Simple Diffusion as the Mechanism of Okadaic Acid Uptake by the Mussel Digestive Gland
Abstract
:1. Introduction
2. Results
2.1. Dissociation and Cell Culture Viability
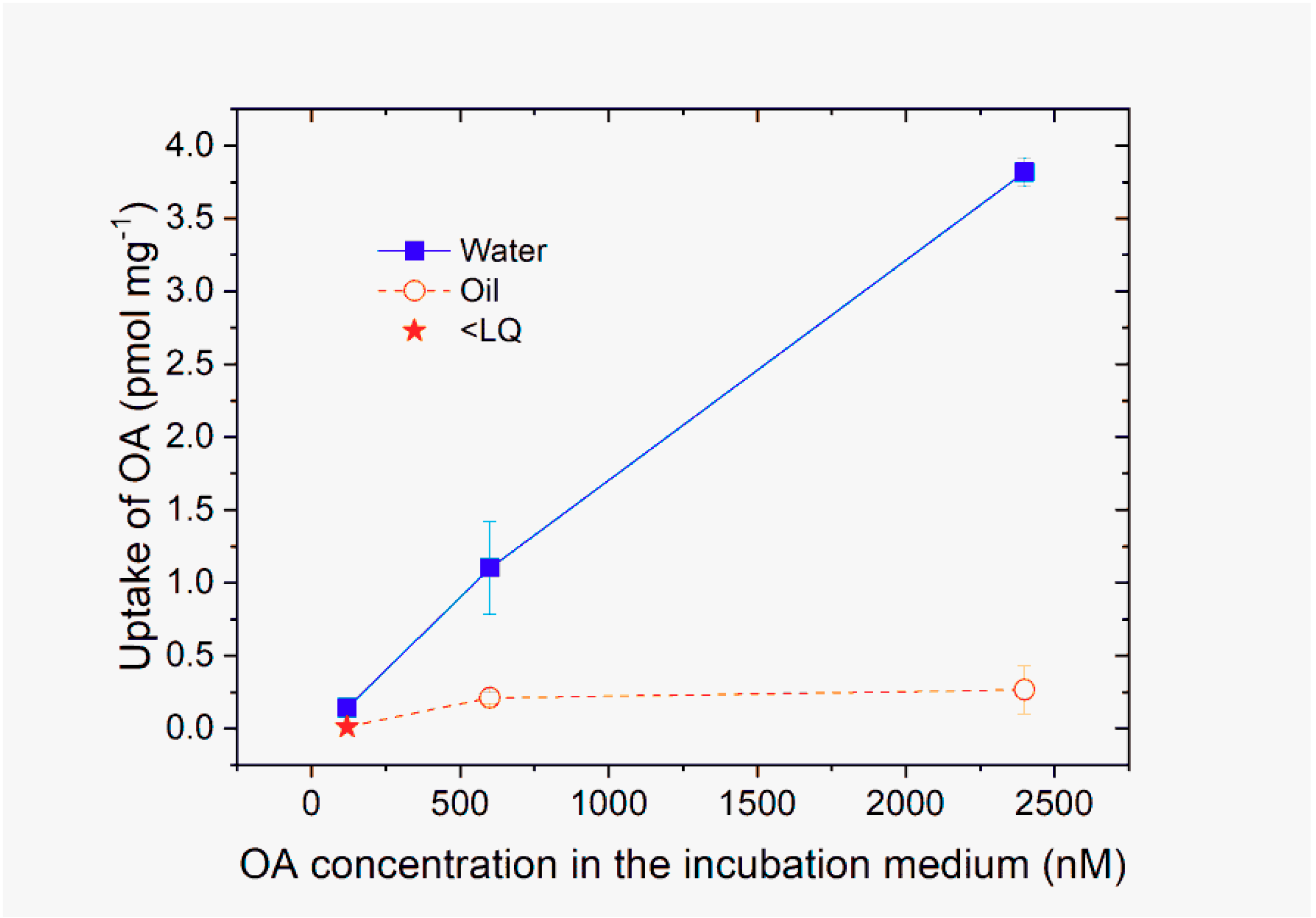
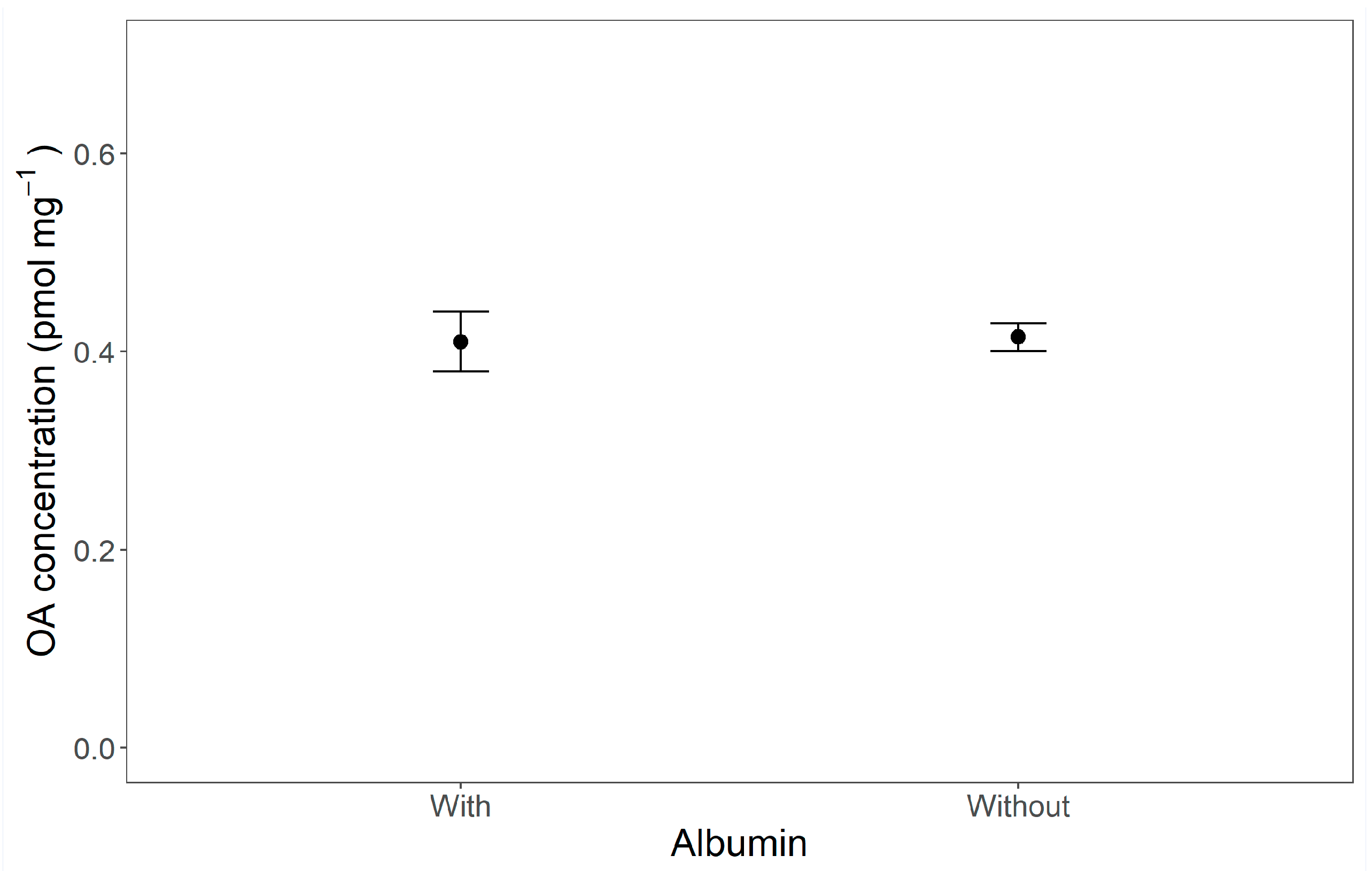
2.2. Uptake of OA Dissolved in Water and Oil
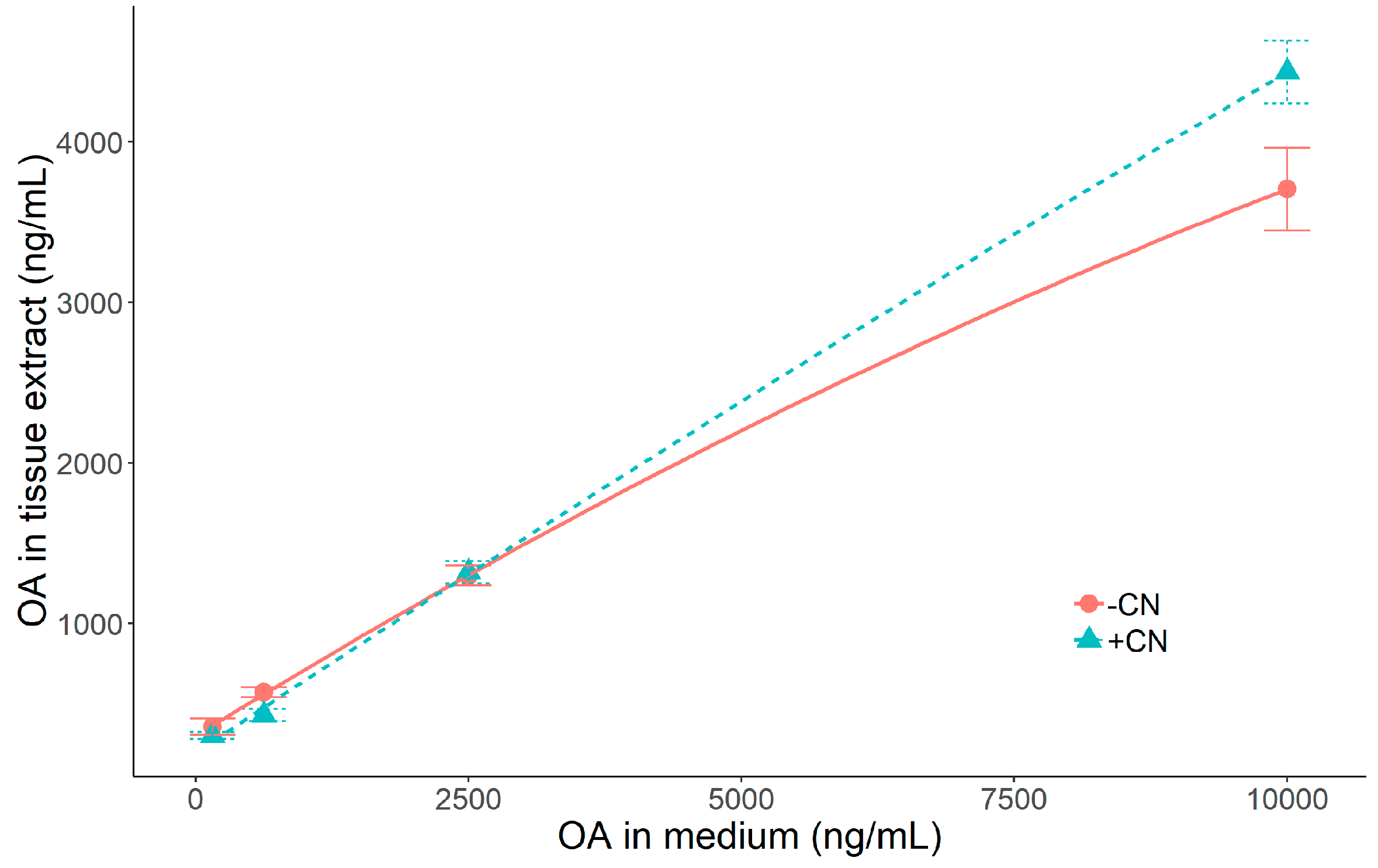
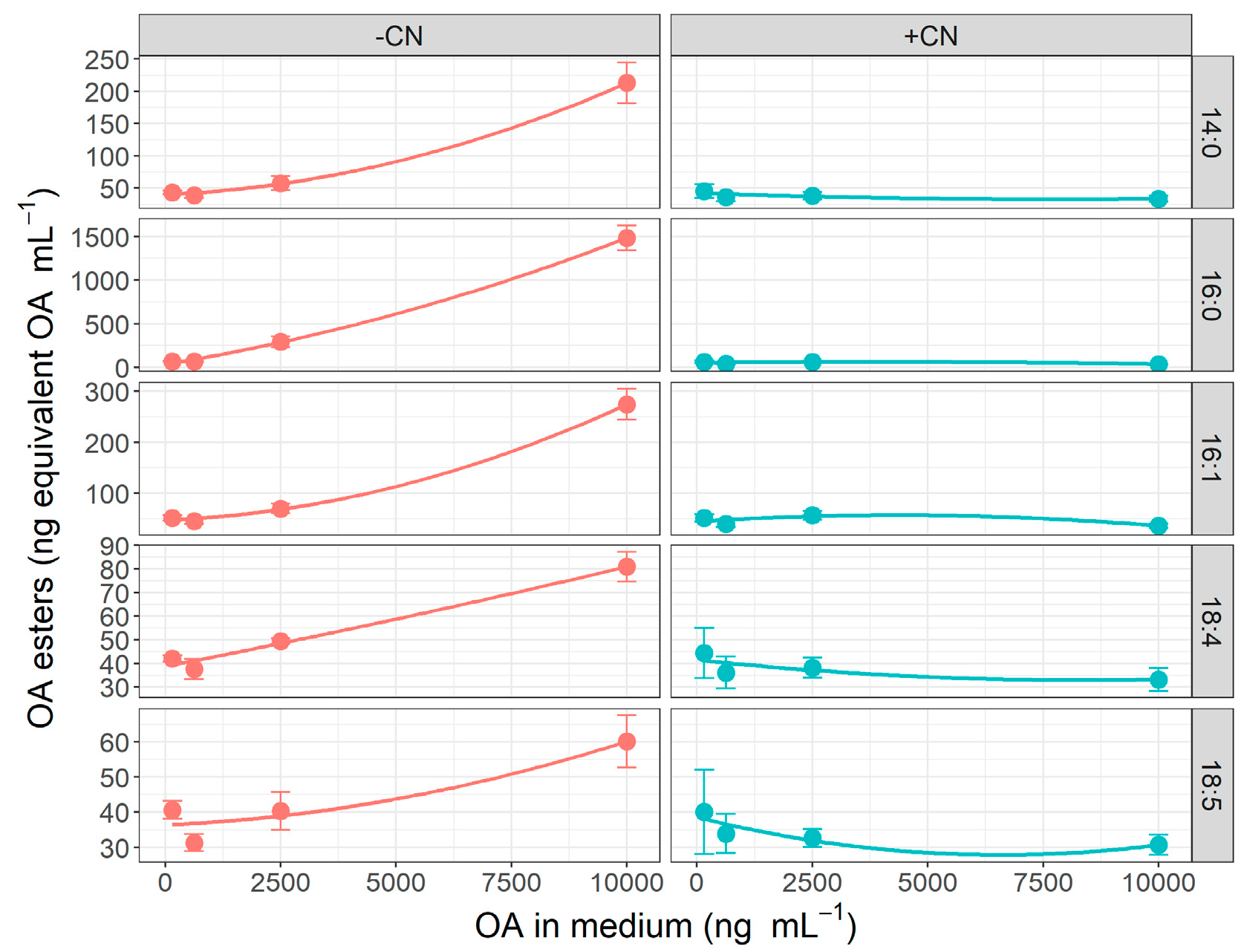
2.3. Effect of Okadaic Acid Concentration and Cyanide on OA Uptake
3. Discussion
4. Materials and Methods
4.1. Biological Material and Chemicals
4.2. Experiments 1 and 2
4.2.1. Digestive Gland Dissociation
4.2.2. Primary Cell Cultures
4.2.3. Experiment 1: Uptake of Okadaic Acid Dissolved in Water and Oil
4.2.4. Experiment 2: Effect of an Emulsifier (Albumin) on the Uptake of Okadaic Acid Dissolved in Oil
4.3. Experiment 3: Effect of OA Concentration and Sodium Cyanide on the Uptake of the Toxin by Slices of Digestive Gland
4.4. Toxin Analysis
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Della Loggia, R.; Cabrini, M.; del Negro, P.; Honsell, G.; Tubaro, A. Relationship between Dinophysis spp. in seawater and DSP toxins in mussels in the Northern Adriatic Sea. In Toxic Phytoplankton Blooms in the Sea; Smayda, T.J., Shimizu, Y., Eds.; Elsevier Science: New York, NY, USA, 1993; pp. 483–488. [Google Scholar]
- Yasumoto, T.; Oshima, Y.; Sugawara, W.; Fukuyo, Y.; Oguri, H.; Igarashi, T.; Fujita, N. Identification of Dinophysis fortii as the causative organism of diarrhetic shellfish poisoning. Bull. Jpn. Soc. Sci. Fish. 1980, 46, 1405–1411. [Google Scholar] [CrossRef]
- Reguera, B.; Bravo, I.; Fraga, S. Distribution of Dinophysis acuta at the time of a DSP outbreak in the rias of Pontevedra and Vigo (Galicia, NW Spain). ICES CM Shellfish Comm. 1990, 1990/L, 14. [Google Scholar]
- Edler, L.; Hageltorn, M. Identification of the causative organism of a DSP-outbreak on the Swedish West Coast. In Toxic Marine Phytoplankton; Graneli, E.B., Sundstrom, L.E., Anderson, D.M., Eds.; Elsevier Science: New York, NY, USA, 1990; pp. 345–349. [Google Scholar]
- Kat, M. Dinophysis acuminata blooms in the Dutch coastal area related to diarrhetic mussel poisoning in the Dutch Waddensea. Sarsia 1983, 6, 81–84. [Google Scholar] [CrossRef]
- Lassus, P.; Bardouil, M.; Truquet, I.; Truquet, P.; Le Baut, C.; Pierre, M.J. Dinophysis acuminata distribution and toxicity along the Southern Brittany Coast (France): Correlation with hydrological parameters. In Physiological Ecology of Harmful Algal Blooms; Anderson, D.M., Cembella, A., Hallegraeff, G.M., Eds.; Springer: Berlin, Germany, 1985; pp. 159–164. [Google Scholar]
- Giacobbe, M.G.; Penna, A.; Ceredi, A.; Milandri, A.; Poletti, R.; Yang, X. Toxicity and ribosomal DNA of the dinoflagellate Dinophysis sacculus (Dinophyta). Phycologia 2000, 39, 177–182. [Google Scholar] [CrossRef]
- Lee, J.S.; Igarashi, T.; Fraga, S.; Dahl, E.; Hovgaard, P.; Yasumoto, T. Determination of diarrhetic shellfish toxins in various dinoflagellate species. J. Appl. Phycol. 1989, 1, 147–152. [Google Scholar] [CrossRef]
- Reguera, B.; Riobó, P.; Rodríguez, F.; Díaz, P.; Pizarro, G.; Paz, B.; Franco, J.; Blanco, J. Dinophysis Toxins: Causative Organisms, Distribution and Fate in Shellfish. Mar. Drugs 2014, 12, 394–461. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.E.; Bauder, A.G.; Quilliam, M.A.; Cembella, A.D. Prorocentrum lima: A putative link to diarrhetic shellfish poisoning in Nova Scotia, Canadá. In Harmful Algae; Reguera, B., Blanco, J., Fernández, M.L., Wyatt, T., Eds.; Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO: Santiago de Compostela, Spain, 1998; pp. 78–79. [Google Scholar]
- Heredia-Tapia, A.; Arredondo-Vega, B.O.; Nuñez-Vázquez, E.J.; Yasumoto, T.; Yasuda, M.; Ochoa, J.L. Isolation of Prorocentrum lima (syn. Exuviaella lima) and diarrhetic shellfish poisoning (DSP) risk assessment in the Gulf of California, Mexico. Toxicon 2002, 40, 1121–1127. [Google Scholar] [PubMed]
- Gayoso, A.M.; Dover, S.; Morton, S.; Busman, M.; Moeller, P.; Fulco, V.K.; Maranda, L. Diarrhetic shellfish poisoning associated with Prorocentrum lima (Dinophyceae) in Patagonian Gulfs (Argentina). J. Shellfish Res. 2002, 21, 461–463. [Google Scholar]
- Bauder, A.G.; Cembella, A.D.; Bricelj, V.M.; Quilliam, M.A. Uptake and fate of diarrhetic shellfish poisoning toxins from the dinoflagellate Prorocentrum lima in the bay scallop Argopecten irradians. Mar. Ecol. Prog. Ser. 2001, 213, 39–52. [Google Scholar] [CrossRef]
- Koike, K.; Sato, S.; Yamaji, M.; Nagahama, Y.; Kotaki, Y.; Ogata, T.; Kodama, M. Occurrence of okadaic acid-producing Prorocentrum lima on the Sanriku coast, Northern Japan. Toxicon 1998, 36, 2039–2042. [Google Scholar] [CrossRef]
- Quilliam, M.A.; Hardstaff, W.R.; Ishida, N.; McLachlan, J.L.; Reeves, A.R.; Ross, N.W.; Windust, A.J. Production od diarrhetic shellfish poisoning (DSP) toxins by Prorocentrum lima in culture and development of analytical methods. In Harmful and Toxic Algal Blooms; Yasumoto, T., Oshima, Y., Fukuyo, Y., Eds.; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 1996; pp. 289–292. [Google Scholar]
- Yasumoto, T.; Murata, M.; Oshima, Y.; Sano, M.; Matsumoto, G.; Clardy, J. Diarrhetic Shellfish Toxins. Tetrahedron 1985, 41, 1019–1025. [Google Scholar] [CrossRef]
- Hu, T.; Doyle, J.; Jackson, D.M.; Marr, J.; Nixon, E.; Pleasance, S.; Quilliam, M.A.; Walter, J.A.; Wright, J.L.C. Isolation of a new diarrhetic shellfish poison from Irish mussels. J. Chem. Soc. Chem. Commun. 1992, 1, 39–44. [Google Scholar] [CrossRef]
- Quilliam, M.A. Chemical method for lipophilic shellfish toxins. In Manual on Harmful Marine Microalgae; Hallegraeff, G.M., Anderson, D.M., Cembella, A.D., Eds.; UNESCO Publishing: Paris, France, 2003; pp. 211–245. [Google Scholar]
- Blanco, J.; Moroño, A.; Fernández, M.L. Toxic episodes in shellfish, produced by lipophilic phycotoxins: An overview. Revista Galega dos Recursos Mariños Monog. 2005, 1, 70. [Google Scholar]
- MacKenzie, L.; Beuzenberg, V.; Holland, P.; McNabb, P.; Selwood, A. Solid phase adsorption toxin tracking (SPATT): A new monitoring tool that simulates the biotoxin contamination of filter feeding bivalves. Toxicon 2004, 44, 901–918. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J. Accumulation of Dinophysis Toxins in Bivalve Molluscs. Toxins 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Holmes, C.F.; Tsukitani, Y. Okadaic acid: A new probe for the study of cellular regulation. Trends Biochem. Sci. 1990, 15, 98–102. [Google Scholar] [CrossRef]
- Mestrovic, V.; Pavela Vrancic, A. Inhibition of alkaline phosphatase activity by okadaic acid, a protein phosphatase inhibitor. Biochimie 2003, 85, 647–650. [Google Scholar] [CrossRef]
- Fujiki, H.; Suganama, M.; Suguri, H.; Yoshizawa, S.; Takagi, K.; Uda, N.; Wakamatsu, K.; Yamanda, K.; Murata, M.; Yasumoto, T.; et al. Diarrhetic shellfish toxins, dinophysistoxin -1 is a potent tumor promoter on mouse skin. Jpn. J. Cancer Res. 1988, 79, 1089–1093. [Google Scholar] [CrossRef]
- Ten-Hage, L.; Delaunay, N.; Pichon, V.; Coute, A.; Puiseux-Dao, S.; Turquet, J. Okadaic acid production from the marine benthic dinoflagellate Prorocentrum arenarium faust (Dinophyceae) isolated from Europa Island coral reef ecosystem (SW Indian Ocean). Toxicon 2000, 38, 1043–1054. [Google Scholar] [CrossRef]
- Creppy, E.E.; Traore, A.; Baudrimont, I.; Cascante, M.; Carratu, M.-R. Recent advances in the study of epigenetic effects induced by the phycotoxin okadaic acid. Toxicology 2002, 181–182, 433–439. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion of the Panel on Contaminants in the Food Chain on a request from the European Commission on Marine Biotoxins in Shellfish—Summary on regulated marine biotoxins. EFSA J. 2009, 1306, 1–23. [Google Scholar]
- Blanco, J.; Correa, J.; Muñiz, S.; Mariño, C.; Martín, H.; Arévalo, F. Evaluación del impacto de los métodos y niveles utilizados para el control de toxinas en el mejillón. Revista Galega dos Recursos Mariños Art. Inf. Tecn. 2013, 3, 1–55. [Google Scholar]
- Blanco, J.; Mariño, C.; Martín, H.; Acosta, C.P. Anatomical distribution of diarrhetic shellfish poisoning (DSP) toxins in the mussel Mytilus galloprovincialis. Toxicon 2007, 50, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.N.; Willows, R.I. A model for cellular uptake and intracellular behaviour of particulate-bound micropollutants. Mar. Environ. Res. 1998, 46, 509–514. [Google Scholar] [CrossRef]
- Smedes, F. Sampling and partitioning of neutral organic contaminants in surface waters with regard to legislation, environmental quality and flux estimations. J. Environ. Anal. Chem. 1994, 57, 215–229. [Google Scholar] [CrossRef]
- Fux, E. Development and Evaluation of Passive Sampling and LC-MS Based Techniques for the Detection and Monitoring of Lipophilic Marine Toxins in Mesocosm and Field Studies; The Marine Institute; School of Chemical and Pharmaceutical Sciences: Dublin, Ireland, 2008. [Google Scholar]
- Langton, R.W. Digestive rhythms in the mussel Mytilus edulis. Mar. Biol. 1977, 41, 53–58. [Google Scholar] [CrossRef]
- Mathers, N.F. Digestion and pH variation in two species of oysters. J. Molluscan Stud. 1974, 41, 37. [Google Scholar] [CrossRef]
- Daranas, A.H.; Cruz, P.G.; Creus, A.H.; Norte, M.; Fernández, J.J. Self-assembly of okadaic acid as a pathway to the cell. Org. Lett. 2007, 9, 4191–4194. [Google Scholar] [CrossRef]
- Li, A.; Li, M.; Qiu, J.; Song, J.; Ji, Y.; Hu, Y.; Wang, S.; Che, Y. Effect of suspended particulate matter on the accumulation of dissolved diarrhetic shellfish toxins by mussels (Mytilus galloprovincialis) under laboratory conditions. Toxins 2018, 10, 273. [Google Scholar] [CrossRef]
- Blanco, J.; Martin-Morales, E.; Alvarez, G. Stability of okadaic acid and 13-desmethyl spirolide C in seawater and sediment. Mar. Chem. 2018, 207, 21–25. [Google Scholar] [CrossRef]
- Rossignoli, A.E.; Blanco, J. Subcellular distribution of okadaic acid in the digestive gland of Mytilus galloprovincialis: First evidences of lipoprotein binding to okadaic acid. Toxicon 2010, 55, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Paz, B.; Daranas, A.H.; Cruz, P.G.; Franco, J.M.; Pizarro, G.; Souto, M.L.; Norte, M.; Fernandez, J.J. Characterisation of Okadaic Acid Related Toxins by Liquid Chromatography Coupled With Mass Spectrometry. Toxicon 2007, 50, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.Y.; Hiro, M.; Kimura, S.; Fujiki, H.; Imanishi, Y. Permeability of a non-TPA-type tumor promoter, okadaic acid, through lipid bilayer membrane. Carcinogenesis 1990, 11, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- González, J.C.; Vieytes, M.R.; Vieites, J.M.; Botana, L.M. Solubilíty of the diarrheic shellfish toxin okadaic acid in supercritical CO2. J. Am. Oil. Chem. Soc. 2001, 78, 77–81. [Google Scholar] [CrossRef]
- Guéguen, M.; Duinker, A.; Marcaillou, C. A First Approach to Localizing Biotoxins in Mussel Digestive Glands, Proceedings of the 7th International Conference on Molluscan Shellfish Safety, Nantes, France, 14–19 June 2009; Irstea, C., Ifremer, I., Eds.; Éditions Quæ: Nantes, France, 2010. [Google Scholar]
- Fernández, M.L.; Miguez, A.; Moroño, A.; Cacho, E.; Martínez, A.; Blanco, J. Detoxification of low polarity toxins (DTX3) from mussels Mytilus galloprovincialis in Spain. In Harmful Algae; Reguera, B., Blanco, J., Fernández, M.L., Wyatt, T., Eds.; Xunta de Galicia & Intergovernmental Oceanografic Comission of UNESCO: Santiago de Compostela, Spain, 1998; pp. 449–452. [Google Scholar]
- Vale, P. Differential dynamics of dinophysistoxins and pectenotoxins between blue mussel and common cockle: A phenomenon originating from the complex toxin profile of Dinophysis acuta. Toxicon 2004, 44, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Vale, P. Differential dynamics of dinophysistoxins and pectenotoxins, Part II: Offshore bivalve species. Toxicon 2006, 47, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Vale, P.; Sampayo, M.A. Esterification of DSP toxins by Portuguese bivalves from the northwest coast determined by LC-MS-a widespread phenomenon. Toxicon 2002, 40, 33–42. [Google Scholar] [CrossRef]
- Rossignoli, A.E.; Fernández, D.; Regueiro, J.; Mariño, C.; Blanco, J. Esterification of okadaic acid in the mussel Mytilus galloprovincialis. Toxicon 2011, 57, 712–720. [Google Scholar] [CrossRef]
- Birmelin, C.; Pipe, R.K.; Goldfarb, P.S.; Livingstone, D.R. Primary cell-culture of the digestive gland of the marine mussel Mytilus edulis: A time-course study of antioxidant -and biotransformation- enzyme activity and ultrastructural changes. Mar. Biol. 1999, 135, 65–75. [Google Scholar] [CrossRef]
- Faucet, J.; Maurice, M.; Gagnaire, B.; Renault, T.; Burgeot, T. Isolation and primary culture of gill and digestive gland cells from the common mussel Mytilus edulis. Methods Cell Sci. 2004, 25, 177–184. [Google Scholar] [CrossRef]
- Haque, Z.A.; Kinsella, J.O.E. Emulsifying properties of food proteins: Bovine serum albumin. J. Food Sci. 1988, 53, 416–420. [Google Scholar] [CrossRef]
- Schultz, I.R.; Skillman, A.; Sloan-Evans, S.; Woodruff, D. Domoic acid toxicokinetics in Dungeness crabs: New insights into mechanisms that regulate bioaccumulation. Aquat. Toxicol. 2013, 140, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Gerssen, A.; Mulder, P.P.J.; McElhinney, M.; Boer, J. Liquid chromatography-tandem mass spectrometry method for the detection of marine lipophilic toxins under alkaline conditions. J. Chromatogr. A 2009, 9, 1421–1430. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, J.; Martín, H.; Mariño, C.; Rossignoli, A.E. Simple Diffusion as the Mechanism of Okadaic Acid Uptake by the Mussel Digestive Gland. Toxins 2019, 11, 395. https://doi.org/10.3390/toxins11070395
Blanco J, Martín H, Mariño C, Rossignoli AE. Simple Diffusion as the Mechanism of Okadaic Acid Uptake by the Mussel Digestive Gland. Toxins. 2019; 11(7):395. https://doi.org/10.3390/toxins11070395
Chicago/Turabian StyleBlanco, Juan, Helena Martín, Carmen Mariño, and Araceli E. Rossignoli. 2019. "Simple Diffusion as the Mechanism of Okadaic Acid Uptake by the Mussel Digestive Gland" Toxins 11, no. 7: 395. https://doi.org/10.3390/toxins11070395
APA StyleBlanco, J., Martín, H., Mariño, C., & Rossignoli, A. E. (2019). Simple Diffusion as the Mechanism of Okadaic Acid Uptake by the Mussel Digestive Gland. Toxins, 11(7), 395. https://doi.org/10.3390/toxins11070395





