Massive Occurrence of the Harmful Benthic Dinoflagellate Ostreopsis cf. ovata in the Eastern Adriatic Sea
Abstract
1. Introduction
2. Results
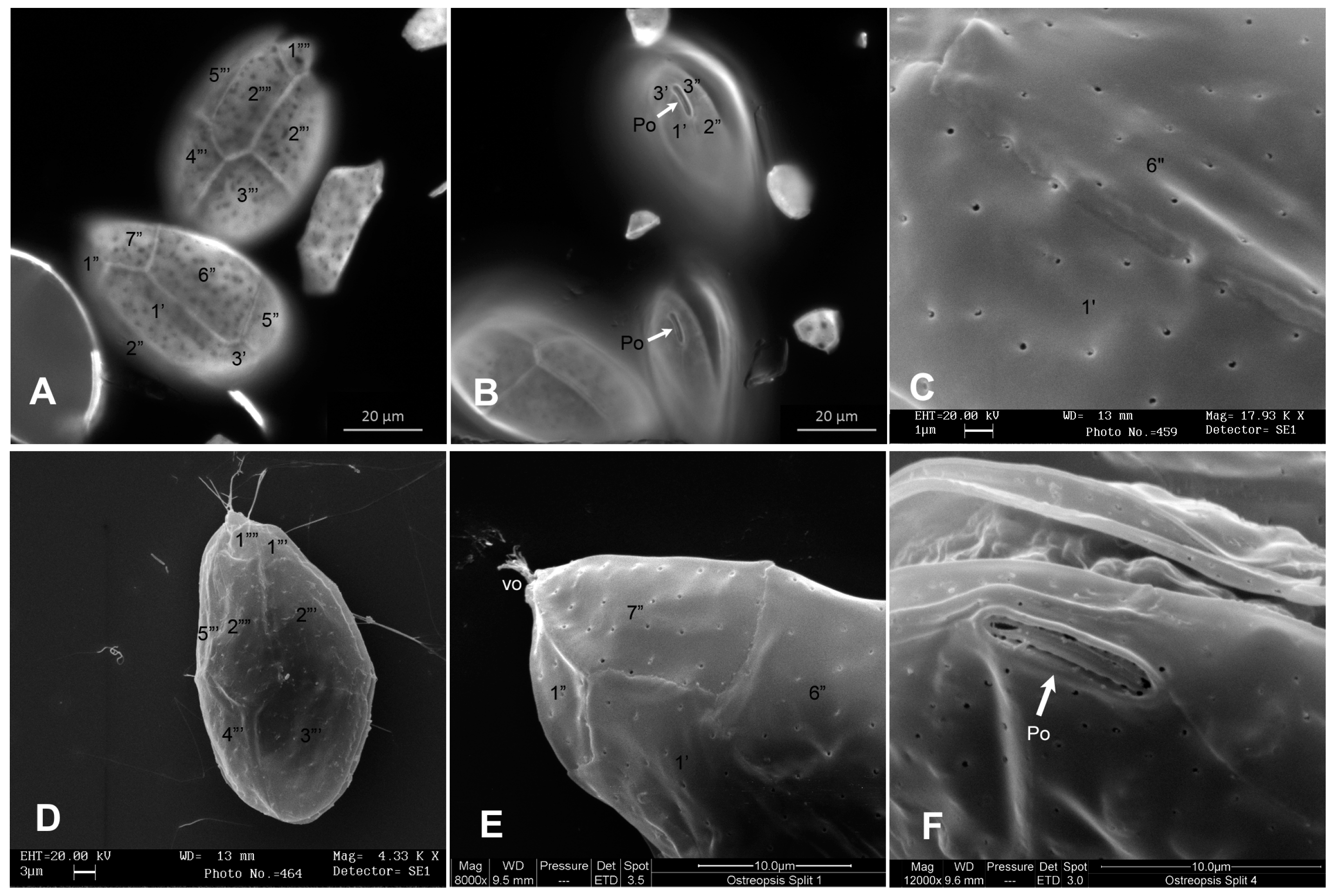
2.1. Microscopy Analyses
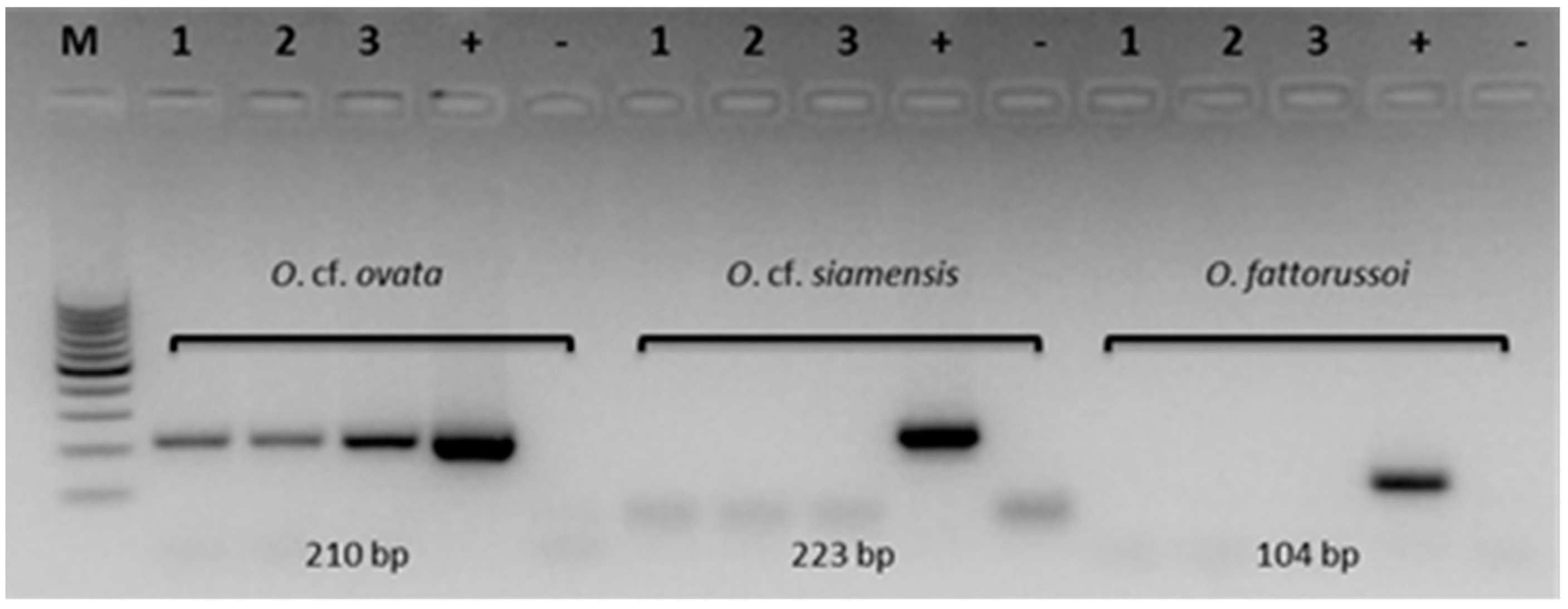
2.2. Molecular Analyses
2.3. Ostreopsis cf. ovata Abundance and Phytoplankton Community Composition
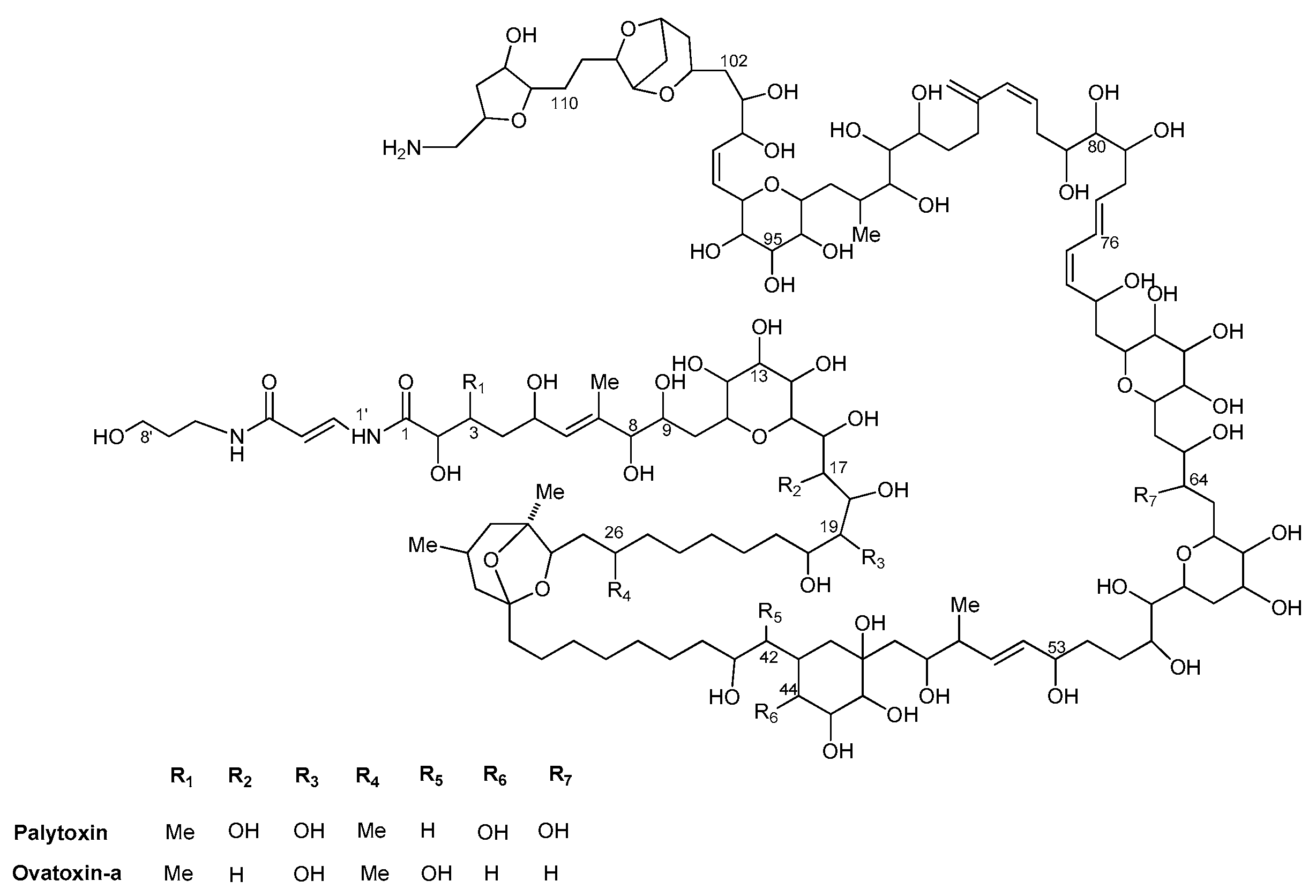
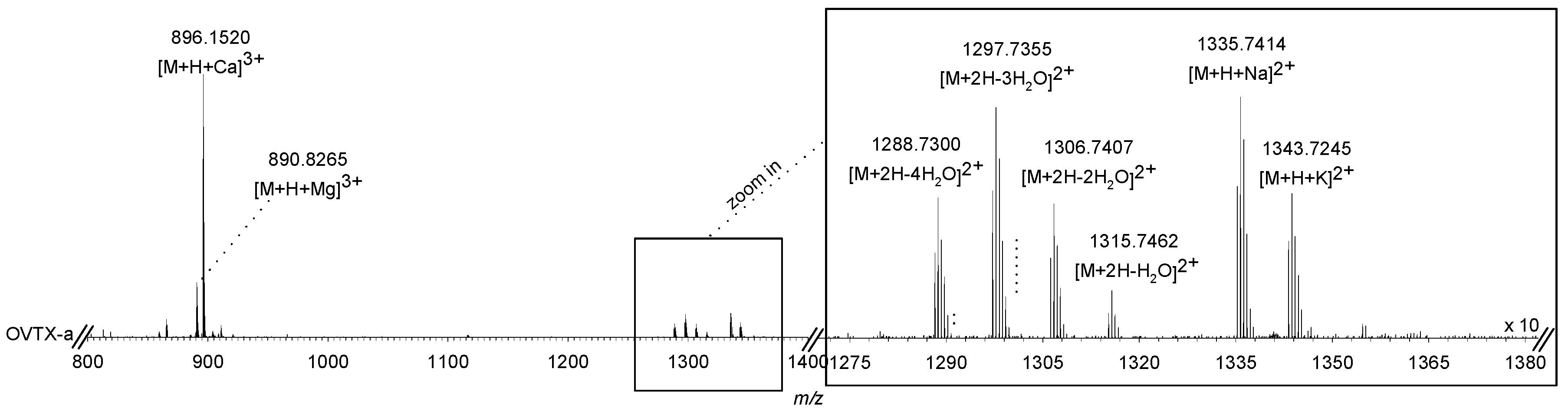
2.4. Concentration and Characterization of Ostreopsis Toxins
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Sampling
5.2. Microscopy Determinations
5.3. Molecular Analyses
5.4. Chemical and Immunoenzymatic Analyses
5.4.1. Extraction
5.4.2. Indirect Sandwich Immunoenzymatic Assay (ELISA)
5.4.3. Liquid Chromatography–High-Resolution Multiple Stage Mass Spectrometry (LC-HRMSn)
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vila, M.; Garcés, E.; Masó, M. Potentially toxic epiphytic dinoflagellates assemblages on macroalgae in the NW Mediterranean. Aquat. Microb. Ecol. 2001, 26, 51–60. [Google Scholar] [CrossRef]
- Mangialajo, L.; Ganzin, N.; Accoroni, S.; Asnaghi, V.; Blanfuné, A.; Cabrini, M.; Cattaneo-Vietti, R.; Chavanon, F.; Chiantore, M.; Cohu, S.; et al. Trends in Ostreopsis proliferation along the Northern Mediterranean coasts. Toxicon 2011, 57, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Battocchi, C.; Totti, C.; Vila, M.; Masó, M.; Capellacci, S.; Accoroni, S.; Reñé, A.; Scardi, M.; Penna, A. Monitoring toxic microalgae Ostreopsis (dinoflagellate) species in coastal waters of the Mediterranean Sea using molecular PCR-based assay combined with light microscopy. Mar. Pollut. Bull. 2010, 60, 1074–1084. [Google Scholar] [CrossRef]
- Totti, C.; Accoroni, S.; Cerino, F.; Cucchiari, E.; Romagnoli, T. Ostreopsis ovata bloom along the Conero Riviera (northern Adriatic Sea): Relationships with environmental conditions and substrata. Harmful Algae 2010, 9, 233–239. [Google Scholar] [CrossRef]
- Penna, A.; Fraga, S.; Battocchi, C.; Casabianca, S.; Perini, F.; Cappellacci, S.; Casabianca, A.; Riobó, P.; Giacobbe, M.G.; Totti, C.; et al. Genetic diversity of the genus Ostreopsis Schmidt: Phylogeographical considerations and molecular methodology applications for field detection in the Mediterranean Sea. Cryptogamie Algol. 2012, 33, 153–163. [Google Scholar] [CrossRef]
- Penna, A.; Vila, M.; Fraga, S.; Giacobbe, M.G.; Andreoni, F.; Riobó, P.; Vernesi, C. Characterization of Ostreopsis and Coolia (Dinophyceae) isolates in the Western Mediterranean Sea based on morphology, toxicity and internal transcribed spacer 5.8 S rDNA sequences. J. Phycol. 2005, 41, 212–225. [Google Scholar] [CrossRef]
- Penna, A.; Fraga, S.; Battocchi, C.; Casabianca, S.; Giacobbe, M.G.; Riobó, P.; Vernesi, C. A phylogeographical study of the toxic benthic dinoflagellate genus Ostreopsis Schmidt. J. Biogeogr. 2010, 37, 830–841. [Google Scholar] [CrossRef]
- Accoroni, S.; Romagnoli, T.; Penna, A.; Capellacci, S.; Ciminiello, P.; Dell’Aversano, C.; Tartaglione, L.; Abboud-Abi Saab, M.; Giussani, V.; Asnaghi, V.; et al. Ostreopsis fattorussoi sp. nov. (Dinophyceae), a new benthic toxic Ostreopsis species from the eastern Mediterranean Sea. J. Phycol. 2016, 52, 1064–1084. [Google Scholar] [CrossRef] [PubMed]
- Tichadou, L.; Glaizal, M.; Armengaud, A.; Grossel, H.; Lemée, R.; Kantin, R.; Lasalle, J.L.; Drouet, G.; Rambaud, L.; Malfait, P.; et al. Health impact of unicellular algae of the Ostreopsis genus blooms in the Mediterranean Sea: Experience of the French Mediterranean coast surveillance network from 2006 to 2009. Clin. Toxicol. 2010, 48, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Durando, P.; Ansaldi, F.; Oreste, P.; Moscatelli, P.; Marensi, L.; Grillo, C.; Gasparini, R.; Icardi, G. Ostreopsis ovata and human health: Epidemiological and clinical features of respiratory syndrome outbreaks from a two year syndromic surveillance, 2005–2006, in northwest Italy. Euro Surveill. 2007, 12, 3212. [Google Scholar]
- Del Favero, G.; Sosa, S.; Pelin, M.; D’Orlando, E.; Florio, C.; Lorenzon, P.; Poli, M.; Tubaro, A. Sanitary problems related to the presence of Ostreopsis spp. in the Mediterranean Sea: A multidisciplinary scientific approach. Ann. Ist. Super. Sanita 2012, 48, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Dell’Aversano, C.; Dello Iacovo, E.; Fattorusso, E.; Forino, M.; Tartaglione, L.; Benedettini, G.; Onorari, M.; Serena, F.; Battocchi, C.; Casabianca, S.; Penna, A. First finding of Ostreopsis cf. ovata toxins in marine aerosols. Environ. Sci. Technol. 2014, 48, 3532–3540. [Google Scholar] [CrossRef] [PubMed]
- Tubaro, A.; Durando, P.; Del Favero, G.; Ansaldi, F.; Icardi, G.; Deeds, J.R.; Sosa, S. Case definitions for human poisonings postulated to palytoxins exposure. Toxicon 2011, 57, 478–495. [Google Scholar] [CrossRef] [PubMed]
- Casabianca, S.; Casabianca, A.; Riobó, P.; Franco, J.; Vila, M.; Penna, A. Quantification of the toxic dinoflagellate Ostreopsis spp. by qPCR assay in marine aerosol. Environ. Sci. Technol. 2013, 47, 3788–3795. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Murata, M.; Inoue, A.; Gawel, M.; Yasumoto, T. Occurrence of palytoxin in the trigger fish Melichtys vidua. Toxicon 1987, 25, 1121–1124. [Google Scholar] [CrossRef]
- Kodama, A.M.; Hokama, Y.; Yasumoto, T.; Fukui, M.; Manea, S.J.; Sutherland, N. Clinical and laboratory findings implicating palytoxin as cause of ciguatera poisoning due to Decapterus macrosoma (mackerel). Toxicon 1989, 27, 1051–1053. [Google Scholar] [CrossRef]
- Onuma, Y.; Satake, M.; Ukena, T.; Roux, J.; Chanteau, S.; Rasolofonirina, N.; Ratsimaloto, M.; Naoki, H.; Yasumoto, T. Identification of putative palytoxin as the cause of clupeotoxism. Toxicon 1999, 37, 55–65. [Google Scholar] [CrossRef]
- Taniyama, S.; Arakawa, O.; Terada, M.; Nishio, S.; Takatani, T.; Mahmud, Y.; Noguchi, T. Ostreopsis sp., a possible origin of palytoxin (PTX) in parrotfish Scarus ovifrons. Toxicon 2003, 42, 29–33. [Google Scholar] [CrossRef]
- Wu, M.L.; Yang, C.C.; Deng, J.F.; Wang, K.Y. Hyperkalemia, hyperphosphatemia, acute kidney injury, and fatal dysrhythmias after consumption of palytoxin-contaminated goldspot herring. Ann. Emerg. Med. 2014, 64, 633–636. [Google Scholar] [CrossRef]
- Yasumoto, T.; Yasumura, D.; Ohizumi, Y.; Takahashi, M.; Alcala, A.C.; Alcala, L.C. Palytoxin in two species of xanthid crab from the Philippines. Agric. Biol. Chem. 1986, 50, 163–167. [Google Scholar]
- Alcala, A.C.; Alcala, L.C.; Garth, J.S.; Yasumura, D.; Yasomoto, T. Human fatality due to ingestion of the crab Demania rynaudii that contained a palytoxin-like toxin. Toxicon 1988, 26, 105–107. [Google Scholar] [CrossRef]
- Ciminiello, P.; Dell’Aversano, C.; Fattorusso, E.; Forino, M.; Magno, G.S.; Tartaglione, L.; Grillo, C.; Melchiorre, N. The Genoa 2005 outbreak. Determination of Putative Palytoxin in Mediterranean Ostreopsis ovata by a New Liquid Chromatography Tandem Mass Spectrometry Method. Anal. Chem. 2006, 78, 6153–6159. [Google Scholar] [CrossRef] [PubMed]
- García-Altares, M.; Tartaglione, L.; Dell’Aversano, C.; Carnicer, O.; de la Iglesia, P.; Forino, M.; Diogéne, J.; Ciminiello, P. The Novel Ovatoxin-g and Isobaric Palytoxin (so far referred to as Putative Palytoxin) from Ostreopsis cf. ovata (NW Mediterranean Sea): Structural Insights by LC-High Resolution MSn. Anal. Bioanal. Chem. 2015, 407, 1191–1204. [Google Scholar]
- Ciminiello, P.; Dell’Aversano, C.; Fattorusso, E.; Forino, M.; Tartaglione, L.; Grillo, C.; Melchiorre, N. Putative palytoxin and its new analogue, ovatoxin-a, in Ostreopsis ovata collected along the Ligurian coasts during the 2006 toxic outbreak. J. Am. Soc. Mass Spectrom. 2008, 19, 111–120. [Google Scholar] [CrossRef]
- Ciminiello, P.; Dell’Aversano, C.; Dello Iacovo, E.; Fattorusso, E.; Forino, M.; Grauso, L.; Tartaglione, L.; Guerrini, F.; Pistocchi, R. Complex palytoxin-like profile of Ostreopsis ovata. Identification of four new ovatoxins by high-resolution liquid chromatography/mass spectrometry. Rapid Commun. Mass Sp. 2010, 24, 2735–2744. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Dell’Aversano, C.; Dello Iacovo, E.; Fattorusso, E.; Forino, M.; Grauso, L.; Tartaglione, L.; Guerrini, F.; Pezzolesi, L.; Pistocchi, R.; et al. Isolation and structure elucidation of ovatoxin-a, the major toxin produced by Ostreopsis ovata. J. Am. Chem. Soc. 2012, 134, 1869–1875. [Google Scholar] [CrossRef]
- Rossi, R.; Castellano, V.; Scalco, E.; Serpe, L.; Zingone, A.; Soprano, V. New palytoxin-like molecules in Mediterranean Ostreopsis cf. ovata (dinoflagellates) and in Palythoa tuberculosa detected by liquid chromatography-electrospray ionization time-of-flight mass spectrometry. Toxicon 2010, 56, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Funari, E.; Manganelli, M.; Testai, E. Ostreopsis cf. ovata blooms in coastal water: Italian guidelines to assess and manage the risk associated to bathing waters and recreational activities. Harmful Algae 2015, 50, 45–56. [Google Scholar] [CrossRef]
- Pelin, M.; Forino, M.; Brovedani, V.; Tartaglione, L.; Dell’Aversano, C.; Pistocchi, R.; Poli, M.; Sosa, S.; Florio, C.; Ciminiello, P.; et al. Ovatoxin-a, a palytoxin analogue isolated from Ostreopsis cf. ovata Fukuyo: Cytotoxic activity and ELISA detection. Environ. Sci. Technol. 2016, 50, 1544–1551. [Google Scholar] [CrossRef]
- Marasović, I. Proportion of dinoflagellates in the phytoplankton community of the Middle Adriatic with special regard to “red tide” and toxic species (in Croatian). Ph.D. Thesis, University of Zagreb, Zagreb, Croatia, May 1990. [Google Scholar]
- Pfannkuchen, M.; Godrijan, J.; Marić Pfannkuchen, D.; Iveša, L.; Kružić, P.; Ciminiello, P.; Dell’Aversano, C.; Dello Iacovo, E.; Fattorusso, E.; Forino, M.; et al. Toxin-producing Ostreopsis cf. ovata are likely to bloom undetected along Coastal Areas. Environ. Sci. Technol. 2012, 46, 5574–5582. [Google Scholar] [CrossRef] [PubMed]
- Zingone, A.; Siano, R.; Alelio, D.D.; Sarno, D. Potentially toxic and harmful microalgae from coastal waters of the Campania region (Tyrrhenian Sea, Mediterranean Sea). Harmful Algae 2006, 5, 321–337. [Google Scholar] [CrossRef]
- Abboud-Abi Saab, M. Les dinoflagellés des eaux côtières libanaises-espèces rares ou nouvelles du phytoplancton marin. Leban. Sci. Bull. 1989, 5, 5–16. [Google Scholar]
- Tognetto, L.; Bellato, S.; Moro, I.; Andreoli, C. Occurrence of Ostreopsis ovata (Dinophyceae) in the Tyrrhenian Sea during summer 1994. Bot. Mar. 1995, 38, 291–295. [Google Scholar] [CrossRef]
- Vila, M.; Camp, J.; Garcés, E.; Masó, M.; Delgado, M. High resolution spatial-temporal detection of HABs in confined waters of the NW Mediterranean. J. Plankton Res. 2001, 23, 497–514. [Google Scholar] [CrossRef]
- Vila, M.; Masó, M.; Sampedro, N.; Illoul, H.; Arin, L.; Garcés, E.; Giacobbe, M.G.; Alvarez, J.; Camp, J. The genus Ostreopsis in the recreational waters of the Catalan Coast and Balearic Islands (NW Mediterranean Sea): Is this the origin of human respiratory difficulties? In Proceedings of the 12th International Conference of Harmful Algae, Copenhagen, Denmark, 4–8 September 2006; International Society for the Study of Harmful Algae and Intergovernmental Oceanographic Commission of UNESCO: Copenhagen, Denmark, 2008; pp. 334–336. [Google Scholar]
- Sansoni, G.; Borghini, B.; Camici, G.; Casotti, M.; Righini, P.; Rustighi, C. Fioriture algali di Ostreopsis ovata (Gonyaulacales: Dinophyceae): Un problema emergente. Biologia Ambientale 2003, 17, 17–23. [Google Scholar]
- Turki, S.; Harzallah, A.; Sammari, C. Occurrence of harmful dinoflagellates in two different Tunisian ecosystems: The lake of Bizerte and the Gulf of Gabes. Cah. Biol. Mar. 2006, 47, 253–259. [Google Scholar]
- Abboud-Abi Saab, M.; Fakhri, M.; Kassab, M.T.; Matar, N. Seasonal and spatial variations of the dinoflagellate Ostreopsis siamensis in the Lebanese coastal waters (Eastern Mediterranean). Cryptogamie Algol. 2013, 34, 57–67. [Google Scholar] [CrossRef]
- Turki, S. Distribution of toxic dinoflagellates along the leaves of seagrass Posidonia oceanica and Cymodocea nodosa from the Gulf of Tunis. Cah. Biol. Mar. 2005, 46, 29–34. [Google Scholar]
- Simoni, F.; Gaddi, A.; Di Paolo, C.; Lepri, L. Harmful epiphytic dinoflagellates on Tyrrhenian Sea. Harmful Algae News 2003, 24, 13–14. [Google Scholar]
- Gallitelli, M.; Ungaro, N.; Addante, L.M.; Procacci, V.; Silver, N.G.; Sabbà, C. Respiratory illness as a reaction to tropical algal blooms occurring in a temperate climate. Jama 2005, 293, 2595–2600. [Google Scholar]
- Aligizaki, K.; Nikolaidis, G. The presence of the potentially toxic genera Ostreopsis and Coolia (Dinophyceae) in the North Aegean Sea, Greece. Harmful Algae 2006, 5, 717–730. [Google Scholar] [CrossRef]
- Aligizaki, K.; Katikou, P.; Nikolaidis, G.; Panou, A. First episode of shellfish contamination by palytoxin-like compounds from Ostreopsis species (Aegean Sea, Greece). Toxicon 2008, 51, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Ismael, A.; Halim, Y. Potentially harmful Ostreopsis spp. in the coastal waters of Alexandria- Egypt. Medit. Mar. Sci. 2012, 13, 208–212. [Google Scholar] [CrossRef]
- Spatharis, S.; Dolapsakis, N.P.; Economou-Amilli, A.; Tsirtsis, G.; Danielidis, D.B. Dynamics of potentially harmful microalgae in a confined Mediterranean Gulf - Assessing the risk of bloom formation. Harmful Algae 2009, 8, 736–743. [Google Scholar] [CrossRef]
- Amzil, Z.; Sibat, M.; Chomerat, N.; Grossel, H.; Marco-Miralles, F.; Lemee, R.; Nezan, E.; Sechet, V. Ovatoxin-a and palytoxin accumulation in seafood in relation to Ostreopsis cf. ovata blooms on the French. Mar. Drugs 2012, 10, 477–496. [Google Scholar] [CrossRef] [PubMed]
- Mangialajo, L.; Bertolotto, R.; Cattaneo-Vietti, R.; Chaintore, M.; Grillo, C.; Lemee, R.; Melchiorre, N.; Moretto, P.; Povero, P.; Ruggieri, N. The toxic benthic dinoflagellate Ostreopsis ovata: Quantification of proliferation along the coastline of Genoa, Italy. Mar. Pollut. Bull. 2008, 56, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Arin, L.; Battocchi, C.; Bravo, I.; Fraga, S.; Penna, A.; Reñé, A.; Riobó, P.; Rodriguez, F.; Sala, M.M.; et al. Management of Ostreopsis blooms in recreational waters along the Catalan coast (NW Mediterranean Sea): Cooperation between a research project and a monitoring program. Cryptogamie Algol. 2012, 33, 143–152. [Google Scholar] [CrossRef]
- Turki, S.; Balti, N.; Aissaoui, A.; Armi, Z. Ostreopsis cf. siamensis proliferations in coastal water of Bizerte, Northern Tunisia. Harmful Algae News 2010, 42, 4–5. [Google Scholar]
- Abbate, M.; Bordone, A.; Cerrati, G.; Lisca, A.; Peirano, A. Variabilità della distribuzione e densità di Ostreopsis ovata nel Golfo della Spezia. Biol. Mar. Mediterr. 2007, 14, 286–287. [Google Scholar]
- Totti, C.; Cucchiari, E.; Romagnoli, T.; Penna, A. Bloom of Ostreopsis ovata on the Conero riviera (NW Adriatic Sea). Harmful Algae News 2007, 33, 12–13. [Google Scholar]
- Monti, M.; Minocci, M.; Beran, A.; Iveša, L. First record of Ostreopsis cfr. ovata on macroalgae in the Northern Adriatic Sea. Mar. Pollut. Bull. 2007, 54, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Cohu, S.; Thibaut, T.; Mangialajo, L.; Labat, J.P.; Passafiume, O.; Blanfuné, A.; Simon, N.; Cottalorda, J.M.; Lemée, R. Occurrence of the toxic dinoflagellate Ostreopsis cf. ovata in relation with environmental factors in Monaco (NW Mediterranean). Mar. Pollut. Bull. 2011, 62, 2681–2691. [Google Scholar] [CrossRef]
- Halim, Y. First IOC/HANA workshop on harmful algal blooms in North Africa. Harmful Algae News 2007, 35, 1–2. [Google Scholar]
- Ungano, N.; Assennato, G.; Blonda, M.; Cudillo, B.; Petruzzelli, M.R.; Mariani, M.; Pastorelli, A.M.; Aliquò, M.R.; D’Angela, A.; Aiello, C.; et al. Occurrence of the potentially toxic dinoflagellate Ostreopsis ovata along the apulian coastal areas (Southern Italy) and relationship with anthropogenic pollution. Fresenius Environ. Bull. 2010, 19, 1813–1821. [Google Scholar]
- Milandri, A.; Ceredi, A.; Riccardi, E.; Gasperetti, L.; Susini, F.; Casotti, M.; Faiman, L.; Pigozzi, S. Impact of Ostreopsis ovata on marine benthic communities: Accumulation of palytoxins in suffering mussels, sea urchins and octopuses from Italy. In Abstract Book of the 14th International Conference on Harmful Algae, Hersonissons-Crete, Greece, 1–5 November 2010; Pagou, K., Hallegraeff, G., Eds.; International Society for the Study of Harmful Algae and Intergovernmental Oceanographic Commission of UNESCO: Copenhagen, Denmark, 2013; p. 92. ISBN 978-87-990827-3-5. [Google Scholar]
- Ingarao, C.; Lanciani, G.; Teodori, A.; Pagliani, T. First presence of Ostreopsis cf. ovata (Dinophyceae) along Abruzzo coasts (W Adriatic Sea). Biol. Mar. Medit. 2009, 16, 172–173. [Google Scholar]
- Illoul, H.; Masó, M.; Demestre, M.; Fortuño, J.M.; De Juan, S. Harmful algae in Bou-Ismaïl Bay coastal waters during August 2008 cruise (Algerian coast). (AECI/ MESRS A/010153/07 Project). In Proceedings of the IOC/HANA Second workshop on Harmful Algal Blooms in North Africa, Alexandria, Egypt, 7–9 November 2009. [Google Scholar]
- Vila, M.; Riobó, P.; Bravo, I.; Masó, M.; Penna, A.; Reñé, A.; Sala, M.; Battocchi, C.; Fraga, S.; Rodriguez, F.; et al. A three-year time series of toxic Ostreopsis blooming in a NW Mediterranean coastal site: Preliminary results. In Proceedings of the 14th International Conference on Harmful Algae, Hersonissons-Crete, Greece, 1–5 November 2010; Pagou, K., Hallegraeff, G., Eds.; International Society for the Study of Harmful Algae and Intergovernmental Oceanographic Commission of UNESCO: Copenhagen, Denmark, 2013; pp. 102–104, ISBN 978-87-990827-3-5. [Google Scholar]
- Mabrouk, L.; Hamza, A.; Brahim, M.B.; Bradai, M.N. Temporal and depth distribution of microepiphytes on Posidonia oceanica (L.) Delile leaves in a meadow off Tunisia. Mar. Ecol. 2011, 32, 148–161. [Google Scholar] [CrossRef]
- Cohu, S.; Mangialajo, L.; Thibaut, T.; Blanfuné, A.; Marro, S.; Lemée, R. Proliferation of the toxic dinoflagellate Ostreopsis cf. ovata in relation to depth, biotic substrate and environmental factors in the North West Mediterranean Sea. Harmful Algae 2013, 24, 32–44. [Google Scholar] [CrossRef]
- Bushati, M.; Koni, E.; Miho, A.; Bregaj, M. Temporal distribution of potentially toxic algae (dinoflagellates and diatoms) in Butrinti lagoon. Nat. Montenegr. 2010, 9, 307–319. [Google Scholar]
- Pagliara, P.; Caroppo, C. Toxicity assessment of Amphidinium carterae, Coolia cfr. monotis and Ostreopsis cfr. ovata (Dinophyta) isolated from the northern Ionian Sea (Mediterranean Sea). Toxicon 2012, 60, 1203–1214. [Google Scholar] [CrossRef]
- Illoul, H.; Hernández, F.R.; Vila, M.; Adjas, N.; Younes, A.A.; Bournissa, M.; Koroghli, A.; Marouf, N.; Rabia, S.; Ameur, F.L.K. The genus Ostreopsis along the Algerian coastal waters (SW Mediterranean Sea) associated with a human respiratory intoxication episode. Cryptogamie Algol. 2012, 33, 209–217. [Google Scholar] [CrossRef]
- Blasutto, O.; Celio, M.; Honsell, G.; Suraci, C.; Venuti, M.; Zanolin, B.; Acquavita, A.; Mattassi, G. Gulf of Trieste, northern Adriatic Sea: First record of Ostreopsis ovata bloom. In Proceedings of the 14th International Conference on Harmful Algae, Hersonissons-Crete, Greece, 1–5 November 2010; Pagou, K., Hallegraeff, G., Eds.; International Society for the Study of Harmful Algae and Intergovernmental Oceanographic Commission of UNESCO: Copenhagen, Denmark, 2013; pp. 13–15, ISBN 978-87-990827-3-5. [Google Scholar]
- Honsell, G.; De Bortoli, M.; Boscolo, S.; Dell’Aversano, C.; Battocchi, C.; Fontanive, G.; Penna, A.; Berti, F.; Sosa, S.; Yasumoto, T.; et al. Harmful dinoflagellate Ostreopsis cf. ovata Fukuyo: Detection of ovatoxins in field samples and cell immunolocalization using antipalytoxin antibodies. Environ. Sci. Technol. 2011, 45, 7051–7059. [Google Scholar] [CrossRef] [PubMed]
- Accoroni, S.; Romagnoli, T.; Colombo, F.; Pennesi, C.; Gioia, C.; Camillo, D.; Marini, M.; Battocchi, C.; Ciminiello, P.; Dell, C.; et al. Ostreopsis cf. ovata bloom in the northern Adriatic Sea during summer 2009: Ecology, molecular characterization and toxin profile. Mar. Pollut. Bull. 2011, 62, 2512–2519. [Google Scholar] [CrossRef] [PubMed]
- Perini, F.; Casabianca, A.; Battocchi, C.; Accoroni, S.; Totti, C.; Penna, A. New approach using the real-time PCR method for estimation of the toxic marine dinoflagellate Ostreopsis cf. ovata in marine environment. PLoS ONE 2011, 6, e17699. [Google Scholar] [CrossRef] [PubMed]
- Accoroni, S.; Colombo, F.; Pichierri, S.; Romagnoli, T.; Marini, M.; Battocchi, C.; Penna, A.; Totti, C. Ecology of Ostreopsis cf. ovata blooms in the northwestern Adriatic Sea. Cryptogamie Algol. 2012, 33, 191–198. [Google Scholar] [CrossRef]
- Giussani, V.; Regionale, L.A.; Asnaghi, V. Management of harmful benthic dinoflagellates requires targeted sampling methods and alarm thresholds. Harmful Algae 2017, 68, 97–104. [Google Scholar] [CrossRef]
- Bizsel, N.; Aligizaki, K. Detection of Ostreopsis cf. ovata in coastal waters of Turkey (East Aegean Sea). In Proceedings of the International Conference on Ostreopsis Development, Villefranche-sur-Mer, France, 4–8 April 2011; Chiantore, M., Lemée, R., Mangialajo, L., Eds.; 2011; p. 53. [Google Scholar]
- Brissard, C.; Herrenknecht, C.; Séchet, V.; Hervé, F.; Pisapia, F.; Harcouet, J.; Lémée, R.; Chomérat, N.; Hess, P.; Amzil, Z. Complex toxin profile of French Mediterranean Ostreopsis cf. ovata strains, seafood accumulation and ovatoxins prepurification. Mar. Drugs 2014, 12, 2851–2876. [Google Scholar] [CrossRef]
- Abdennadher, M.; Zouari, A.B.; Sahnoun, W.F.; Alverca, E.; Penna, A.; Hamza, A. Ostreopsis cf. ovata in the Gulf of Gabès (south-eastern Mediterranean Sea): Morphological, molecular and ecological characterization. Harmful Algae 2017, 63, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Satta, C.T.; Padedda, B.M.; Stacca, D.; Simeone, S.; De Falco, G.; Penna, A.; Capellacci, S.; Pulina, S.; Perilli, A.; Sechi, N.; et al. Assessment of harmful algal species using different approaches: The case study of the Sardinian coasts. AIOL 2014, 5, 60–78. [Google Scholar] [CrossRef]
- Vila, M.; Abós-Herràndiz, R.; Isern-Fontanet, J.; Àlvarez, J.; Berdalet, E. Establishing the link between Ostreopsis cf. ovata blooms and human health impacts using ecology and epidemiology. Sci. Mar. 2016, 80, 107–115. [Google Scholar] [CrossRef]
- Giussani, V.; Kletou, D.; Casabianca, S.; Cappellacci, S.; Asnaghi, V.; Penna, A.; Ciminiello, P.; Dell’Aversano, C.; Mazzeo, A.; Tartaglione, L.; et al. New Ostreopsis species recorded along Cyprus coasts: Toxic effect and preliminary characterization of chemical-molecular aspects. In Abstract book of 16th International Conference on Harmful Algae, Wellington, New Zealand, 27–31 October 2014; International Society for the Study of Harmful Algae and Intergovernmental Oceanographic Commission of UNESCO: Copenhagen, Denmark, 2014; p. 146. [Google Scholar]
- Ben-Gharbia, H.; Yahia, O.K.D.; Amzil, Z.; Chomérat, N.; Abadie, E.; Masseret, E.; Sibat, M.; Triki, H.Z.; Nouri, H.; Laabir, M. Toxicity and growth assessments of three thermophilic benthic dinoflagellates (Ostreopsis cf. ovata, Prorocentrum lima and Coolia monotis) developing in the Southern Mediterranean basin. Toxins 2016, 8, 297. [Google Scholar] [CrossRef]
- ISPRA 2017, Monitoraggio della microalga potenzialmente tossica Ostreopsis cf. ovata lungo le coste italiane. In Anno 2016; ISPRA Rapporti 275/2017; Istituto Superiore per la Protezione e la Ricerca Ambientale: Rome, Italy, 2017.
- Penna, A.; Battocchi, C.; Capellacci, S.; Fraga, S.; Aligizaki, K.; Lemée, R.; Vernesi, C. Mitochondrial, but not rDNA, genes fail to discriminate dinoflagellate species in the genus Ostreopsis. Harmful Algae 2014, 40, 40–50. [Google Scholar] [CrossRef]
- Accoroni, S.; Totti, C. The toxic benthic dinoflagellates of the genus Ostreopsis in temperate areas: A review. Adv. Oceanogr. Limnol. 2016, 7, 1–15. [Google Scholar] [CrossRef]
- Balech, E. E’tude des Dinoflagelle´s du sable de Roscoff. Rev. Algol. 1956, 2, 29–52. [Google Scholar]
- Fukuyo, Y. Taxonomical study on benthic dinoflagellates collected in coral reefs. Bull. Japan. Soc. Sci. Fish. 1981, 47, 967–978. [Google Scholar] [CrossRef]
- Besada, E.G.; Loeblich, L.A.; Loeblich, A.R., III. Observations on tropical, benthic dinoflagellates from ciguatera-endemic areas: Coolia, Gambierdiscus, and Ostreopsis. Bull. Mar. Sci. 1982, 32, 723–735. [Google Scholar]
- Fraga, S.; Rodríguez, F.; Caillaud, A.; Diogène, J.; Raho, N.; Zapata, M. Gambierdiscus excentricus sp. nov. (Dinophyceae), a benthic toxic dinoflagellate from the Canary Islands (NE Atlantic Ocean). Harmful Algae 2011, 11, 10–20. [Google Scholar] [CrossRef]
- Escalera, L.; Benvenuto, G.; Scalco, E.; Zingone, A.; Montresor, M. Ultrastructural Features of the Benthic Dinoflagellate Ostreopsis cf. ovata (Dinophyceae). Protist 2014, 165, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Faust, M.A.; Morton, S.L.; Quod, J.P. Further study of marine dinoflagellates: The genus Ostreopsis (Dinophyceae). J. Phycol. 1996, 32, 1053–1065. [Google Scholar] [CrossRef]
- Sato, S.; Nishimura, T.; Uehara, K.; Sakanari, H.; Tawong, W.; Hariganeya, N.; Smith, K.; Rhodes, L.; Yasumoto, T.; Taira, Y.; et al. Phylogeography of Ostreopsis along West Pacific coast, with special reference to a novel clade from Japan. PLoS ONE 2011, 6, e27983. [Google Scholar] [CrossRef]
- Kang, N.S.; Jeong, H.J.; Lee, S.Y.; Lim, A.S.; Lee, M.J.; Kim, H.S.; Yih, W. Morphology and molecular characterization of the epiphytic benthic dinoflagellate Ostreopsis cf. ovata in the temperate waters off Jeju Island, Korea. Harmful Algae 2013, 27, 98–112. [Google Scholar] [CrossRef]
- Simoni, F.; Di Paolo, C.; Gori, L.; Lepri, L.; Mancino, A.; Falaschi, A. Further investigation on blooms of Ostreopsis ovata, Coolia monotis, Prorocentrum lima on the macroalgae of artificial and natural reefs in the Northern Tyrrhenian Sea. Harmful Algae News 2004, 26, 6–7. [Google Scholar]
- Congestri, R.; Penna, A.; Zingone, A. BENTOX-NET: A research and management initiative on Ostreopsis spp. and other benthic microalgal blooms along the Italian coast. Harmful Algae News 2006, 32, 11–12. [Google Scholar]
- Hariganeya, N.; Tanimoto, Y.; Yamaguchi, H.; Nishimura, T.; Tawong, W.; Sakanari, H.; Yoshimatsu, T.; Sato, S.; Preston, C.M.; Adachi, M. Quantitative PCR Method for Enumeration of Cells of Cryptic Species of the Toxic Marine Dinoflagellate Ostreopsis spp. in Coastal Waters of Japan. PLoS ONE 2013, 8, e57627. [Google Scholar] [CrossRef] [PubMed]
- Ramos, V.; Salvi, D.; Machado, J.P.; Vale, M.; Azevedo, J.; Vasconcelos, V. Culture-Independent Study of the Late-Stage of a Bloom of the Toxic Dinoflagellate Ostreopsis cf. ovata: Preliminary Findings Suggest Genetic Differences at the Sub-Species Level and Allow ITS2 Structure Characterization. Toxins 2015, 7, 2514–2533. [Google Scholar] [CrossRef] [PubMed]
- Casabianca, S.; Perini, F.; Casabianca, A.; Battocchi, C.; Giussani, V.; Chiantore, M.; Penna, A. Monitoring toxic Ostreopsis cf. ovata in recreational waters using a qPCR based assay. Mar. Pollut. Bull. 2014, 88, 102–109. [Google Scholar] [CrossRef]
- Guerrini, F.; Pezzolesi, L.; Feller, A.; Riccardi, M.; Ciminiello, P.; Dell’Aversano, C.; Tartaglione, L.; Dello Iacovo, E.; Fattorusso, E.; Forino, M.; et al. Comparative growth and toxin profile of cultured Ostreopsis ovata from the Tyrrhenian and Adriatic Seas. Toxicon 2010, 55, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Grbec, B.; Morović, M.; Kušpilić, G.; Matijević, S.; Matić, F.; Beg Paklar, G.; Ninčević, Ž. The relationship between the atmospheric variability and productivity in the Adriatic Sea area. J. Mar. Biol. Assoc. UK 2009, 89, 1549–1558. [Google Scholar] [CrossRef]
- Occhipinti-Ambrogi, A. Global change and marine communities: Alien species and climate change. Mar. Pollut. Bull. 2007, 55, 342–352. [Google Scholar] [CrossRef]
- Granéli, E.; Vidyarathna, N.K.; Funari, E.; Cumaranatunga, P.R.T.; Scenati, R. Can increases in temperature stimulate blooms of the toxic benthic dinoflagellate Ostreopsis ovata? Harmful Algae 2011, 10, 165–172. [Google Scholar] [CrossRef]
- Grbec, B.; Matić, F.; Beg Paklar, G.; Morović, M.; Popović, R.; Vilibić, I. Long-Term Trends, Variability and Extremes of In Situ Sea Surface Temperature Measured Along the Eastern Adriatic Coast and its Relationship to Hemispheric Processes. Pure Appl. Geophys. 2018, 176, 1–16. [Google Scholar] [CrossRef]
- Herring, S.C.; Hoell, A.; Hoerling, M.P.; Kossin, J.P.; Schreck, C.J., III; Stott, P.A. (Eds.) Explaining Extreme Events of 2015 from a Climate Perspective. Bull. Am. Meteorol. Soc. 2016, 97, S1–S145. [Google Scholar]
- Meroni, L.; Chiantore, M.; Petrillo, M.; Asnaghi, V. Habitat effects on Ostreopsis cf. ovata bloom dynamics. Harmful Algae 2018, 80, 64–71. [Google Scholar] [CrossRef]
- Tartaglione, L.; Dello Iacovo, E.; Mazzeo, A.; Casabianca, S.; Ciminiello, P.; Penna, A.; Dell’Aversano, C. Variability in Toxin Profiles of the Mediterranean Ostreopsis cf. ovata and in Structural Features of the Produced Ovatoxins. Environ. Sci. Technol. 2017, 51, 13920–13928. [Google Scholar] [CrossRef]
- Utermöhl, H. Zur Vervollkommnung der quantitativen Phytoplankton Methodik. Mit. Int. Ver. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Vassalli, M.; Penna, A.; Sbrana, F.; Casabianca, S.; Gjeci, N.; Capellacci, S.; Asnaghi, V.; Ottaviani, E.; Giussani, V.; Pugliese, L.; et al. Intercalibration of counting methods for Ostreopsis spp. blooms in the Mediterranean Sea. Ecol. Indic. 2018, 85, 1092–1100. [Google Scholar] [CrossRef]
- Boscolo, S.; Pelin, M.; De Bortoli, M.; Fontanive, G.; Barreras, A.; Berti, F.; Sosa, S.; Chaloin, O.; Bianco, A.; Yasumoto, T.; et al. Sandwich ELISA assay for the quantitation of palytoxin and its analogs in natural samples. Environ. Sci. Technol. 2013, 47, 2034–2042. [Google Scholar] [CrossRef]



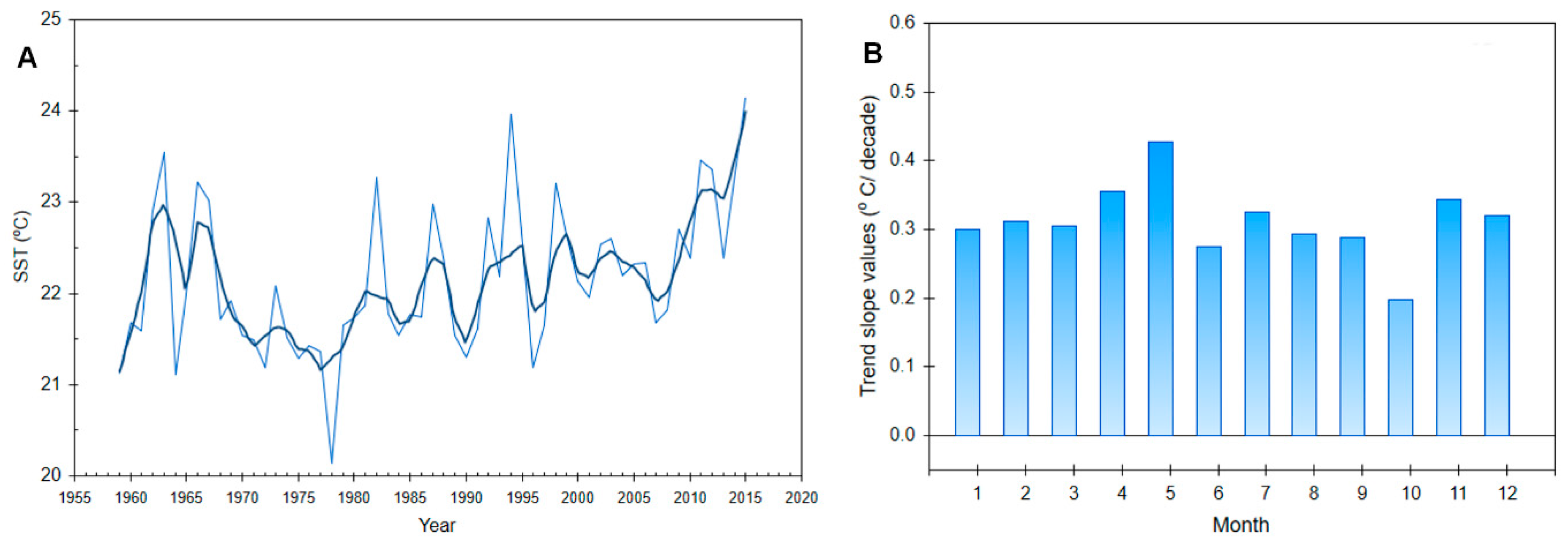
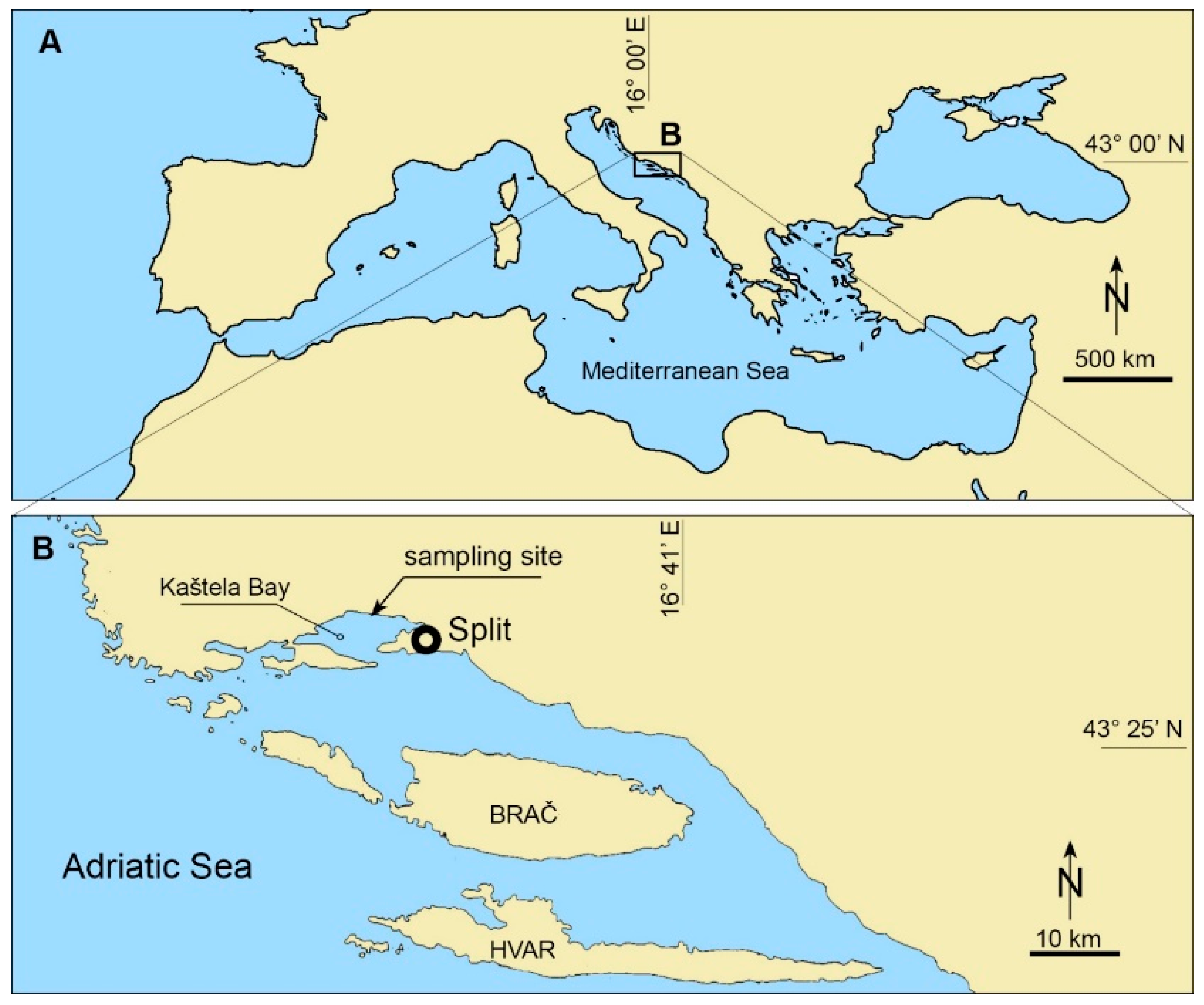
| Year Month | Location | Authors | Species | Water Column (Cells L−1) | Macrophyte (Cells g−1) |
|---|---|---|---|---|---|
| 1972 | Villefranche-sur-Mer | Taylor’s personal communication cited in [32] | O. cf. ovata | ||
| 1979 | Lebanese water | [33] | Ostreopsis sp. | ||
| 1984 | Kaštela Bay | [30] | O. cf. ovata | ||
| 1994 Aug | Tyrrhenian Sea | [34] | O. cf. ovata | 8000 | |
| 1995–1999 | Catalan Sea (Garraf and Blanes harbor) | [35,36] | Ostreopsis spp. | 78,000; 98,000 | 590,000 |
| 1997 July | Catalan Sea | [1] | Ostreopsis sp. | 596,000 | |
| 1998 Aug | Catalan Sea (Llavaneres) | [36] | O. cf. ovata O. siamensis | 200,000 | |
| 1998 Aug | Coast of Tuscany | [37] | O. cf. ovata | 50,000 (3,000,000,000 in the resuspended mat) | |
| 2000 Oct | Gulf of Gabés, Tunisia | [38] | O. siamensis | ≈150 | |
| 2001 | Balearic islands | [36] | Ostreopsis spp. | 25,000 | |
| 2001 July | Lebanese waters | [39] | O. siamensis | 10,560 | |
| 2001 Aug–Sept | Gulf of Tunis, Tunisia | [40] | O. siamensis | 3600 | |
| 2002 Aug | Tyrrhenian Sea Marina di Massa | [41] | O.cf. ovata | 10,550 | |
| 2003 Aug 2004 Sept | South Italy, coasts of Bari | [42] | Ostreopsis spp. | >1,000,000 | |
| 2003–2004 Sept | North Aegean Sea | [43] | O. ovata, O. cf. siamensis | 16,000 | 405,000 |
| 2004 Aug | Catalan Sea | [36] | O. cf. siamensis, O. ovata | 106,655 | |
| 2004 Aug | Balearic islands | [36] | O. cf. siamensis, O. ovata | 1280 | |
| 2004 July | Tyrrhenian Sea, Gulf of Naples | [32] | O. ovata | ||
| 2004 Sept 2005 Sept | North Aegean Sea | [44] | Ostreopsis spp. | 1000; 3600 | 39,493; 33,212 |
| 2005 July | Ligurian Sea, Gulf of Genoa | [22] | O. ovata | 1,800,000 | |
| 2005 July | Alexandria, Egypt | [45] | Ostreopsis spp. | 9053 | |
| 2005 May | Aegean Sea (Gulf of Kalloni) | [46] | O. cf. ovata | 600 | |
| 2005–2007 | Egyptian Mediterranean waters | [45] | O. cf. ovata | ||
| 2006 Aug | Morgiret, Iles de Frioul, off the coast of Marseille, France | [47] | Ostreopsis spp. | 900,000 | |
| 2006 July | French coast: Frioul Island, Marseille | [9] | Ostreopsis spp. | >30,000 | |
| 2006 July | Ligurian Sea | [24,48] | O. cf. ovata | 87,000 (± 27,000) | 2,541,000 (±588,000) |
| 2006 July | Catalan Sea (monitoring of 14 beaches) (beach Ses Illeters | [49] | Ostreopsis spp. (O.cf. ovata, O. cf. siamensis) | 34,445 | |
| 2006 July | Alexandria, Egypt | [45] | Ostreopsis spp. | ≈3500 | |
| 2006 July | Tunis Lake Bizerte | [50] | O. cf. siamensis | 24,700 | |
| 2006 Sept | Ligurian Sea Gulf La Spezia | [51] | O. cf. ovata | 12,000,000 | |
| 2006 Sept | Adriatic Sea Conero riviera | [52] | O. cf. ovata | 2000 | 20,000 |
| 2006 | Adriatic Sea Gulf of Trieste and close to Rovinj (Croatia) | [53] | O. cf. ovata | ||
| 2007 Aug, July; 2008 July, Oct; 2009 July, Aug | Balearic Sea | [2] | O. cf. ovata, O. cf. siamensis | 33,908; 80,272; 385,601 | 2,600,239; 7,248,635; 4,008,204 |
| 2007–2008 | Monaco (Larvotto beach) | [54] | O. cf. ovata | 213,000 | 2,800,000 |
| 2007 Aug–Sept | NW Adriatic Sea (Conero Riviera) | [3] | Ostreopsis spp. (O. ovata and O. cf. siamensis) | 25,000 (± 4000) (average values) | 160,000 (±28,000) (average values) |
| 2007 Aug, July; 2008 Aug; 2009 Sept, July | Gulf of Lion | [2] | O. cf. ovata | 46,600; 36,900; 116,200 | 105,923; 186,480; 392,756 |
| Eastern Harbour of Alexandria, Egipt | [55] | Ostreopsis spp. | |||
| 2007 July | Alexandria, Egypt | [45] | Ostreopsis spp. | ≈4500 | |
| 2007 July | Tunis Lake Bizerte | [50] | O. cf. siamensis | 37,500 | |
| 2007 July; 2008 July, Aug; 2009 July | Ligurian Sea | [2] | O. cf. ovata | 43,278; 104,000; 16,100 | 1,592,511; 1,433,470; 1,610,462 |
| 2007 July–Aug | Morgiret, Iles de Frioul, off the coast of Marseille, France | [47] | Ostreopsis spp. | ≈8000 | ≈100,000 |
| 2007 June–Aug | French coast: Aygulf Beach, Fréjus; Larvotto Beach, Monaco; Méjan Beach, Toulon | [9] | Ostreopsis spp. | >30,000 | |
| 2007 May–Aug | Catalan Sea | [3] | Ostreopsis spp. (O. ovata, O. cf. siamensis) | 20,000 (±3000) | 3,000,000 (±540,000) |
| 2007 Oct | Adriatic Sea Conero Riviera | [4] | O.cf. ovata | 25,200 (13,500,000 in the resuspended mat) | 1,700,000 |
| 2007 Sept | South Adriatic (Puglia region) | [56] | O. cf. ovata | 4900 (bottom water 421,200) | |
| 2007 Sept–Oct 2009 Sept–Oct | Adriatic Sea (Ancona) | [2] | O. cf. ovata | 25,279; 92,483 | 1,701,614; 1,626,621 |
| 2007–2010 | NW Mediterranean Sea (Catalan coast) | [49] | Ostreopsis sp. | ||
| 2007–2010 | Italian region Marche | ISPRA 2010, 2011 cited in [28] | O. cf. ovata | 641,000–7,000,000 | |
| 2007–2011 | Italian region Puglia | ISPRA 2010, 2011, 2012, cited in [28] | O. cf. ovata | 36,400–7,500,000 | |
| 2008 Aug | Coast of Tuscany | [57] | O. cf. ovata | 95,200 | |
| 2008 Aug | Ionian Sea (Puglia region) | [56] | O. cf. ovata | 7680 (bottom water 160,000) | |
| 2008 Aug | Abruzzo coast (Ortona) | [58] | O. cf. ovata | 3600 | |
| 2008 Aug | Western Algiers area Bou-Ismaïl Bay waters | [59] | Ostreopsis spp. | 3000 | |
| 2008 Aug–Sept | South Adriatic (Puglia region) | [56] | O. cf. ovata | 304,000 (bottom water 5,000,000) | |
| 2008 July | Catalan Sea (monitoring of 14 beaches) (beach Llavaneres) | [49,60] | Ostreopsis spp. (O. cf. ovata, O. cf. siamensis) | 205,632 | several millions (EBITOX) |
| 2008 July–Sept | French coast: Marinière Beach, Villefranche; Réserve Beach, Nice; Frioul Island, Marseille | [9] | Ostreopsis spp. | >30,000 | |
| 2008 July 2009 Jan | Eastern Tunisia Mahdia | [61] | O. cf. siamensis | 1–5 (average values) | |
| 2008 July–Aug | Morgiret, Iles de Frioul, off the coast of Marseille, France | [47] | Ostreopsis spp. | ≈5,000,000 | ≈300,000 |
| 2008 June–Aug | Ligurian Sea Genoa; Villefranche-sur-Mer; Nice; Saint Raphael; Ramatuelle | [62] | O. cf. ovata | 68,000; 7000; 12,000; 400; 3000 | 2,810,000; 8,540,000; 1,980,000; 20,000; 10,000 |
| 2008 June–Aug | Gulf of Lyon | [62] | O. cf. ovata | 1000 | 60,000 |
| 2008–2009 | Albania Butrinti lagoon | [63] | Ostreopsis spp. | ||
| 2009 Aug | Ionian Sea | [64] | O.cf. ovata | 757,800 (±114,300) (average values) | 422,300 (±120,000) (average values) |
| 2009 July | SW Mediterranean Algerian beaches | [65] | Ostreopsis spp. | 5920 | 20,000 |
| 2009 July | Catalan Sea (monitoring of 14 beaches) (beach Alguer) | [49,60] | Ostreopsis spp. (O.cf. ovata, O. cf. siamensis) | 2400 | |
| 2009 July–Sept | French coast: Marinière Beach, Villefranche; Frioul Island, Marseille | [9] | Ostreopsis spp. | >30,000 | |
| 2009 July–Sept | Morgiret, Iles de Frioul, off the coast of Marseille, France | [47] | Ostreopsis spp. | ≈120,000 | ≈400,000 |
| 2009 Oct, Sept | Adriatic Sea (North Eastern part | [2] | O. cf. ovata | 280 | 333,793 |
| 2009 Sept | Adriatic Sea (Gulf of Trieste) | [66,67] | O. cf. ovata | 3,076,416 6,700,000 | |
| 2009 Sept | Adriatic Sea (Conero Riviera) | [68] | O. cf. ovata | 92,000 | 1,313,000 |
| 2009 Sept | Adriatic Sea (Conero Riviera) | [5,69] | O.cf. ovata | >120,000 | >70,000 |
| 2010 Aug | Adriatic Sea (Conero Riviera) | [70] | O.cf. ovata | 10,200 | 1,200,000 |
| 2010 | Italian region Liguria | ISPRA 2011 cited in [28] | O. cf. ovata | 10,200,000 | |
| 2010 Aug | Catalan Sea (monitoring of 14 beaches) (beach Castelldefels) | [49,60] | Ostreopsis spp. (O.cf. ovata, O. cf. siamensis) | 1680 | |
| 2010 July–Aug | SW Mediterranean Algerian beaches | [65] | Ostreopsis spp. | 21,680 | 79,000 |
| 2010 July–Aug | Genoa, Italy Quarto dei Mille | [71] | O. cf. ovata | 20,670 | 733,678 |
| 2010 May–Dec | Lebanese waters | [39] | O. siamensis | about 250 | |
| 2010 Oct | Cesme Bay (Eastern Aegean coast) | [72] | O. cf. ovata | 65,000 | |
| 2010 Sep–Oct | Adriatic Sea (northern Adriatic, public beach close to the city of Rovinj, Croatia | [31] | O. cf. ovata | 42,600 | 334,306 |
| 2011 July | Villefranche-sur-Mer | [73] | O. cf. ovata | 28,000 | 3,700,000 |
| 2011 July | Villefranche-sur-Mer | [73] | O. cf. ovata | 70,000 | 490,000 |
| 2011 July | Genoa, Italy Quarto dei Mille | [71] | O. cf. ovata | 4770 | 412,930 |
| 1997–2012 Oct–Nov | Tunisia (Gulf of Gabes) | [74] | O. cf. siamensis | 5000–8000 | |
| 2012 July | Genoa, Italy Quarto dei Mille | [71] | O. cf. ovata | 24,740 | 1,919,740 |
| 2012 July–Aug | Sardinian coast, Italy | [75] | O. cf. ovata | 1100 | |
| 2013 July–Aug | Genoa, Italy Quarto dei Mille | [71] | O. cf. ovata | 24,520 | 973,882 |
| 2016 Aug | Catalan coast Sant Andreu de Llavaneres | [76] | O. cf. ovata | ≈500,000 | ≈500,000 |
| 2014 July | Genoa, Italy Quarto dei Mille | [71] | O. cf. ovata | 7340 | 218,365 |
| 2014 | Greece and Cyprus coasts | [77] | New genotype Ostreopsis sp. | ||
| 2014 | Southern Mediterranean, Bizerte Bay | [78] | O. cf. ovata | ||
| 2015 July | Genoa, Italy Quarto dei Mille | [71] | O. cf. ovata | 51,719 | 2,289,100 |
| 2015 June–July | Cyprus and Lebanon | [8] | O. fattorussoi | 840 | 28,000 |
| 2016 | Italian region Veneto | [79] | O. cf. ovata | 820 | |
| 2016 Aug | Italian region Puglia | [79] | O. cf. ovata | 7,362,000 | |
| 2016 Aug | Italian region Calabria | [79] | O. cf. ovata | 4000 | 6,878 |
| 2016 Aug | Sardinia | [79] | O. cf. ovata | 40,333 | 841,270 |
| 2016 July | Italian region Campania | [79] | O. cf. ovata | 39,362 | 371,696 |
| 2016 July | Italian region Lazio | [79] | O. cf. ovata | 141,140 | 10,008,076 |
| 2016 July | Italian region Tuscany | [79] | O. cf. ovata | 634,800 | |
| 2016 July | Sicily | [79] | O. cf. ovata | 225,503 ± 20,976 | 410,580 ± 54,010 |
| 2016 July–Aug | Italian region Liguria | [79] | O. cf. ovata | 101,760 | 349,463 |
| 2016 Sept | Italian region Marche | [79] | O. cf. ovata | 6,860,000 | 58,960 |
| Basic Statistic | DV (µm) | AP (µm) | DV/AP |
|---|---|---|---|
| Av ± SD | 54.81 ± 5.07 | 25.41 ± 2.27 | 2.17 ± 0.20 |
| min–max | 40.00–63.73 | 21.20–31.80 | 1.57–2.54 |
| Phytoplankton Species | Abundance (Cells L−1) | |
|---|---|---|
| 18 September | 1 October | |
| Diatoms | ||
| Bacteriastrum sp. | 2560 | 5120 |
| Chaetoceros affinis | ||
| Chaetoceros sp. | 40,960 | |
| Cylindrotheca closterium | 7680 | 23,040 |
| Dactyliosolen fragilissimus | 17,920 | |
| Guinardia delicatula | 19,200 | |
| Guinardia flaccida | 1280 | 1280 |
| Guinardia striata | 20,480 | 76,800 |
| Hemiaulus haucki | 2560 | |
| Leptocylindrus danicus | 14,080 | 12,800 |
| Leptocylindrus mediterraneus | 1280 | |
| Licmophora flabelata | 1280 | 1280 |
| Navicula sp. | 17,920 | 10,240 |
| Pennatae indeterm | 10,240 | 12,800 |
| Pleurosigma sp. | 1280 | 1280 |
| Proboscia alata | 10,240 | 7680 |
| Pseudo-nitzschia spp. | 98,560 | 136,960 |
| Striatella unipunctata | 1280 | |
| Thalassionema nitzschioides | 6400 | 5120 |
| Dinoflagellates | ||
| Alexandrium minutum | 1280 | |
| Amphidinium carterae | 1280 | |
| Coolia sp. | 1120 | 1600 |
| Dinophysis fortii | 1280 | |
| Gymnodinium sp.1 | 2560 | |
| Gymnodinium sp.2 (<20 µm) | 5120 | |
| Gyrodinium fusiforme | 1280 | |
| Ostreopsis sp. | 28,560 | 1920 |
| Prorocentrum sp. | 1280 | |
| Coccolithophorids | ||
| Rhabdosphaera clavigera | 1280 | |
| Syracosphaera pulchra | 1280 | |
| Euglenophyta | ||
| Eutreptiella sp. | 1280 | |
| LC-HRMS (pg cell−1) | ELISA (pg PLTX eq. cell−1) | |||||
|---|---|---|---|---|---|---|
| OVTX-a | OVTX-b | OVTX-c | OVTX-d/e | Isobaric PLTX | Total | |
| 3.6 | 1.3 | 0.2 | 1.1 | 0.1 | 6.3 | 5.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ninčević Gladan, Ž.; Arapov, J.; Casabianca, S.; Penna, A.; Honsell, G.; Brovedani, V.; Pelin, M.; Tartaglione, L.; Sosa, S.; Dell’Aversano, C.; et al. Massive Occurrence of the Harmful Benthic Dinoflagellate Ostreopsis cf. ovata in the Eastern Adriatic Sea. Toxins 2019, 11, 300. https://doi.org/10.3390/toxins11050300
Ninčević Gladan Ž, Arapov J, Casabianca S, Penna A, Honsell G, Brovedani V, Pelin M, Tartaglione L, Sosa S, Dell’Aversano C, et al. Massive Occurrence of the Harmful Benthic Dinoflagellate Ostreopsis cf. ovata in the Eastern Adriatic Sea. Toxins. 2019; 11(5):300. https://doi.org/10.3390/toxins11050300
Chicago/Turabian StyleNinčević Gladan, Živana, Jasna Arapov, Silvia Casabianca, Antonella Penna, Giorgio Honsell, Valentina Brovedani, Marco Pelin, Luciana Tartaglione, Silvio Sosa, Carmela Dell’Aversano, and et al. 2019. "Massive Occurrence of the Harmful Benthic Dinoflagellate Ostreopsis cf. ovata in the Eastern Adriatic Sea" Toxins 11, no. 5: 300. https://doi.org/10.3390/toxins11050300
APA StyleNinčević Gladan, Ž., Arapov, J., Casabianca, S., Penna, A., Honsell, G., Brovedani, V., Pelin, M., Tartaglione, L., Sosa, S., Dell’Aversano, C., Tubaro, A., Žuljević, A., Grbec, B., Čavar, M., Bužančić, M., Bakrač, A., & Skejić, S. (2019). Massive Occurrence of the Harmful Benthic Dinoflagellate Ostreopsis cf. ovata in the Eastern Adriatic Sea. Toxins, 11(5), 300. https://doi.org/10.3390/toxins11050300









