Exposure Assessment and Risk Characterization of Aflatoxin M1 Intake through Consumption of Milk and Yoghurt by Student Population in Serbia and Greece
Abstract
1. Introduction
2. Results and Discussion
2.1. Demographic Profile and Consumption Patterns
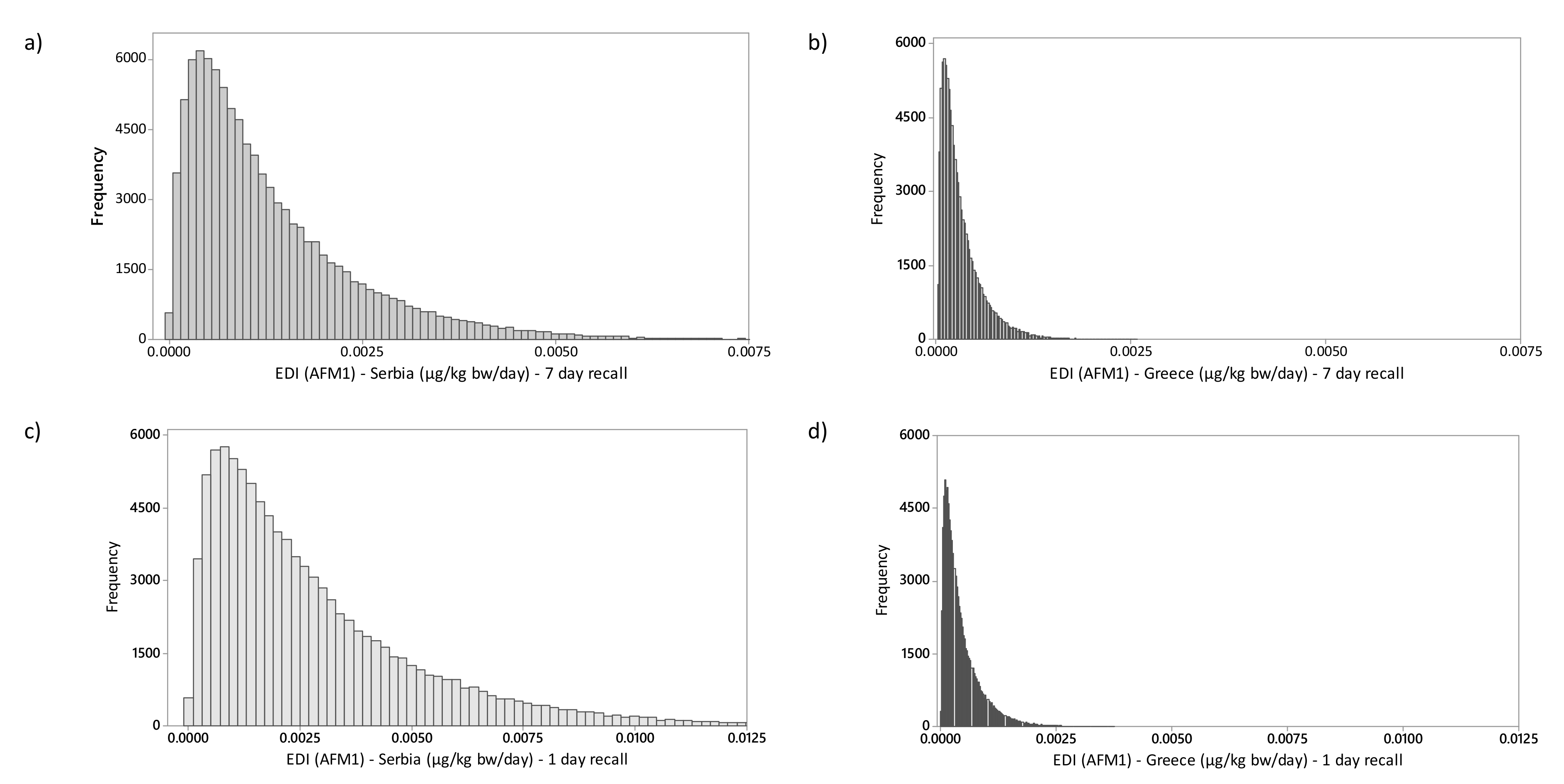
2.2. Exposure Assessment and Risk Characterization
3. Conclusions
4. Materials and Methods
4.1. Consumer Survey
4.2. Exposure Assessment
4.3. Risk Characterization
4.4. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cullen, J.M.; Newberne, P.M. Acute Hepatotoxicity of Aflatoxins. In The Toxicology of Aflatoxins: Human Health, Veterinary, and Agricultural Significance; Eaton, D.L., Groopman, J.D., Eds.; Academic Press: San Diego, FL, USA, 1994; pp. 3–26. [Google Scholar]
- Battacone, G.; Nudda, A.; Palomba, M.; Pascale, M.; Nicolussi, P.; Pulina, G. Transfer of Aflatoxin B1 from Feed to Milk and from Milk to Curd and Whey in Dairy Sheep Fed Artificially Contaminated Concentrates. J. Dairy Sci. 2005, 88, 3063–3069. [Google Scholar] [CrossRef]
- IARC. IARC monographs on the evaluation of carcinogenic risks to humans. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. IARC Press 2002, 82, 1–556. [Google Scholar]
- Hsieh, D.P.H.; Cullen, J.M.; Ruebner, B.H. Comparative hepatocarcinogenicity of aflatoxins B1 and M1 in the rat. Food Chem. Toxicol. 1984, 22, 1027–1028. [Google Scholar] [CrossRef]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar] [CrossRef] [PubMed]
- Prandini, A.; Tansini, G.; Sigolo, S.; Filippi, L.; Laporta, M.; Piva, G. On the occurrence of aflatoxin M1 in milk and dairy products. Food Chem. Toxicol. 2009, 47, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Thorning, T.K.; Raben, A.; Tholstrup, T.; Soedamah-Muthu, S.S.; Astrup, I.; Givens, A.; Arne, A. Milk and dairy products: Good or bad for human health? An assessment of the totality of scientific evidence. Food Nutr. Res. 2016, 60, 32527. [Google Scholar] [CrossRef] [PubMed]
- European Commission Commission regulation 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 32–43.
- Ministry of Agriculture Forestry and Water Management Amendment to Legislation; Ministry of Agriculture, Forestry and Water Management: Belgrade, Serbia, 2017; Volume 81, p. 1.
- Hill, K.; Hoffman, D.; Rex, T. The Value of Higher Education: Individual and Societal Benefit; Arizona State University: Tempe, AZ, USA, 2005. [Google Scholar]
- Tomasevic, I.; Petrovic, J.; Jovetic, M.; Raicevic, S.; Milojevic, M.; Miocinovic, J. Two year survey on the occurrence and seasonal variation of aflatoxin M1 in milk and milk products in Serbia. Food Control 2015, 56, 64–70. [Google Scholar] [CrossRef]
- Cano-Sancho, G.; Marin, S.; Ramos, A.J.; Peris-Vicente, J.; Sanchis, V. Occurrence of aflatoxin M1 and exposure assessment in Catalonia (Spain). Rev. Iberoam. Micol. 2010, 27, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.; Basil, M.D.; Basil, D.Z. Factors Influencing Healthy Eating Habits Among College Students: An Application of the Health Belief Model Among College Students: An Application of the Health Belief Model. Health Mark. Q. 2009, 26, 145–64. [Google Scholar] [CrossRef]
- Hellenic Statistical Authority. Greece in Figures; Hellenic Statistical Authority: Piraeus, Greece, 2018. [Google Scholar]
- Statistical Office of the Republic of Serbia. Statistical Yearbook; Statistical Office of the Republic of Serbia: Belgrade, Serbia, 2017.
- Milicevic, D.; Spiric, D.; Radicevic, T.; Velebit, B.; Stefanovic, S.; Milojevic, L.; Jankovic, S. A review of the current situation of aflatoxin M1 in cow’s milk in Serbia: Risk assessment and regulatory aspects. Food Addit. Contam. Part A 2017, 34, 1617–1631. [Google Scholar] [CrossRef] [PubMed]
- Skrbic, B.; Zivancev, J.; Antic, I.; Godula, M. Levels of aflatoxin M1 in different types of milk collected in Serbia: Assessment of human and animal exposure. Food Control 2014, 40, 113–119. [Google Scholar] [CrossRef]
- Kos, J.; Levic, J.; Duragic, O.; Kokic, B.; Miladinovic, I. Occurrence and estimation of aflatoxin M1 exposure in milk in Serbia. Food Control 2014, 38, 41–46. [Google Scholar] [CrossRef]
- Torovic, L. Aflatoxin M 1 in processed milk and infant formulae and corresponding exposure of adult population in Serbia in 2013–2014. Food Addit. Contam. Part B 2015, 3210, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tsakiris, I.N.; Tzatzarakis, M.N.; Alegakis, A.K.; Vlachou, M.I.; Renieri, E.A.; Tsatsakis, A.M. Risk assessment scenarios of children’ s exposure to aflatoxin M1 residues in different milk types from the Greek market. Food Chem. Toxicol. 2013, 56, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Roussi, V.; Govaris, A.; Varagouli, A.; Botsoglou, N.A. Occurrence of aflatoxin M1 in raw and market milk commercialized in Greece Occurrence of aflatoxin M1 in raw and market milk commercialized in Greece. Food Addit. Contam. 2002, 19, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Malissiova, E.; Maraidoni, E.; Kyriazi, D.; Gonidakis, M.; Manouras, A.; Gortzi, O.; Deligiannis, C. Alatoxin M1 levels in milk and dairy products in Greece in relation to public health. In Proceedings of the 38th European Society for Clinical Nutrition, Copenhagen, Norway, 17–20 September 2016; pp. 66–67. [Google Scholar]
- WHO. Global Hepatitis Report, 2017; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- JECFA Safety evaluation of certain mycotoxins in food. Fifty-sixth meeting of the Joint FAO/WHO Expert Committee on food additives. In FAO Food Nutric Paper; FAO: Rome, Italy, 2001. [Google Scholar]
- WHO/GEMS/Food Consumption Database. Available online: https://www.who.int/nutrition/landscape_analysis/nlis_gem_food/en/#.XJy5WBFvZnw.mendeley (accessed on 28 March 2019).
- Cano-Sancho, G.; Sanchis, V.; Marín, S.; Ramos, A.J. Occurrence and exposure assessment of aflatoxins in Catalonia (Spain). Food Chem. Toxicol. 2013, 51, 188–193. [Google Scholar] [CrossRef]
- Leblanc, J.C.; Tard, A.; Volatier, J.L.; Verger, P. Estimated dietary exposure to principal food mycotoxins from The First French Total Diet Study. Food Addit. Contam. 2005, 22, 652–672. [Google Scholar] [CrossRef] [PubMed]
- Shundo, L.; Navas, S.A.; Lamardo, L.C.A.; Ruvieri, V.; Sabino, M. Estimate of aflatoxin M 1 exposure in milk and occurrence in Brazil. Food Control 2009, 20, 655–657. [Google Scholar] [CrossRef]
- Udovicki, B.; Audenaert, K.; De Saeger, S.; Rajkovic, A. Overview on the Mycotoxins Incidence in Serbia in the Period 2004–2016. Toxins 2018, 10, 279. [Google Scholar] [CrossRef]
- Janic Hajnal, E.; Kos, J.; Krulj, J.; Krstovic, S.; Jajic, I.; Pezo, L.; Saric, B.; Nedeljkovic, N. Aflatoxins contamination of maize in Serbia: The impact of weather conditions in 2015. Food Addit. Contam. 2017, 34, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Miocinovic, J.; Keskic, T.; Miloradovic, Z.; Kos, A.; Pudja, P.; Miocinovic, J.; Keskic, T.; Miloradovic, Z.; Kos, A. The aflatoxin M1 crisis in the Serbian dairy sector: The year after. Food Addit. Contam. Part B 2017, 10, 1–4. [Google Scholar] [CrossRef]
- Ahlberg, S.; Grace, D.; Kiarie, G.; Kirino, Y.; Lindahl, J. A risk assessment of Aflatoxin M1 exposure in low and mid-income dairy consumers in Kenya. Toxins 2018, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- EFSA General principles for the collection of national food consumption data in the view of a pan-European dietary surveys. EFSA J. 2009, 7, 1435. [CrossRef]
- Nakatani, Y.; Satoh, T.; Saito, S.; Watanabe, M.; Yoshiike, N.; Kumagai, S.; Sugita-Konishi, Y. Simulation of deoxynivalenol intake from wheat consumption in Japan using the Monte Carlo method. Food Addit. Contam. Part A 2011, 28, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Djekic, I.; Miocinovic, J.; Pisinov, B.; Ivanovic, S.; Smigic, N. Quality characteristics of selected dairy products in Serbia. Mljekarstvo 2013, 63, 228–236. [Google Scholar]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet: A Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef] [PubMed]
- Granados-Chinchilla, F. Insights into the Interaction of Milk and Dairy Proteins with Aflatoxin M1. In Milk Proteins—From Structure to Biological Properties and Health Aspects; Isabel, G., Ed.; IntechOpen: Rijeka, Croatia, 2016; Volume 2, pp. 265–286. ISBN 9789537619992. [Google Scholar]
- FAO/WHO Application of Risk Analysis to Food Standards Issues: Report of the Joint FAO/WHO Expert Consultation; FAO/WHO: Geneva, Switzerland, 1995.
- Iha, M.H.; Barbosa, C.B.; Okada, I.A.; Trucksess, M.W. Aflatoxin M1 in milk and distribution and stability of aflatoxin M1 during production and storage of yoghurt and cheese. Food Control 2013, 29, 1–6. [Google Scholar] [CrossRef]
- Bakirci, I. A study on the occurrence of aflatoxin M1 in milk and milk products produced in Van province of Turkey. Food Control 2001, 12, 47–51. [Google Scholar] [CrossRef]
- Van Egmond, H.P.; Paulsch, W.E.; Veringa, H.A.; Schuller, P.L.; Sizoo, E.A.; Wilhelmina, E.; Deyll, E.; Nannings, B. The effect of processing on the aflatoxin M1 content of milk and milk products. Arch. Inst. Pasteur Tunis 1977, 4, 381–390. [Google Scholar]
- Gibb, H.; Devleesschauwer, B.; Bolger, P.M.; Wu, F.; Ezendam, J.; Cliff, J.; Zeilmaker, M.; Verger, P.; Pitt, J.; Baines, J.; et al. World Health Organization estimates of the global and regional disease burden of four foodborne chemical toxins, 2010: A data synthesis. F1000Research 2016, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- JECFA Evaluation of certain food additives and contaminants (Forty-ninth report of the Joint FAO/WHO Expert Committee on Food Additives). In FAO/WHO Expert Committee on Food Additives; FAO/WHO: Geneva, Switzerland, 1999.
- EFSA Opinion of the Scientific Committee on a request from EFSA related to A Harmonised Approach for Risk Assessment of Substances Which are both Genotoxic and Carcinogenic. EFSA J. 2005, 3, 282. [CrossRef]
- Cullen, J.M.; Ruebner, B.H.; Hsieh, L.S.; Hyde, D.M.; Hsieh, D.P. Carcinogenicity of Dietary Aflatoxin M1 in Male Fischer Rats Compared to Aflatoxin B1. Cancer Res. 1987, 47, 1913–1917. [Google Scholar] [PubMed]
- Kuiper-Goodman, T. Uncertainties in the risk assessment of three myco-toxins: Aflatoxin, ochratoxin and zearalenone. Can. J. Physiol. Pharmacol. 1990, 68, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Schaffner, D.W.; Yue, T. Quantification of aflatoxin risk associated with Chinese spices: Point and probability risk assessments for aflatoxin B1. Food Control 2013, 33, 366–377. [Google Scholar] [CrossRef]
- Morales, J.S.S.; Rojas, R.M.; Pérez-Rodríguez, F.; Casas, A.A.; López, M.A.A. Risk assessment of the lead intake by consumption of red deer and wild boar meat in Southern Spain. Food Addit. Contam. Part A 2011, 28, 1021–1033. [Google Scholar] [CrossRef] [PubMed]
| Demographic Characteristics | Total | Serbia | Greece | |
|---|---|---|---|---|
| Gender | Male | 523 (42.0%) | 281 (37.8%) | 242 (48.2%) |
| Female | 723 (58.0%) | 463 (62.2%) | 260 (51.8%) | |
| Age | Less than 22 years | 648 (52.0%) | 270 (36.3%) | 378 (75.3%) |
| 22–27 years | 598 (48.0%) | 474 (63.7%) | 124 (24.7%) | |
| Total | 1246 (100%) | 744 (100%) | 502 (100%) | |
| Average body weight [kg] | 68.2 ± 13.8 | 68.0 ± 13.9 | 68.4 ± 13.9 | |
| Country | At Least Once A Day (Milk/Yogurt) | At Least Once A Week (Milk/Yogurt) | On A Monthly Basis or Rarer (Milk/Yogurt) | Total |
|---|---|---|---|---|
| Serbia A | 150/203 (20.2%/27.3%) | 360/426 (48.4%/57.3%) | 234/115 (31.5%/15.5%) | 744 (100%) |
| Greece B | 102/28 (20.3%/5.6%) | 176/232 (35.1%/46.2%) | 224/242 (44.6%/48.2%) | 502 (100%) |
| Overall | 252/231 (20.2%/18.5%) | 536/658 (43.0%/52.8%) | 458/357 (36.8%/28.7%) | 1244 (100%) |
| χ2 = 26.524; p < 0.05/χ2 = 195.319; p < 0.05 | ||||
| Gender | ||||
| Male | 117/85 (22.4%/16.3%) | 238/271 (45.5%/51.8) | 168/167 (32.1%/31.9%) | 523 (100%) |
| Female | 135/146 (18.7%/20.2%) | 298/387 (41.2%/53.5%) | 290/190 (40.1%/26.3%) | 723 (100%) |
| Age | ||||
| Young (<22 years) | 137/97 (21.1%/15%) | 272/327 (42.0%/50.5%) | 239/224 (36.9%/24.6%) | 648 (100%) |
| Older (>22 years) | 115/134 (19.2%/22.4%) | 264/331 (44.1%/55.4%) | 219/133 (36.6%/22.2%) | 598 (100%) |
| Average daily intake of milk and yoghurt (1-day recall) | Per person (mL) | Per kg bw (mL) | ||
| Serbia | Greece | Serbia | Greece | |
| Male | 612.9 ± 871.9 a | 711.2 ± 1189.2 a | 7.6 ± 11.1 a | 9.4 ± 16.3 a |
| Female | 503.9 ± 656.0 a | 694.4 ± 1156.8 a | 8.6 ± 11.7 b | 11.5 ± 18.8 a |
| Total | 545.1 ± 746.9 A | 702.5 ± 1172.5 A | 8.2 ± 11.5 A | 10.5 ± 17.7 B |
| Average daily intake of milk and yoghurt (7-day recall) | Per person (mL) | Per kg bw (mL) | ||
| Serbia | Greece | Serbia | Greece | |
| Male | 322.0 ± 211.3 a | 463.9 ± 239.1 a | 3.9 ± 2.6 a | 6.2 ± 3.5 a |
| Female | 300.4 ± 242.4 b | 501.4 ± 288.1 a | 5.1 ± 4.3 b | 8.4 ± 4.9 b |
| Total | 308.6 ± 231.4 A | 483.4 ± 266.3 B | 4.7 ± 3.8 A | 7.4 ± 4.4 B |
| Country | Type of Commodity | Number of Samples | Positive Samples | Mean (μg kg−1) | Range (μg kg−1) | Production Year | Reference |
|---|---|---|---|---|---|---|---|
| Serbia | UHT/pasteurized milk | 42 | 39 | 0.270 | 0.010–0.800 | 2013 | [17] |
| UHT/pasteurized milk | 104 | 104 | 0.200–0.670 * | 0.020–1.200 | 2013 | [18] | |
| Organic milk | 6 | 6 | 0.030–0.040 * | 0.010–0.080 | 2013 | [18] | |
| UHT/pasteurized milk | 20 | 20 | 0.133 | 0.024–0.319 | 2013 | [19] | |
| UHT milk | 223 | 180 | 0.071 | 0.005–>1.000 | 2013 | [11] | |
| UHT/pasteurized milk | 60 | 54 | 0.026 | 0.005–0.104 | 2014 | [19] | |
| UHT milk | 105 | 17 | 0.022 | 0.005–>1.000 | 2014 | [11] | |
| Heat treated milk | 468 | 364 | 0.027 | <0.005–0.278 | 2015 | [16] | |
| Heat treated milk | 765 | 753 | 0.039 | <0.005–0.280 | 2016 | [16] | |
| Total | 1793 | 1537 | |||||
| Greece | Refrigerated milk | 32 | NR | NR | 0.0002-0.018 | 2016 | [22] |
| Pasteurized | 82 | 70 | 0.010 | <0.005–0.05 | 1999–2000 | [21] | |
| UHT | 17 | 14 | 0.020 | <0.005–0.05 | 1999–2001 | [21] | |
| Conventional | 154 | 75 | 0.009 | 0.006–0.013 | 2012 | [20] | |
| Total | 285 | 159 |
| AFM1 Intake and Risk Characterization | AFM1 ng/kg bw/Day (1-Day Recall) * | AFM1 ng/kg bw/Day (7-Day Recall) * | Range of HCC Cases/Year/105 Individuals (1.2–2.6% of HBsAg+) (1-Day Recall) | Range of HCC Cases/Year/105 Individuals (1.2–2.6% of HBsAg+) (7-Day Recall) | Range of HI Values (1–7-Day Recall) | MOE Values (1–7-Day Recall) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | Serbia | Greece | Serbia | Greece | Serbia | Greece | Serbia | Greece | Serbia | Greece | Serbia | Greece |
| Mean | 2.674 | 0.499 | 1.238 | 0.350 | 0.0036–0.0047 | 0.0007–0.0009 | 0.0017–0.0022 | 0.0005–0.0007 | 13.4–6.2 | 2.5–1.8 | 213.2–460.4 | 1142.3–1628.6 |
| 5th percentile | 0.302 | 0.078 | 0.144 | 0.080 | 0.0004–0.0005 | 0.0001–0.0001 | 0.0002–0.0003 | 0.0001–0.0001 | 1.5–0.7 | 0.4–0.4 | 1887.4–3958.3 | 7307.7–7125 |
| 1st quartile | 0.933 | 0.203 | 0.448 | 0.161 | 0.0013–0.0016 | 0.0003–0.0004 | 0.0006–0.0008 | 0.0002–0.0003 | 4.7–2.2 | 1.0–0.8 | 610.9–1272.3 | 2807.9–3540.4 |
| 3rd quartile | 3.636 | 0.673 | 1.675 | 0.461 | 0.0049–0.0064 | 0.0009–0.0012 | 0.0023–0.0029 | 0.0006–0.0008 | 18.2–8.4 | 3.4–2.3 | 156.8–340.3 | 846.9–1236.4 |
| 95th percentile | 7.841 | 1.353 | 3.698 | 0.873 | 0.0106–0.0138 | 0.0018–0.0024 | 0.0050–0.0065 | 0.0012–0.0015 | 39.2–18.5 | 6.8–4.4 | 72.7–154.1 | 421.3–652.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udovicki, B.; Djekic, I.; Kalogianni, E.P.; Rajkovic, A. Exposure Assessment and Risk Characterization of Aflatoxin M1 Intake through Consumption of Milk and Yoghurt by Student Population in Serbia and Greece. Toxins 2019, 11, 205. https://doi.org/10.3390/toxins11040205
Udovicki B, Djekic I, Kalogianni EP, Rajkovic A. Exposure Assessment and Risk Characterization of Aflatoxin M1 Intake through Consumption of Milk and Yoghurt by Student Population in Serbia and Greece. Toxins. 2019; 11(4):205. https://doi.org/10.3390/toxins11040205
Chicago/Turabian StyleUdovicki, Bozidar, Ilija Djekic, Eleni P. Kalogianni, and Andreja Rajkovic. 2019. "Exposure Assessment and Risk Characterization of Aflatoxin M1 Intake through Consumption of Milk and Yoghurt by Student Population in Serbia and Greece" Toxins 11, no. 4: 205. https://doi.org/10.3390/toxins11040205
APA StyleUdovicki, B., Djekic, I., Kalogianni, E. P., & Rajkovic, A. (2019). Exposure Assessment and Risk Characterization of Aflatoxin M1 Intake through Consumption of Milk and Yoghurt by Student Population in Serbia and Greece. Toxins, 11(4), 205. https://doi.org/10.3390/toxins11040205








