A New Topical Eye Drop Containing LyeTxI-b, A Synthetic Peptide Designed from A Lycosa erithrognata Venom Toxin, Was Effective to Treat Resistant Bacterial Keratitis
Abstract
1. Introduction
2. Results
2.1. Antimicrobial Activity in Planktonic and Biofilm Culture
2.2. Ocular Toxicity of the LyeTxI-b by HET-CAM Assay
2.3. LyeTxI-b Was Not Irritant for Topical Administration
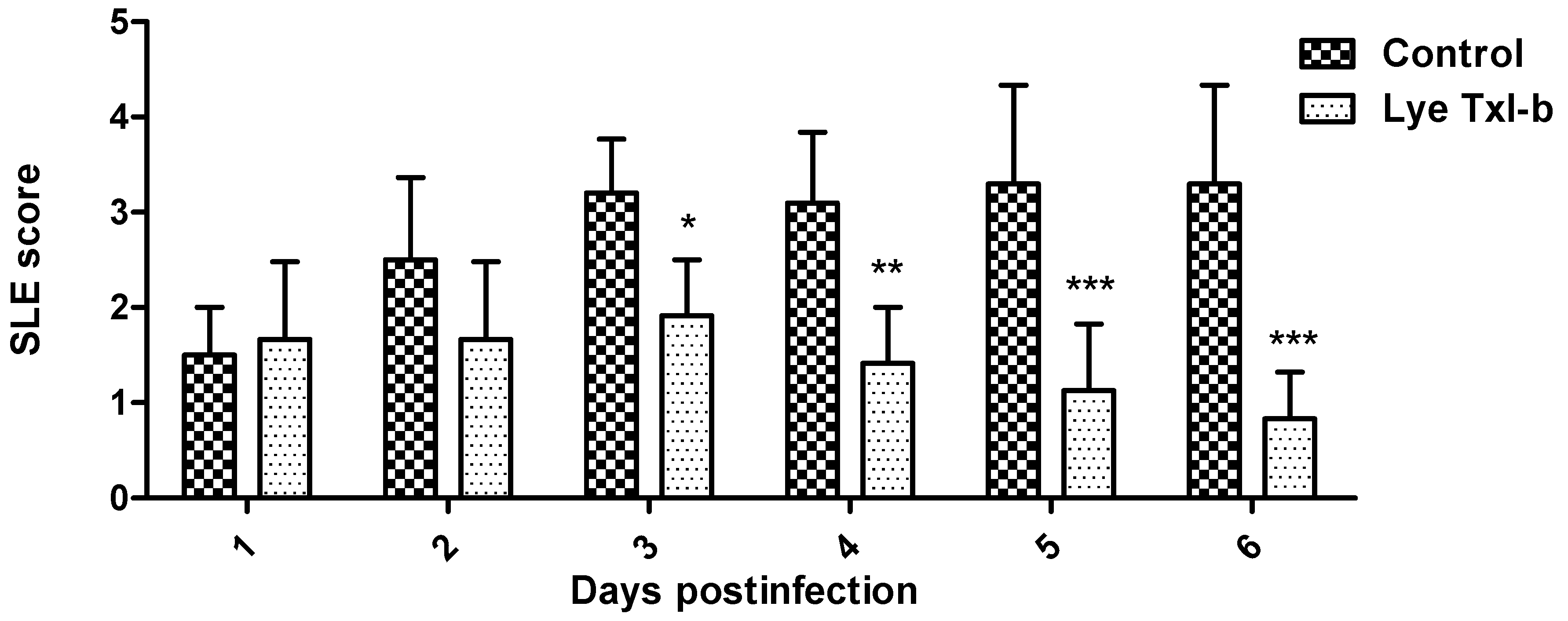
2.4. LyeTxI-b Reduces Bacterial Growth in Ocular Keratitis
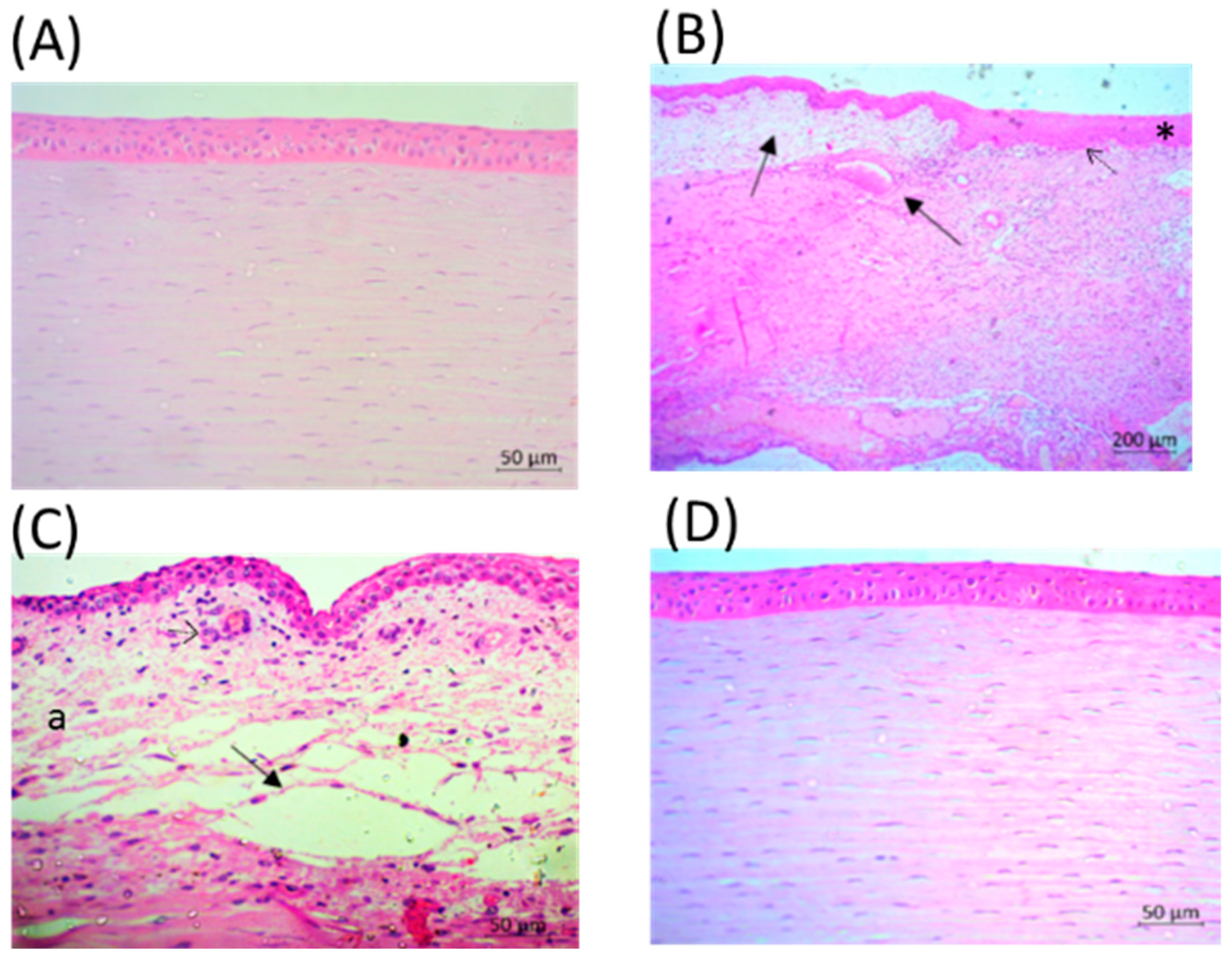
2.5. Eyes after LyeTxI-b Treatment Show Tissue Repair
2.6. PMN Infiltration was Lower After Treatment with LyeTxI-b Eye Drops
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. In Vitro Antimicrobial Test
4.2.2. Evaluation of LyeTxI-b Toxicity by Chorioallantoic Membrane Test (HET-CAM)
4.2.3. Ocular Tolerability of LyeTxI-b
4.2.4. Induction of Bacterial Keratitis and Treatment with LyeTxI-b
4.2.5. Slit Lamp Examination (SLE)
4.2.6. Histological Analysis
4.2.7. Inflammatory Analysis: MPO and NAG Activity
4.2.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Callaghan, R.J. The Pathogenesis of Staphylococcus aureus Eye Infections. Pathogens 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Whitcher, J.P.; Srinivasan, M.; Upadhyay, M.P. Corneal blindness: A global perspective. Bull. World Health Organ. 2001, 79, 214–221. [Google Scholar] [PubMed]
- Rachwalik, D.; Pleyer, U. Bacterial Keratitis. Klinische Monatsblätter Augenheilkunde 2015, 232, 738–744. [Google Scholar]
- Keay, L.; Edwards, K.; Naduvilath, T.; Taylor, H.; Snibson, G.; Forde, K.; Stapleton, F. Microbial keratitis predisposing factors and morbidity. Ophthalmology 2006, 113, 109–116. [Google Scholar]
- McGhee, C.N.J.; Niederer, R. Resisting susceptibility: Bacterial keratitis and generations of antibiotics. Clin. Exp. Ophthalmol. 2006, 34, 3–5. [Google Scholar] [PubMed]
- De Oliveira Fulgêncio, G.; Viana, F.A.; Silva, R.O.; Lobato, F.C.; Ribeiro, R.R.; Fanca, J.R.; Byrro, R.M.; Faraco, A.A.; da Silva Cunha-Júnior, A. Mucoadhesive chitosan films as a potential ocular delivery system for ofloxacin: Preliminary in vitro studies. Vet. Ophthalmol. 2014, 17, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Dajcs, J.J.; Moreau, J.M.; Thibodeaux, B.A.; Traidej, M.; Austin, M.S.; Marquart, M.E.; Stroman, D.W.; O’callaghan, R.J. Effectiveness of Ciprofloxacin and Ofloxacin in a prophylaxis model of Staphylococcus Keratitis. Cornea 2001, 20, 878–880. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A.; Ackermann, M.; McCray, P.B., Jr.; Tack, B.F. Antimicrobial peptides in animals and their role in host defences. Int. J. Antimicrob. Agents 2003, 22, 465–478. [Google Scholar] [CrossRef]
- Kang, S.J.; Park, S.J.; Mishig-Ochir, T.; Lee, B.J. Antimicrobial peptides: Therapeutic potentials. Expert Rev. Ant. Infect. Ther. 2014, 12, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Lakshmaiah, N.J.; Chen, J.Y. Antimicrobial peptides: Possible anti-infective agents. Peptides 2015, 72, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Atia, R.; Jouve, L.; Knoeri, J.; Georgeon, C.; Laroche, L.; Borderie, V.; Bouheraoua, N. Corneal collagen cross-linking to treat infectious keratitis. J. Fr. Ophtalmol. 2018, 41, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.M.; Verly, R.M.; Pilo-Veloso, D.; De Maria, M.; De Carvalho, M.A.R.; Cisalpino, P.S.; Soares, B.M.; Diniz, C.G.; Farias, L.M.; Moreira, D.F.; et al. LyeTx I, a potent antimicrobial peptide from the venom of the spider Lycosa erythrognatha. Amino Acids 2010, 39, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Consuegra, J.; de Lima, M.E.; Santos, D.; Sinisterra, R.D.; Cortés, M.E. Peptides: B-cyclodextrin inclusion compounds as highly effective antimicrobial and anti-epithelial proliferation agents. J. Periodontol. 2013, 84., 1858–1868. [Google Scholar] [CrossRef]
- Cruz Olivo, E.A.; Santos, D.; de Lima, M.E.; Dos Santos, V.L.; Sinisterra, R.D.; Cortés, M.E. Antibacterial Effect of Synthetic Peptide LyeTxI and LyeTxI/β-Cyclodextrin Association Compound Against Planktonic and Multispecies Biofilms of Periodontal Pathogens. J. Periodontol. 2017, 88, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.V.M.; Boff, D.; Verly, R.M.; Melo-Braga, M.N.; Cortés, M.E.; Santos, D.M.; Pimenta, A.M.D.C.; Amaral, F.A.; Resende, J.M.; de Lima, M.E. LyeTxI-b, a Synthetic Peptide Derived from Lycosa erythrognatha Spider Venom, Shows Potent Antibiotic Activity in Vitro and in Vivo. Front. Microbiol. 2018, 9, 667. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.R.; Paiva, M.R.B.; Dourado, L.F.N.; Silva, R.O.; da Silva, C.N.; da Costa, B.L.; Toledo, C.R.; de Lima, M.E.; da Silva-Cunha, A. Intravitreal injection of the synthetic peptide LyeTx I b, derived of a spider toxin, in rabbit’s eye is safety and prevents neovascularization on Chorio-allantoic Membrane Model. J. Venom. Anim. Toxins Include. Trop. Dis. 2018, 24, 31. [Google Scholar] [CrossRef] [PubMed]
- Habboush, Y.; Guzman, N. Antibiotic Resistance. SourceStatPearls [Internet]. 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513277/ (accessed on 3 March 2019).
- Mancl, K.A.; Kirsner, R.S.; Ajdic, D. Wound biofilms: Lessons learned from oral biofilms. Wound Repair Regen 2013, 21, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; McDermott, A.M.; Zasloff, M. Antimicrobial peptides and wound healing: Biological and therapeutic considerations. Exp. Dermatol. 2016, 25, 167–173. [Google Scholar] [CrossRef]
- Wolcott, R.D.; Rhoads, D.D.; Bennett, M.E.; Wolcott, B.M.; Gogokhia, L.; Costerton, J.W.; Dowd, S.E. Chronic wounds and the medical biofilm paradigm. J. Wound Care 2010, 19, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Breidenstein, E.B.; de la Fuente-Nunez, C.; Hancock, R.E. Pseudomonas aeruginosa: All roads lead to resistance. Trends Microbiol. 2011, 19, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.K.; Yeung, A.T.; Hancock, R.E. Antibiotic resistance in Pseudomonas aeruginosa biofilms: Towards the development of novel anti-biofilm therapies. J. Biotechnol. 2014, 191, 121–130. [Google Scholar] [CrossRef]
- Garrett, Q.; Simmons, P.A.; Xu, S.; Vehige, J.; Zhao, Z.; Ehrmann, K.; Willcox, M. Carboxymethylcellulose binds to human corneal epithelial cells and is a modulator of cornealepithelial wound healing. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.; Burrows, L.L.; Deber, C.M. Helix induction in antimicrobial peptides by alginate in biofilms. J. Biol. Chem. 2004, 279, 38749–38754. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, H.; Charron-Mazenod, L.; Lewenza, S. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2008, 4, e1000213. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, H.; Ismail, S.; Hussein, A.; Wu, Z.; Al-Kassas, R.; Alany, R.G. Conjunctival and corneal tolerability assessment of ocular naltrexone niosomes and their ingredients on the hen’s egg chorioallantoic membrane and excised bovine cornea models. Int. J. Pharm. 2012, 432, 1–10. [Google Scholar] [CrossRef]
- Vargas, A.; Zeisser-Labouèbe, M.; Lange, N.; Gurny, R.; Delie, F. The chick embryo and its chorioallantoic membrane (CAM) for the in vivo evaluation of drug delivery systems. Adv. Drug Deliv. Rev. 2007, 59, 1162–1176. [Google Scholar] [CrossRef]
- Sloop, G.D.; Moreau, J.M.; Conerly, L.L.; Dajcs, J.J.; O’Callaghan, R.J. Acute inflammation of the eyelid and cornea in Staphylococcus keratitis in the rabbit. Investig. Ophthalmol. Vis. Sci. 1999, 40, 385–391. [Google Scholar]
- Hume, E.B.; Dajcs, J.J.; Moreau, J.M.; Sloop, G.D.; Willcox, M.D.; O’Callaghan, R.J. Staphylococcus corneal virulence in a new topical model of infection. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2904–2908. [Google Scholar]
- O’brien, P.J. Peroxidases. Chemico-Biol. Interact. 2000, 129, 113–139. [Google Scholar] [CrossRef]
- Giovannini, S.; Onder, G.; Leeuwenburgh, C.; Carter, C.; Marzetti, E.; Russo, A.; Capoluongo, E.; Pahor, M.; Bernabei, R.; Landi, F.; et al. Myeloperoxidase levels and mortality in frail community-living elderly individuals. J. Gerontol. Med. Sci. 2010, 65A, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Oguido, A.P.M.T.; Hohmann, M.S.N.; Pinho-Ribeiro, F.A.; Crespigio, J.; Domiciano, T.P.; Verri, W.A.; Casella, A.M. Naringenin Eye Drops Inhibit Corneal Neovascularization by Anti-Inflammatory and Antioxidant Mechanisms. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5764–5776. [Google Scholar] [CrossRef] [PubMed]
- Toté, K.; Horemans, T.; Vanden Berghe, D.; Maes, L.; Cos, P. Inhibitory Effect of Biocides on the Viable Masses and Matrices of Staphylococcus aureus and Pseudomonas aeruginosa Biofilms. Appl. Environ. Microbiol. 2010, 76, 3135–3142. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Gilleron, L.; Coecke, S.; Sysmans, M.; Hansen, E.; Van Oproy, S.; Marzin, D.; Van Cauteren, H.; Vanparys, P. Evaluation of a modified HET-CAM assay as a screening test for eye irritancy. Toxicol. In Vitro 1996, 10, 431–446. [Google Scholar] [CrossRef]
- Fangueiro, J.F.; Calpena, A.C.; Clares, B.; Andreani, T.; Egea, M.A.; Veiga, F.J.; Garcia, M.L.; Silva, A.M.; Souto, E.B. Biopharmaceutical evaluation of epigallocatechin gallate-loaded cationic lipid nanoparticles(EGCG-LNs): In vivo, in vitro and ex vivo studies. Int. J. Pharm. 2016, 502, 161–169. [Google Scholar] [CrossRef]
- Ferreira, A.E.; Castro, B.F.; Vieira, L.C.; Cassali, G.D.; Souza, C.M.; Fulgêncio, G.O.; Ayres, E.; Oréfice, R.L.; Jorge, R.; Silva-Cunha, A.; et al. Antiangiogenic activity of a bevacizumab-loaded polyurethane device in animal neovascularization models. J. Fr. Ophtalmol. 2017, 40, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Draize, J.H.; Woodward, G.; Calvery, H.O. Methods for the study of irritation and toxicity of articles applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther. 1944, 82, 377–390. [Google Scholar]
- Fialho, S.L.; da Silva-Cunha, A. New vehicle based on a microemulsion for topical ocular administration of dexamethasone. Clin. Exp. Ophthalmol. 2004, 32, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Hazlett, L.D.; Moon, M.M.; Strejc, M.; Berk, R.S. Evidence for N-acetylmannosamine as an ocular receptor for, P. aeruginosa adherence to scarified cornea. Investig. Ophthalmol. Vis. Sci. 1987, 28, 1978–1985. [Google Scholar]
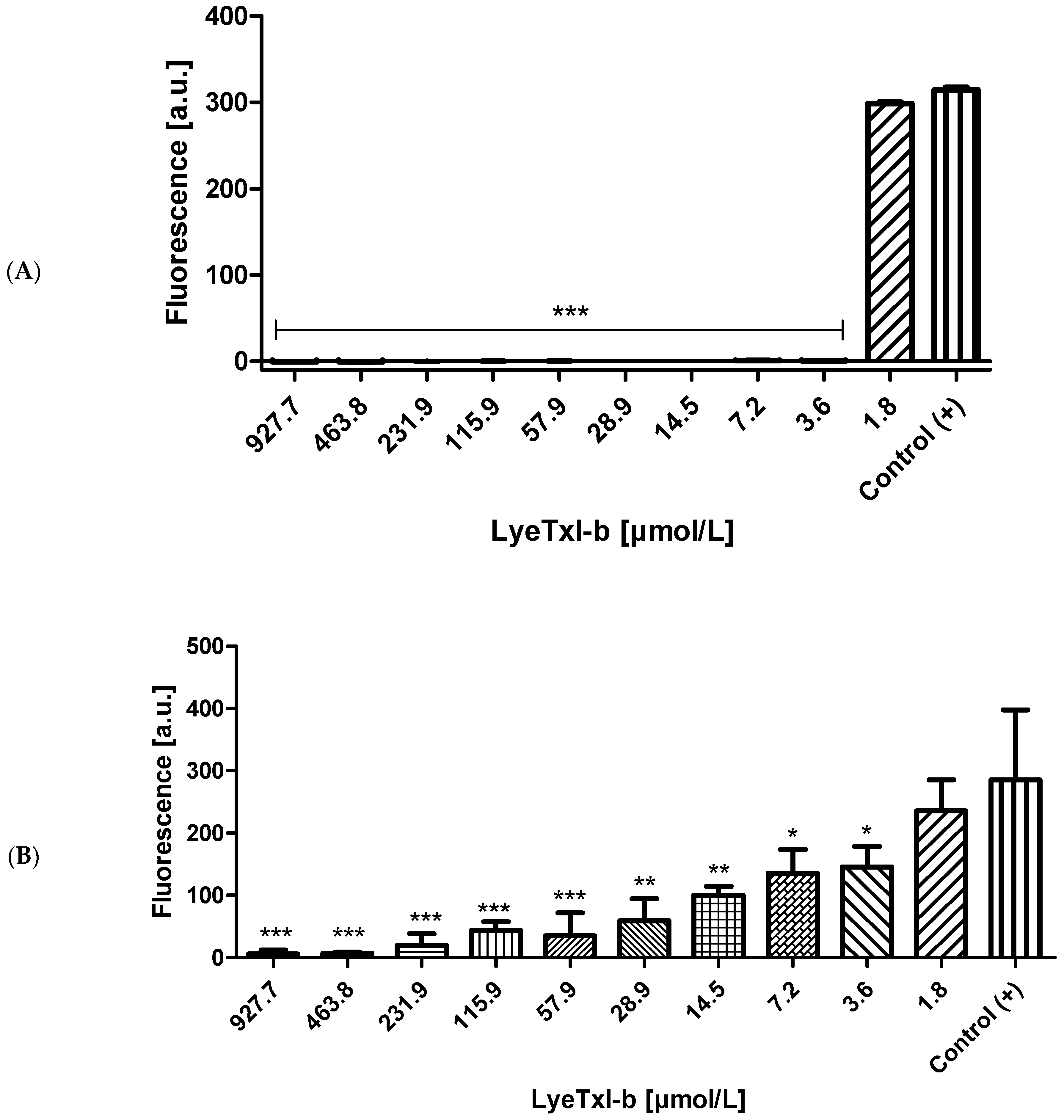
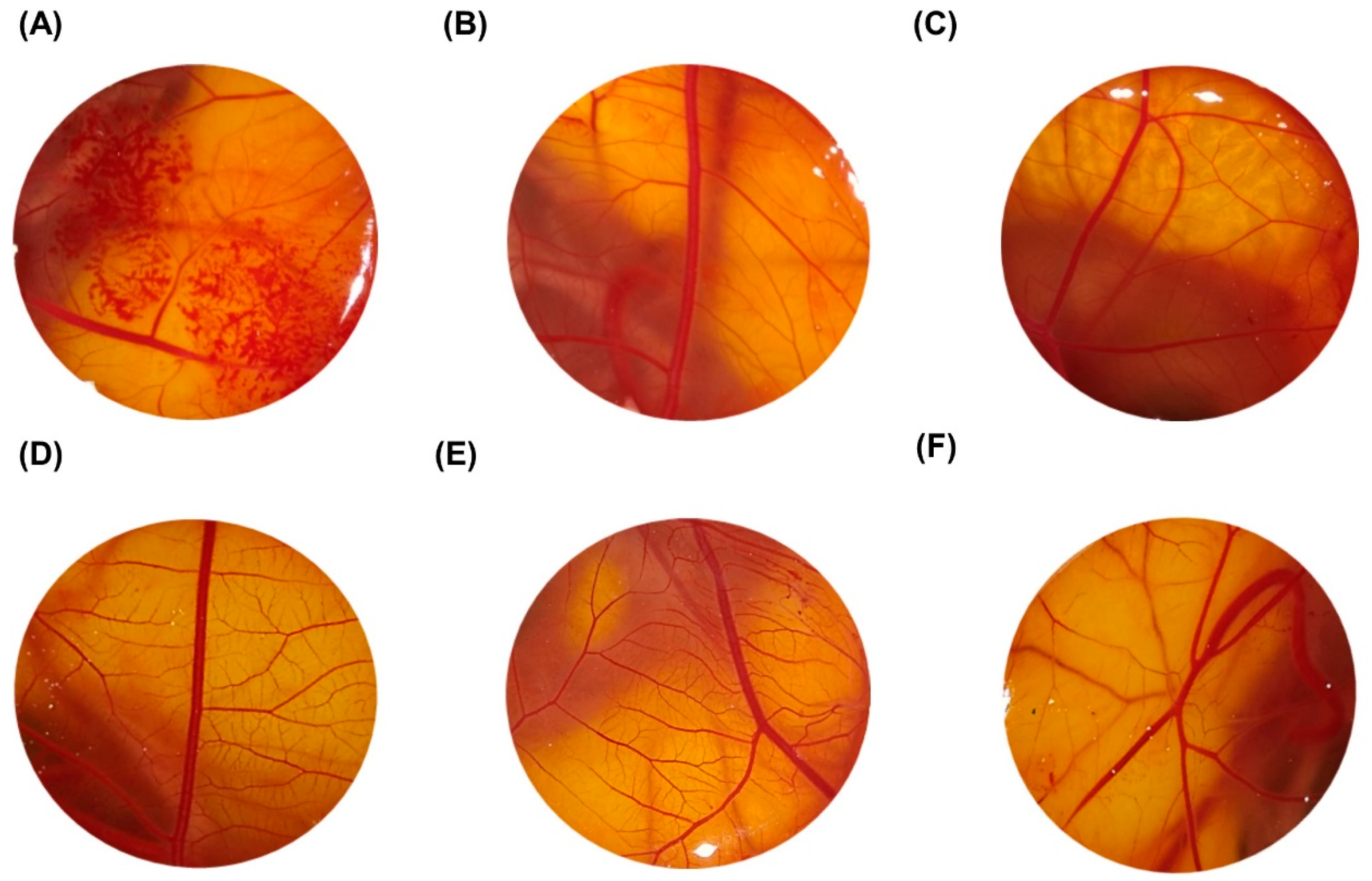



| Tested Solution/Dispersion | OII ± SEM | Irritancy Classification |
|---|---|---|
| 0.1 M NaOH (Positive control) | 21.11 ± 0.32 | SI |
| 0.9% NaCl +CMC 5% (Negative control) | ≤0.9 ± 0.0 | NI |
| LyeTxI-b—14.5 μmol/L | ≤0.9 ± 0.0 | NI |
| LyeTxI-b—28.9 μmol/L | ≤0.9 ± 0.0 | NI |
| LyeTxI-b—57.9 μmol/L | ≤0.9 ± 0.0 | NI |
| LyeTxI-b—115.9 μmol/L | ≤0.9 ± 0.0 | NI |
| Location | Concentrations (μmol/L) | |||
|---|---|---|---|---|
| 14.5 | 28.9 | 57.9 | 115.9 | |
| Cornea opacity | 0 | 0 | 0 | 0 |
| Iris inflammation degree | 0 | 0 | 0 | 0 |
| Conjunctival congestion | 0 | 0 | 0 | 0.6 |
| Conjunctival swelling | 0 | 0 | 0 | 0 |
| Conjunctival discharge | 0 | 0 | 0 | 0 |
| Total score | 0 | 0 | 0 | 0.6 |
| 0 = Clear or slight opacity, partially covering pupil. +1 = Slight opacity covering anterior segment. +2 = Moderate to dense opacity, partially or fully covering entire corneal and over pupil. +3 = Dense opacity, covering entire anterior segment. +4 = Corneal erosion or phthisis. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, C.N.d.; Silva, F.R.d.; Dourado, L.F.N.; Reis, P.V.M.d.; Silva, R.O.; Costa, B.L.d.; Nunes, P.S.; Amaral, F.A.; Santos, V.L.d.; de Lima, M.E.; et al. A New Topical Eye Drop Containing LyeTxI-b, A Synthetic Peptide Designed from A Lycosa erithrognata Venom Toxin, Was Effective to Treat Resistant Bacterial Keratitis. Toxins 2019, 11, 203. https://doi.org/10.3390/toxins11040203
Silva CNd, Silva FRd, Dourado LFN, Reis PVMd, Silva RO, Costa BLd, Nunes PS, Amaral FA, Santos VLd, de Lima ME, et al. A New Topical Eye Drop Containing LyeTxI-b, A Synthetic Peptide Designed from A Lycosa erithrognata Venom Toxin, Was Effective to Treat Resistant Bacterial Keratitis. Toxins. 2019; 11(4):203. https://doi.org/10.3390/toxins11040203
Chicago/Turabian StyleSilva, Carolina Nunes da, Flavia Rodrigues da Silva, Lays Fernanda Nunes Dourado, Pablo Victor Mendes dos Reis, Rummenigge Oliveira Silva, Bruna Lopes da Costa, Paula Santos Nunes, Flávio Almeida Amaral, Vera Lúcia dos Santos, Maria Elena de Lima, and et al. 2019. "A New Topical Eye Drop Containing LyeTxI-b, A Synthetic Peptide Designed from A Lycosa erithrognata Venom Toxin, Was Effective to Treat Resistant Bacterial Keratitis" Toxins 11, no. 4: 203. https://doi.org/10.3390/toxins11040203
APA StyleSilva, C. N. d., Silva, F. R. d., Dourado, L. F. N., Reis, P. V. M. d., Silva, R. O., Costa, B. L. d., Nunes, P. S., Amaral, F. A., Santos, V. L. d., de Lima, M. E., & Silva Cunha Júnior, A. d. (2019). A New Topical Eye Drop Containing LyeTxI-b, A Synthetic Peptide Designed from A Lycosa erithrognata Venom Toxin, Was Effective to Treat Resistant Bacterial Keratitis. Toxins, 11(4), 203. https://doi.org/10.3390/toxins11040203






