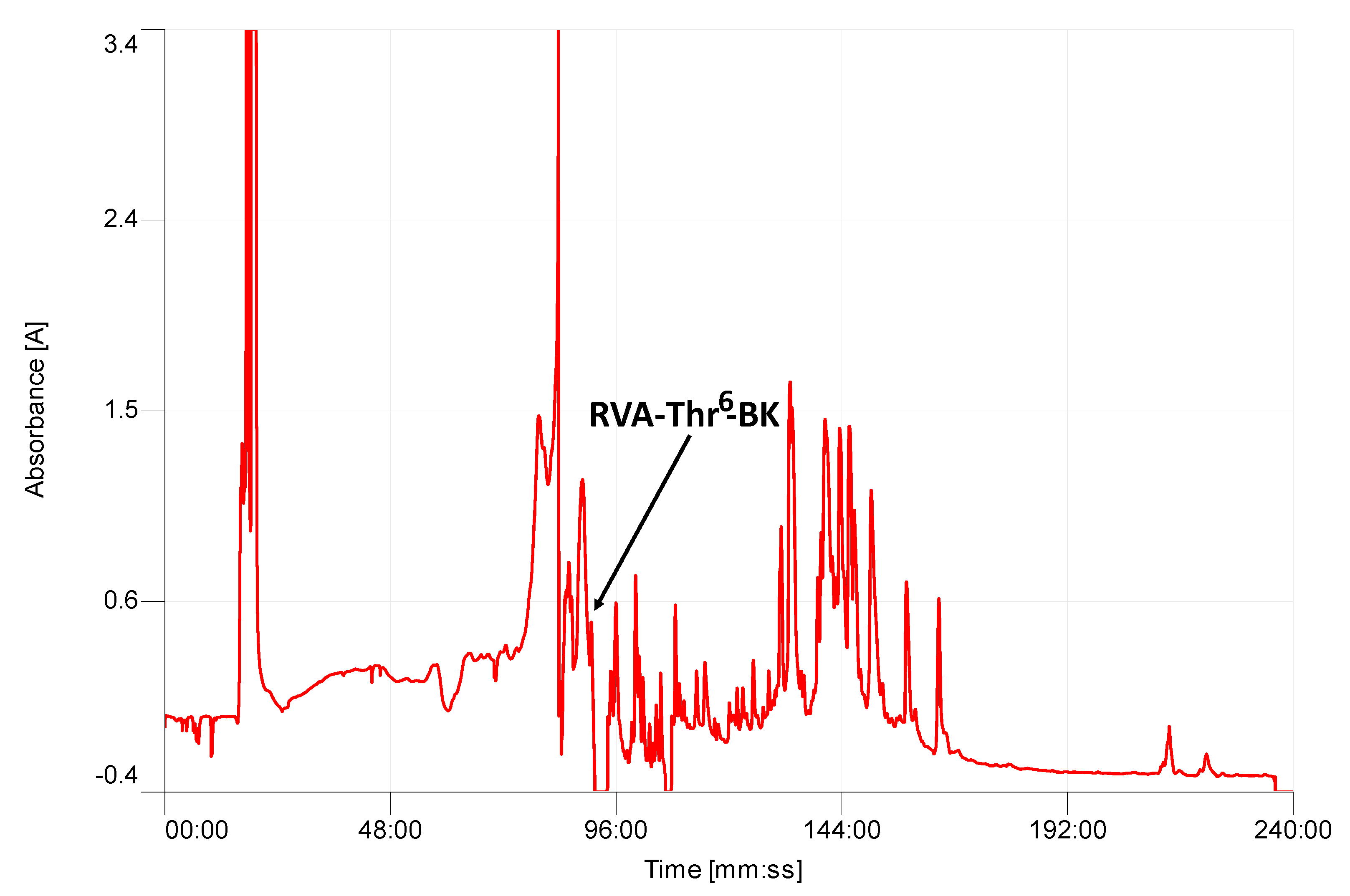
2.2. Isolation and Structural Characterization of RVA-Thr6-BK from RP-HPLC Fractions of Skin Secretion
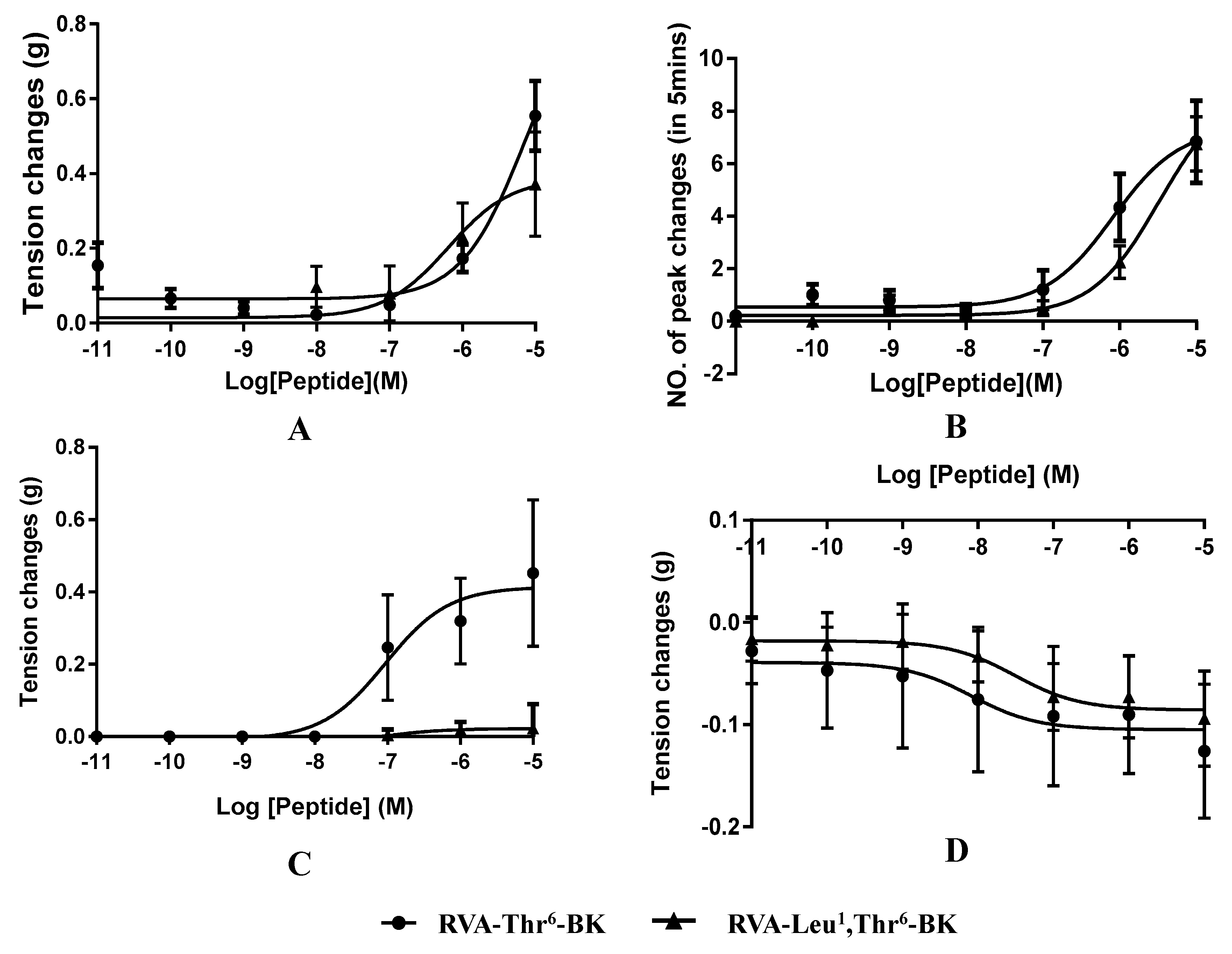
2.3. Myotropic Activities of RVA-Thr6-BK and RVA-Leu1, Thr6-BK
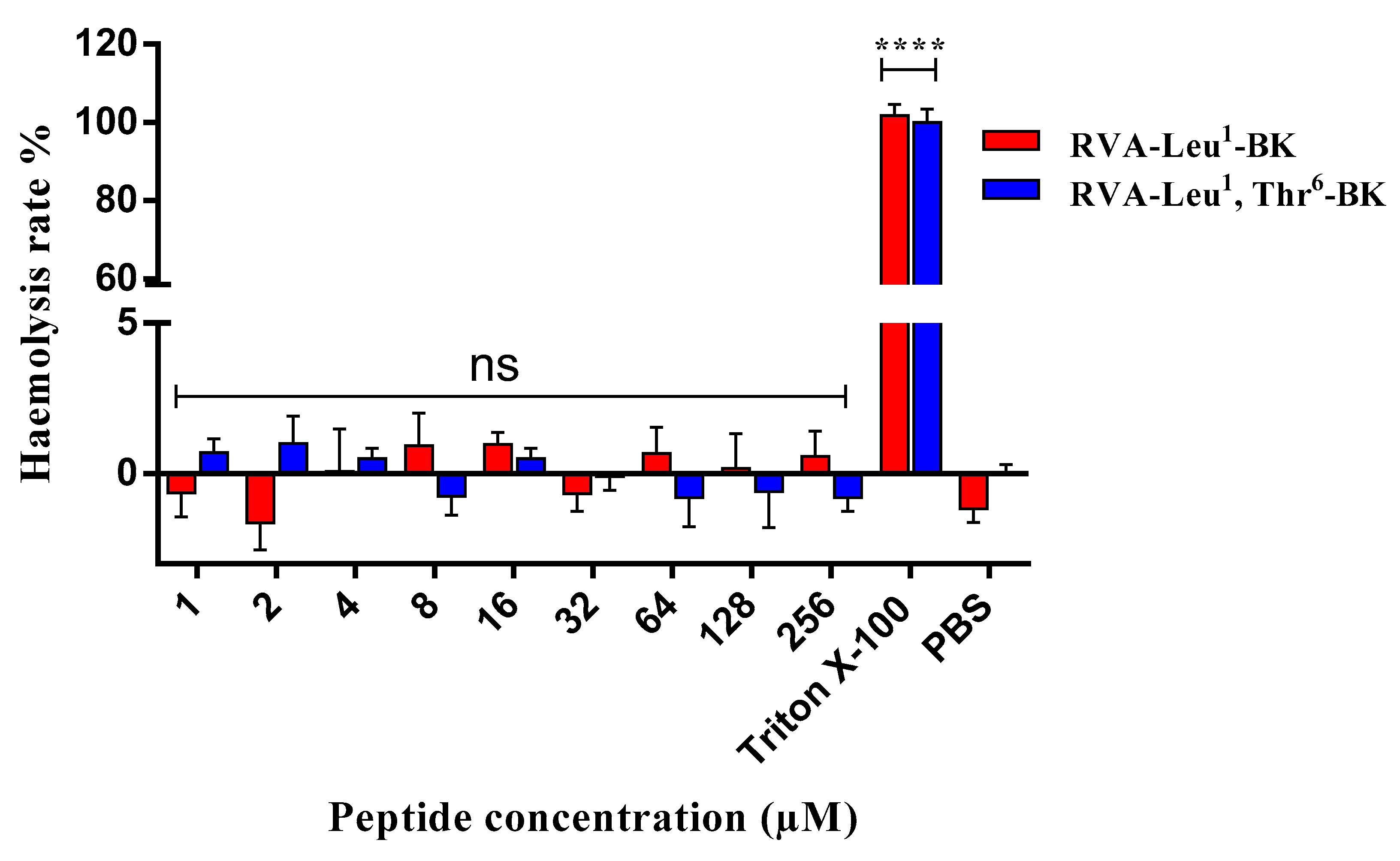
2.4. Assessment of Cytotoxic Effect on Mammalian Erythrocytes
3. Discussion
4. Materials and Methods
4.1. Skin Secretion Acquisition
4.2. “Shotgun” Cloning of cDNA Encoding RVA-Thr6-BK Biosynthetic Precursor from Skin Secretion
4.3. Isolation and Structural Characterization of RVA-Thr6-BK from Skin Secretion
4.4. RVA-Thr6-BK and Its Site-Substituted Analogue, RVA-Leu1, Thr6-BK: Synthesis and Purification
4.5. Myotropic Activity Evaluation on Smooth Muscles
4.6. Haemolysis Assay
4.7. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Mandle, R.J.; Colman, R.W.; Kaplan, A.P. Identification of prekallikrein and high-molecular-weight kininogen as a complex in human plasma. Proc. Natl. Acad. Sci. USA 1976, 73, 4179–4183. [Google Scholar] [CrossRef] [PubMed]
- Bhoola, K.D.; Figueroa, C.D.; Worthy, K. Bioregulation of kinins: Kallikreins, kininogens, and kininases. Pharmacol. Rev. 1992, 44, 1–80. [Google Scholar] [PubMed]
- Kaplan, A.P.; Joseph, K.; Silverberg, M. Pathways for bradykinin formation and inflammatory disease. J. Allergy Clin. Immunol. 2002, 109, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Regoli, D.; Barabe, J. Pharmacology of bradykinin and related kinins. Pharmacol. Rev. 1980, 32, 1–46. [Google Scholar] [PubMed]
- Rabito, S.F.; Binia, A.; Segovia, R. Plasma kininogen content of toads, fowl and reptiles. Comp. Biochem. Physiol. A. 1972, 41, 281–284. [Google Scholar] [CrossRef]
- Seki, T.; Miwa, I.; Nakajima, T.; Erdos, E. Plasma kallikrein-kinin system in nonmammalian blood: Evolutionary aspects. Am. J. Physiol. 1973, 224, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Li, B.; Chen, T.; Kwok, H. A review on bradykinin-related peptides isolated from amphibian skin secretion. Toxins 2015, 7, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Anastasi, A.; Erspamer, V.; Bertaccini, G. Occurence of bradykinin in the skin of Rana temporaria. Comp. Biochem. Physiol. 1965, 14, 43–52. [Google Scholar] [CrossRef]
- Conlon, J.M.; Aronsson, U. Multiple bradykinin-related peptides from the skin of the frog, rana temporaria. Peptides 1997, 18, 361–365. [Google Scholar] [CrossRef]
- Conlon, J.M.; Jouenne, T.; Cosette, P.; Cosquer, D.; Vaudry, H.; Taylor, C.K.; Abel, P.W. Bradykinin-related peptides and tryptophyllins in the skin secretions of the most primitive extant frog. Ascaphus truei. Gen. Comp. Endocrinol. 2005, 143, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M. Bradykinin-related peptides from frog skin. In Handbook of Biologically Active Peptides, 1st ed.; Kastin, J.A., Ed.; Academic Press: Amsterdam, The Netherlands, 2006; pp. 291–294. [Google Scholar]
- Samgina, T.Y.; Gorshkov, V.; Vorontsov, Y.A.; Artemenko, K.; Zubarev, R.; Lebedev, A. Mass spectrometric study of bradykinin-related peptides (BRPs) from the skin secretion of russian ranid frogs. Rapid Commun. Mass Spectrom. 2011, 25, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Shaw, C. Cloning of the (Thr6)-phyllokinin precursor from Phyllomedusa sauvagei skin confirms a non-consensus tyrosine O-sulfation motif. Peptides 2003, 24, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Bjourson, A.J.; Coulter, D.J.; Chen, T.; Shaw, C.; O’rourke, M.; Hirst, D.G.; Zhang, Y.; Rao, P.; McClean, S. Bradykinin-related peptides, including a novel structural variant, (Val1)-bradykinin, from the skin secretion of guenther’s frog, Hylarana guentheri and their molecular precursors. Peptides 2007, 28, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Rates, B.; Silva, L.P.; Ireno, I.C.; Leite, F.S.F.; Borges, M.H.; Bloch, C., Jr.; De Lima, M.E.; Pimenta, A.M. Peptidomic dissection of the skin secretion of Phasmahyla jandaia (bokermann and sazima, 1978)(Anura, Hylidae, Phyllomedusinae). Toxicon 2011, 57, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.H.; Bjourson, A.J.; Shaw, C.; McClean, S. Bradykinin-related peptides from Phyllomedusa hypochondrialis azurea: Mass spectrometric structural characterisation and cloning of precursor cDNAs. Rapid Commun. Mass Spectrom. 2006, 20, 3780–3788. [Google Scholar] [CrossRef] [PubMed]
- Samgina, T.Y.; Artemenko, K.A.; Gorshkov, V.A.; Ogourtsov, S.V.; Zubarev, R.A.; Lebedev, A.T. De novo sequencing of peptides secreted by the skin glands of the caucasian green frog Rana ridibunda. Rapid Commun. Mass Spectrom. 2008, 22, 3517–3525. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, L.; Zhou, M.; Chen, T.; Ding, A.; Rao, P.; Walker, B.; Shaw, C. Amolopkinins W1 and W2—novel bradykinin-related peptides (BRPs) from the skin of the chinese torrent frog, Amolops wuyiensis: Antagonists of bradykinin-induced smooth muscle contraction of the rat ileum. Peptides 2009, 30, 893–900. [Google Scholar] [CrossRef]
- Bevins, C.L.; Zasloff, M. Peptides from frog skin. Annu. Rev. Biochem. 1990, 59, 395–414. [Google Scholar] [CrossRef]
- König, E.; Bininda-Emonds, O.R.P.; Shaw, C. The diversity and evolution of anuran skin peptides. Peptides 2015, 63, 96–117. [Google Scholar] [CrossRef]
- Jensen, J.; Conlon, J.M. Effects of trout bradykinin on the motility of the trout stomach and intestine: Evidence for a receptor distinct from mammalian B1 and B2 subtypes. Br. J. Pharmacol. 1997, 121, 526–530. [Google Scholar] [CrossRef]
- Li, Z.; Secor, S.M.; Lance, V.A.; Masini, M.A.; Vallarino, M.; Conlon, J.M. Characterization of bradykinin-related peptides generated in the plasma of six sarcopterygian species (african lungfish, amphiuma, coachwhip, bullsnake, gila monster, and gray’s monitor). Gen. Comp. Endocrinol. 1998, 112, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Farmer, S.G.; Burch, R.M. Biochemical and molecular pharmacology of kinin receptors. Annu. Rev. Pharmacol. Toxicol. 1992, 32, 511–536. [Google Scholar] [CrossRef] [PubMed]
- Marceau, F.; Hess, J.F.; Bachvarov, D.R. The B1 receptors for kinins. Pharmacol. Rev. 1998, 50, 357–386. [Google Scholar] [PubMed]
- Marceau, F.; Regoli, D. Bradykinin receptor ligands: Therapeutic perspectives. Nat. Rev. Drug Discov. 2004, 3, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Lintner, K.; Fermandjian, S.; Regoli, D. Conformational features of bradykinin: A circular dichroism study of some peptide fragments and structural analogues of bradykinin. Biochimie 1979, 61, 87–92. [Google Scholar] [CrossRef]
- Bonechi, C.; Ristori, S.; Martini, G.; Martini, S.; Rossi, C. Study of bradykinin conformation in the presence of model membrane by nuclear magnetic resonance and molecular modelling. Biochim. Biophys. Acta Biomembr. 2009, 1788, 708–716. [Google Scholar] [CrossRef]
- Parasuraman, S. Toxicological screening. J. Pharmacol. Pharmacother. 2011, 2, 74. [Google Scholar] [CrossRef]
- Megaw, J.; Thompson, T.P.; Lafferty, R.A.; Gilmore, B.F. Galleria mellonella as a novel in vivo model for assessment of the toxicity of 1-alkyl-3-methylimidazolium chloride ionic liquids. Chemosphere 2015, 139, 197–201. [Google Scholar] [CrossRef]
- Tyler, M.J.; Stone, D.; Bowie, J.H. A novel method for the release and collection of dermal, glandular secretions from the skin of frogs. J. Pharmacol. Toxicol. Methods 1992, 28, 199–200. [Google Scholar] [CrossRef]
- Xiang, J.; Wang, H.; Ma, C.; Zhou, M.; Wu, Y.; Wang, L.; Guo, S.; Chen, T.; Shaw, C. Ex vivo smooth muscle pharmacological effects of a novel bradykinin-related peptide, and its analogue, from Chinese large odorous frog, Odorrana livida skin secretions. Toxins 2016, 8, 283. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Wu, Y.; Zhou, M.; Ma, C.; Xi, X.; Chen, T.; Walker, B.; Shaw, C.; Wang, L. A Bowman-Birk type chymotrypsin inhibitor peptide from the amphibian, Hylarana erythraea. Sci. Rep. 2018, 8, 5851. [Google Scholar] [CrossRef] [PubMed]

| #1 | b(1+) | b(2+) | b(3+) | Seq. | y(1+) | y(2+) | y(3+) | #2 |
|---|---|---|---|---|---|---|---|---|
| 1 | 157.10840 | 79.05784 | 53.04098 | R | 12 | |||
| 2 | 256.17682 | 128.59205 | 86.06379 | V | 1244.68992 | 622.84860 | 415.56816 | 11 |
| 3 | 327.21394 | 164.11061 | 109.74283 | A | 1145.62150 | 573.31439 | 382.54535 | 10 |
| 4 | 483.31506 | 242.16117 | 161.77654 | R | 1074.58438 | 537.79583 | 358.86631 | 9 |
| 5 | 580.36783 | 290.68755 | 194.12746 | P | 918.48326 | 459.74527 | 306.83260 | 8 |
| 6 | 677.42060 | 339.21394 | 226.47838 | P | 821.43049 | 411.21888 | 274.48168 | 7 |
| 7 | 734.44207 | 367.72467 | 245.48554 | G | 724.37772 | 362.69250 | 242.13076 | 6 |
| 8 | 881.51049 | 441.25888 | 294.50835 | F | 667.35625 | 334.18176 | 223.12360 | 5 |
| 9 | 982.55817 | 491.78272 | 328.19091 | T | 520.28783 | 260.64755 | 174.10079 | 4 |
| 10 | 1079.61094 | 540.30911 | 360.54183 | P | 419.24015 | 210.12371 | 140.41823 | 3 |
| 11 | 1226.67936 | 613.84332 | 409.56464 | F | 322.18738 | 161.59733 | 108.06731 | 2 |
| 12 | R | 175.11896 | 88.06312 | 59.04450 | 1 |
| Isolated Tissue | E Max | TC (nM) | EC50 (nM) |
|---|---|---|---|
| RVA-Thr6-BK/RVA-Leu1, Thr6-BK | |||
| Bladder | 0.56/0.38 (g) | 100.00/10.00 | 7102.00/636.70 |
| Uterus | 7.00/7.00 (peaks/5 min) | 10.00/100.00 | 817.20/3270.00 |
| Ileum | 0.43/N (g) | 1.00/N | 93.70/N |
| Tail artery | −0.13/−0.08 (g) | 1.00/1 | 8.28/30.91 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Shi, D.; Chen, X.; Wang, L.; Ying, Y.; Ma, C.; Xi, X.; Zhou, M.; Chen, T.; Shaw, C. A Novel Bradykinin-Related Peptide, RVA-Thr6-BK, from the Skin Secretion of the Hejiang Frog; Ordorrana hejiangensis: Effects of Mammalian Isolated Smooth Muscle. Toxins 2019, 11, 376. https://doi.org/10.3390/toxins11070376
Wu Y, Shi D, Chen X, Wang L, Ying Y, Ma C, Xi X, Zhou M, Chen T, Shaw C. A Novel Bradykinin-Related Peptide, RVA-Thr6-BK, from the Skin Secretion of the Hejiang Frog; Ordorrana hejiangensis: Effects of Mammalian Isolated Smooth Muscle. Toxins. 2019; 11(7):376. https://doi.org/10.3390/toxins11070376
Chicago/Turabian StyleWu, Yue, Daning Shi, Xiaoling Chen, Lei Wang, Yuan Ying, Chengbang Ma, Xinping Xi, Mei Zhou, Tianbao Chen, and Chris Shaw. 2019. "A Novel Bradykinin-Related Peptide, RVA-Thr6-BK, from the Skin Secretion of the Hejiang Frog; Ordorrana hejiangensis: Effects of Mammalian Isolated Smooth Muscle" Toxins 11, no. 7: 376. https://doi.org/10.3390/toxins11070376
APA StyleWu, Y., Shi, D., Chen, X., Wang, L., Ying, Y., Ma, C., Xi, X., Zhou, M., Chen, T., & Shaw, C. (2019). A Novel Bradykinin-Related Peptide, RVA-Thr6-BK, from the Skin Secretion of the Hejiang Frog; Ordorrana hejiangensis: Effects of Mammalian Isolated Smooth Muscle. Toxins, 11(7), 376. https://doi.org/10.3390/toxins11070376






