D. russelii Venom Mediates Vasodilatation of Resistance Like Arteries via Activation of Kv and KCa Channels
Abstract
1. Introduction
2. Results and Discussion
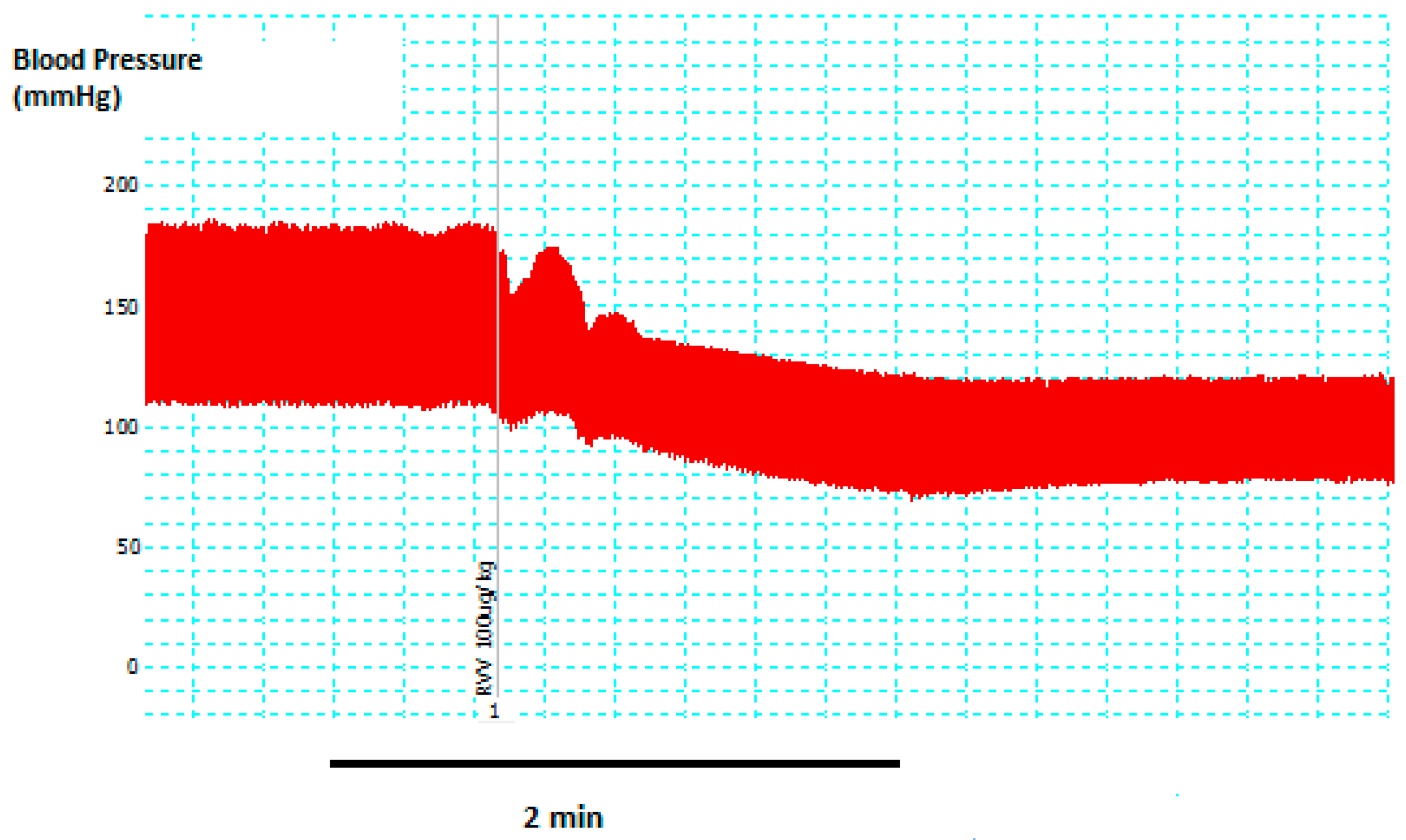
2.1. In Vivo Studies
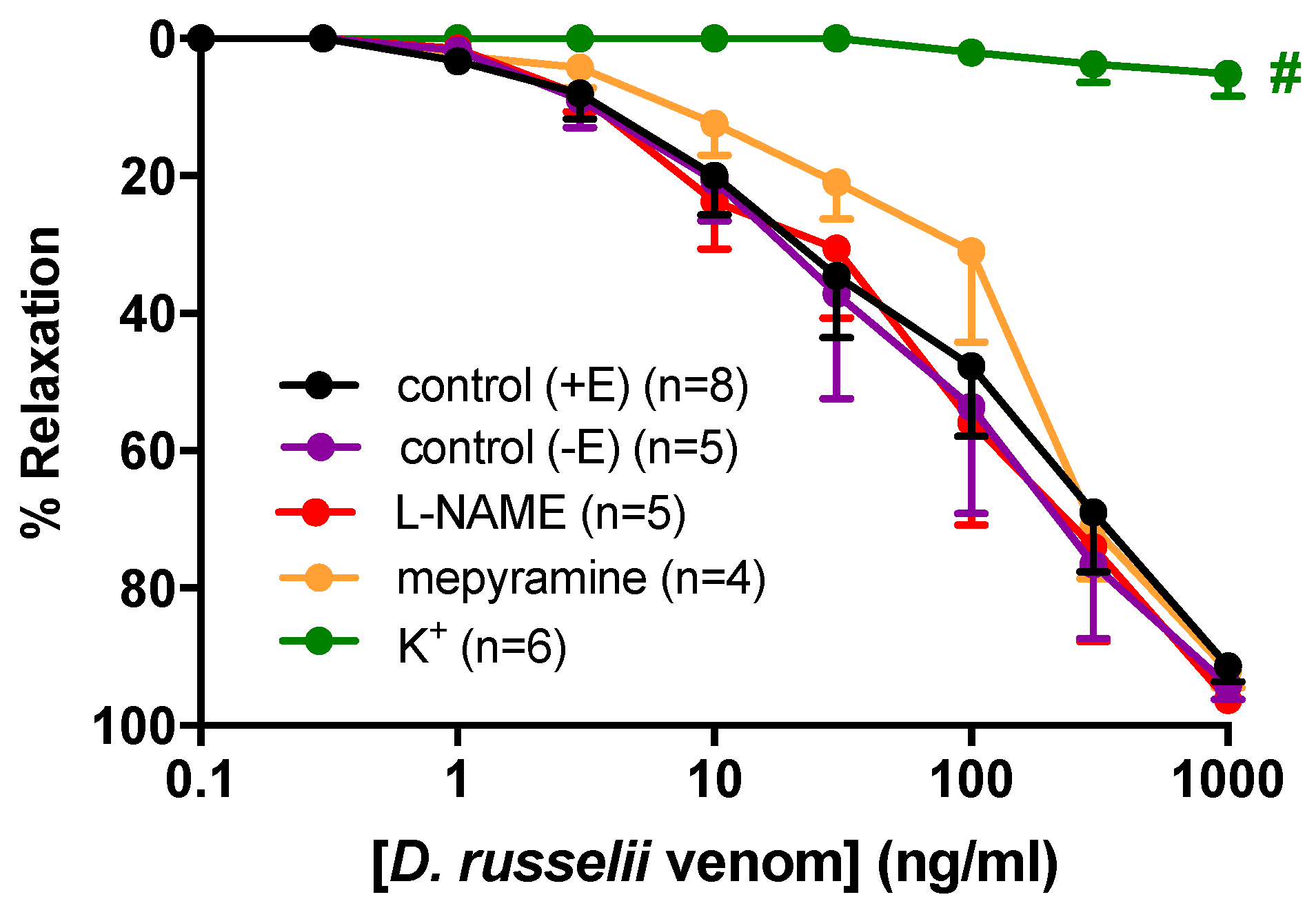
2.2. Vasorelaxant Responses to D. russelii Venom
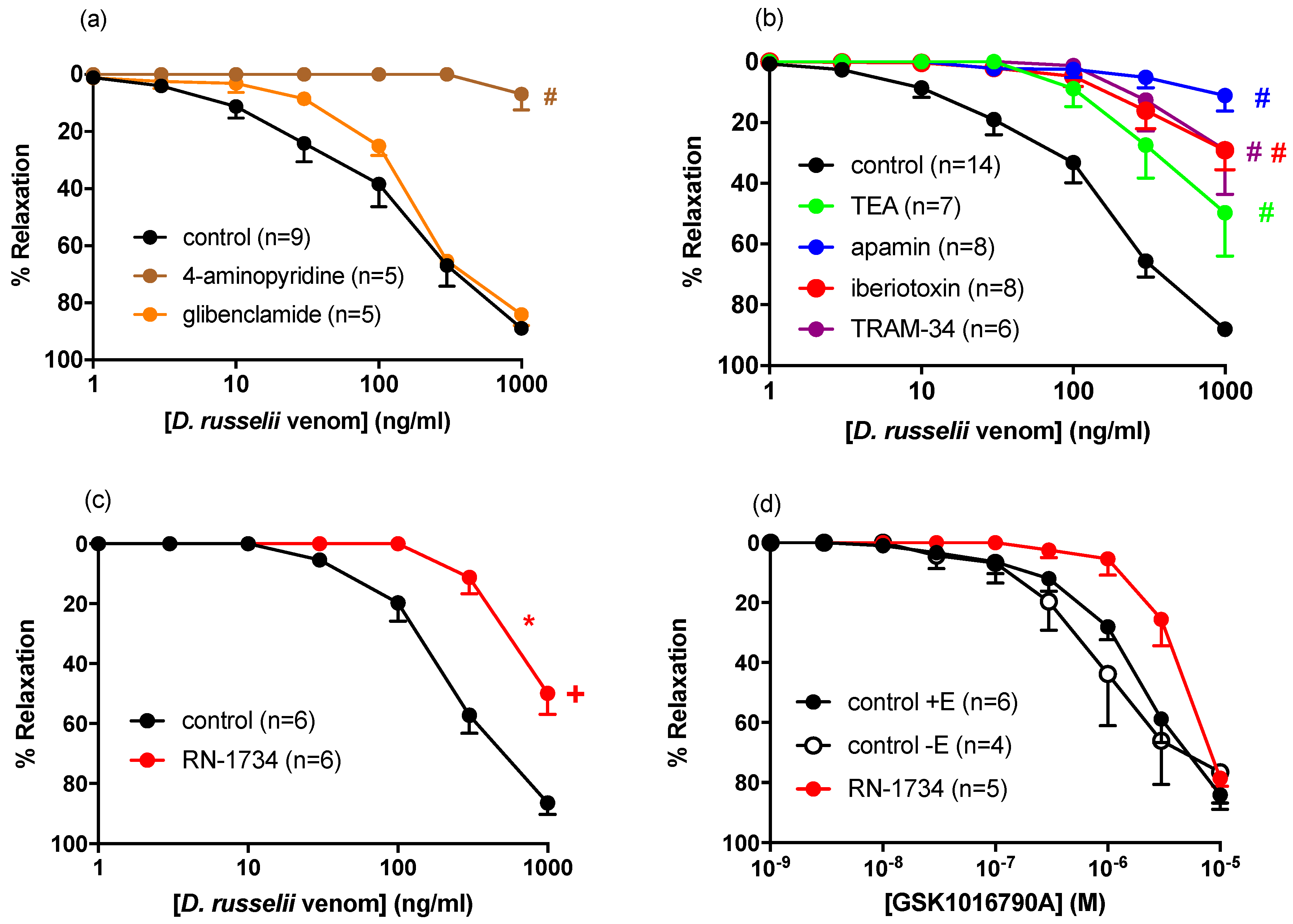
2.3. Contribution of Potassium Channels to D. russelii-Mediated Vasorelaxation
3. Conclusions
4. Materials and Methods
4.1. In Vivo Blood Pressure Experiments
4.2. Isolation of Rat Small Mesenteric Arteries
4.3. Vasorelaxation Experiments
4.4. Data Analysis and Statistical Procedures
4.4.1. In Vivo
4.4.2. In Vitro
4.5. Reagents
Author Contributions
Funding
Conflicts of Interest
References
- Chippaux, J.P. Estimating the global burden of snakebite can help to improve management. PLoS Med. 2008, 5, 221. [Google Scholar] [CrossRef]
- Warrell, D.A. Snake bite. Lancet 2010, 375, 77–88. [Google Scholar] [CrossRef]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S. The toxicogenomic multiverse: Convergent recruitment of proteins into animal venoms. Ann. Rev. Genomics Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef]
- Mackessy, S.P. Handbook of Venoms and Toxins of Reptiles; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Calvete, J.J.; Sanz, L.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M. Venoms, venomics, antivenomics. FEBS Lett. 2009, 583, 1736–1743. [Google Scholar] [CrossRef]
- Chaisakul, J.; Konstantakopoulos, N.; Smith, A.I.; Hodgson, W.C. Isolation and characterisation of P-EPTX-Ap1a and P-EPTX-Ar1a: Pre-synaptic neurotoxins from the venom of the northern (Acanthophis praelongus) and Irian Jayan (Acanthophis rugosus) death adders. Biochem. Pharmacol. 2010, 80, 895–902. [Google Scholar] [CrossRef]
- Kuruppu, S.; Smith, A.I.; Isbister, G.K.; Hodgson, W.C. Neurotoxins from Australo-Papuan elapids: A biochemical and pharmacological perspective. Crit. Rev. Toxicol. 2008, 38, 73–86. [Google Scholar] [CrossRef]
- Barber, C.; Isbister, G.; Hodgson, W. Alpha neurotoxins. Toxicon 2013, 66, 47–58. [Google Scholar] [CrossRef]
- Wickramaratna, J.C.; Fry, B.G.; Aguilar, M.I.; Kini, R.M.; Hodgson, W.C. Isolation and pharmacological characterization of a phospholipase A2 myotoxin from the venom of the Irian Jayan death adder (Acanthophis rugosus). Br. J. Pharmacol. 2003, 138, 333–342. [Google Scholar] [CrossRef]
- Silva, A.; Johnston, C.; Kuruppu, S.; Kneisz, D.; Maduwage, K.; Kleifeld, O.; Smith, A.I.; Siribaddana, S.; Buckley, N.A.; Hodgson, W.C. Clinical and pharmacological investigation of myotoxicity in Sri Lankan Russell’s viper (Daboia russelii) envenoming. PLoS Negl. Trop. Dis. 2016, 10, e0005172. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Lomonte, B. Phospholipase A2 myotoxins from Bothrops snake venoms. Toxicon 1995, 33, 1405–1424. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Lomonte, B. Phospholipases A2: Unveiling the secrets of a functionally versatile group of snake venom toxins. Toxicon 2013, 62, 27–39. [Google Scholar] [CrossRef]
- Isbister, G.K.; Brown, S.G.; MacDonald, E.; White, J.; Currie, B.J. Current use of Australian snake antivenoms and frequency of immediate-type hypersensitivity reactions and anaphylaxis. Med. J. Aust. 2008, 188, 473. [Google Scholar] [PubMed]
- Joseph, R.; Pahari, S.; Hodgson, W.C.; Kini, R.M. Hypotensive agents from snake venoms. Cardiovasc. Hematol. Disord. Drug Targets 2004, 4, 437–459. [Google Scholar] [CrossRef]
- Chaisakul, J.; Isbister, G.K.; Kuruppu, S.; Konstantakopoulos, N.; Hodgson, W.C. An examination of cardiovascular collapse induced by Eastern brown snake (Pseudonaja textilis) venom. Toxicol. Lett. 2013, 221, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Chaisakul, J.; Isbister, G.K.; Konstantakopoulos, N.; Tare, M.; Parkington, H.C.; Hodgson, W.C. In vivo and in vitro cardiovascular effects of Papuan taipan (Oxyuranus scutellatus) venom: Exploring “sudden collapse”. Toxicol. Lett. 2012, 213, 243–248. [Google Scholar] [CrossRef]
- Chaisakul, J.; Isbister, G.K.; Tare, M.; Parkington, H.C.; Hodgson, W.C. Hypotensive and vascular relaxant effects of phospholipase A2 toxins from Papuan taipan (Oxyuranus scutellatus) venom. Eur. J. Pharmacol. 2014, 723, 227–233. [Google Scholar] [CrossRef]
- Tibballs, J. The cardiovascular, coagulation and haematological effects of Tiger snake (Notechis scutatus) venom. Anaesth. Intensive Care 1998, 26, 529. [Google Scholar] [CrossRef]
- Tibballs, J.; Sutherland, S.; Rivera, R.; Masci, P. The cardiovascular and haematological effects of purified prothrombin activator from the common Brown snake (Pseudonaja textilis) and their antagonism with heparin. Anaesth. Intensive Care 1992, 20, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Klabunde, R. Cardiovascular Physiology Concepts; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2011. [Google Scholar]
- Hodgson, W.C.; Isbister, G.K. The application of toxins and venoms to cardiovascular drug discovery. Curr. Opin. Pharmacol. 2009, 9, 173–176. [Google Scholar] [CrossRef]
- De Silva, A.; Ranasinghe, L. Epidemiology of snake-bite in Sri Lanka: A review. Ceylon Med. J. 1983, 28, 144. [Google Scholar]
- Silva, A.; Kuruppu, S.; Othman, I.; Goode, R.J.; Hodgson, W.C.; Isbister, G.K. Neurotoxicity in Sri Lankan Russell’s viper (Daboia russelii) envenoming is primarily due to U1-viperitoxin-Dr1a, a pre-synaptic neurotoxin. Neurotox. Res. 2017, 31, 11–19. [Google Scholar] [CrossRef]
- Alirol, E.; Sharma, S.K.; Bawaskar, H.S.; Kuch, U.; Chappuis, F. Snake bite in South Asia: A review. PLoS Negl. Trop. Dis. 2010, 4, e603. [Google Scholar] [CrossRef]
- Phillips, R.E.; Theakston, R.D.G.; Warrekk, D.A.; Galigedara, Y.; Abeysekera, D.; Dissanayaka, P.; Hutton, R.A.; Aloysius, D.J. Paralysis, rhabdomyolysis and haemolysis caused by bites of Russell’s viper (Vipera russelli pulchella) in Sri Lanka: Failure of Indian (haffkine) antivenom. QJM 1988, 68, 691–715. [Google Scholar]
- Warrell, D.A. Snake venoms in science and clinical medicine 1. Russell’s viper: Biology, venom and treatment of bites. Trans. R. Soc. Trop. Med. Hyg. 1989, 83, 732–740. [Google Scholar] [CrossRef]
- Silva, A.; Maduwage, K.; Sedgwick, M.; Pilapitiya, S.; Weerawansa, P.; Dahanayaka, N.J.; Buckley, N.A.; Siribaddana, S.; Isbister, G.K. Neurotoxicity in Russell’s viper (Daboia russelii) envenoming in Sri Lanka: A clinical and neurophysiological study. Clin. Toxicol. 2016, 54, 411–419. [Google Scholar] [CrossRef]
- Kularatne, S.A.; Silva, A.; Weerakoon, K.; Maduwage, K.; Walathara, C.; Paranagama, R.; Mendis, S. Revisiting Russell’s viper (Daboia russelii) bite in Sri Lanka: Is abdominal pain an early feature of systemic envenoming? PLoS ONE 2014, 9, e90198. [Google Scholar] [CrossRef]
- Kasturiratne, A.; Pathmeswaran, A.; Fonseka, M.; Lalloo, D.; Brooker, S.; De Silva, H. Estimates of disease burden due to land-snake bite in Sri Lankan hospitals. Southeast Asian J. Trop. Med. Public Health 2005, 36, 733. [Google Scholar]
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef]
- Phillips, R.; Warrell, D. Bites by Russell’s viper (Vipera russelli siamensis) in Burma: Haemostatic, vascular, and renal disturbances and response to treatment. Lancet 1985, 326, 1259–1264. [Google Scholar]
- O’Leary, M.A.; Isbister, G.K. A turbidimetric assay for the measurement of clotting times of procoagulant venoms in plasma. J. Pharmacol. Toxicol. Methods 2010, 61, 27–31. [Google Scholar] [CrossRef]
- Maduwage, K.; Isbister, G.K. Current treatment for venom-induced consumption coagulopathy resulting from snakebite. PLoS Negl. Trop. Dis. 2014, 8, e3220. [Google Scholar] [CrossRef]
- Mukherjee, A.K. Characterization of a novel pro-coagulant metalloprotease (RVBCMP) possessing α-fibrinogenase and tissue haemorrhagic activity from venom of Daboia russelli russelli (Russell’s viper): Evidence of distinct coagulant and haemorrhagic sites in RVBCMP. Toxicon 2008, 51, 923–933. [Google Scholar] [CrossRef]
- Standen, N.; Quayle, J. K+ channel modulation in arterial smooth muscle. Acta Physiol. Scand. 1998, 164, 549–557. [Google Scholar] [CrossRef]
- Jackson, W.F. Potassium channels in the peripheral microcirculation. Microcirculation 2005, 12, 113–127. [Google Scholar] [CrossRef]
- Quayle, J.M.; Bonev, A.D.; Brayden, J.E.; Nelson, M.T. Calcitonin gene-related peptide activated ATP-sensitive K+ currents in rabbit arterial smooth muscle via protein kinase A. J. Physiol. 1994, 475, 9–13. [Google Scholar] [CrossRef]
- Michelakis, E.D.; Reeve, H.L.; Huang, J.M.; Tolarova, S.; Nelson, D.P.; Weir, E.K.; Archer, S.L. Potassium channel diversity in vascular smooth muscle cells. Can. J. Physiol. Pharmacol. 1997, 75, 889–897. [Google Scholar] [CrossRef]
- Baylie, R.; Brayden, J. TRPV channels and vascular function. Acta Physiol. 2011, 203, 99–116. [Google Scholar] [CrossRef]
- Inoue, R.; Jensen, L.J.; Shi, J.; Morita, H.; Nishida, M.; Honda, A.; Ito, Y. Transient receptor potential channels in cardiovascular function and disease. Circ. Res. 2006, 99, 119–131. [Google Scholar] [CrossRef]
- Earley, S.; Heppner, T.J.; Nelson, M.T.; Brayden, J.E. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ. Res. 2005, 97, 1270–1279. [Google Scholar] [CrossRef]
- Earley, S.; Pauyo, T.; Drapp, R.; Tavares, M.J.; Liedtke, W.; Brayden, J.E. TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, 1096–1102. [Google Scholar] [CrossRef]
- Andrews, K.L.; Irvine, J.C.; Tare, M.; Apostolopoulos, J.; Favaloro, J.L.; Triggle, C.R.; Kemp-Harper, B.K. A role for nitroxyl (HNO) as an endothelium-derived relaxing and hyperpolarizing factor in resistance arteries. Br. J. Pharmacol. 2009, 157, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Boussery, K.; Delaey, C.; Van de Voorde, J. The vasorelaxing effect of CGRP and natriuretic peptides in isolated bovine retinal arteries. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Kakumanu, R.; Hodgson, W.; Ravi, R.; Alagon, A.; Harris, R.; Brust, A.; Alewood, P.; Kemp-Harper, B.; Fry, B. Vampire venom: Vasodilatory mechanisms of vampire bat (Desmodus rotundus) blood feeding. Toxins 2019, 11, 26. [Google Scholar] [CrossRef]
- Lei, S.; Mulvany, M.J.; Nyborg, N.C.B. Characterization of the CGRP receptor and mechanisms of action in rat mesenteric small arteries. Pharmacol. Toxicol. 1994, 74, 130–135. [Google Scholar] [CrossRef]
- Isomoto, S.; Kondo, C.; Yamada, M.; Matsumoto, S.; Higashiguchi, O.; Horio, Y.; Matsuzawa, Y.; Kurachi, Y. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. J. Biol. Chem. 1996, 271, 24321–24324. [Google Scholar] [CrossRef] [PubMed]
- Yuill, K.H.; Yarova, P.; Kemp-Harper, B.K.; Garland, C.J.; Dora, K.A. A novel role for HNO in local and spreading vasodilatation in rat mesenteric resistance arteries. Antioxid. Redox Signal. 2011, 14, 1625–1635. [Google Scholar] [CrossRef]
- Favaloro, J.L.; Kemp-Harper, B.K. Redox variants of NO (NO· and HNO) elicit vasorelaxation of resistance arteries via distinct mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, 1274–1280. [Google Scholar] [CrossRef]
| Treatment | D. russelii Venom | ||
|---|---|---|---|
| EC50 (ng/mL) | Rmax (%) | n | |
| Control (+E) | 145.4 ± 63.6 | 92 ± 2 | 8 |
| Control (−E) | 137.6 ± 51.6 | 87 ± 7 | 5 |
| L-NAME | 130.7 ± 72.5 | 91 ± 5 | 5 |
| Mepyramine | 159.4 ± 82.3 | 76 ± 10 | 4 |
| K+ | ND | 5 ± 3 # | 5 |
| Control | 276.5 ± 69.3 | 88 ± 2 | 15 |
| TEA | ND | 50 ± 14 # | 7 |
| Apamin | ND | 11 ± 5 # | 8 |
| Iberiotoxin | ND | 29 ± 6 # | 8 |
| TRAM-34 | ND | 29 ± 15 | 6 |
| Control | 328.7 ± 110.5 | 89 ± 2 | 9 |
| Glibenclamide | 237.7 ± 62.1 | 84 ± 4 | 4 |
| 4-Aminopyridine | ND | 7 ± 6 # | 5 |
| Control | 273.6 ± 57.6 | 86 ± 4 | 6 |
| RN-1734 | ND | 50 ± 7 * | 6 |
| GSK1016790A | |||
| Control (+E) | 2.8 ± 0.7 µM | 84 ± 5 | 6 |
| Control (−E) | 1.3 ± 0.6 µM | 77 ± 10 | 4 |
| RN-1734 | 3.5 ± 0.9 µM | 79 ± 3 | 5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakumanu, R.; Kuruppu, S.; Rash, L.D.; Isbister, G.K.; Hodgson, W.C.; Kemp-Harper, B.K. D. russelii Venom Mediates Vasodilatation of Resistance Like Arteries via Activation of Kv and KCa Channels. Toxins 2019, 11, 197. https://doi.org/10.3390/toxins11040197
Kakumanu R, Kuruppu S, Rash LD, Isbister GK, Hodgson WC, Kemp-Harper BK. D. russelii Venom Mediates Vasodilatation of Resistance Like Arteries via Activation of Kv and KCa Channels. Toxins. 2019; 11(4):197. https://doi.org/10.3390/toxins11040197
Chicago/Turabian StyleKakumanu, Rahini, Sanjaya Kuruppu, Lachlan D. Rash, Geoffrey K. Isbister, Wayne C. Hodgson, and Barbara K. Kemp-Harper. 2019. "D. russelii Venom Mediates Vasodilatation of Resistance Like Arteries via Activation of Kv and KCa Channels" Toxins 11, no. 4: 197. https://doi.org/10.3390/toxins11040197
APA StyleKakumanu, R., Kuruppu, S., Rash, L. D., Isbister, G. K., Hodgson, W. C., & Kemp-Harper, B. K. (2019). D. russelii Venom Mediates Vasodilatation of Resistance Like Arteries via Activation of Kv and KCa Channels. Toxins, 11(4), 197. https://doi.org/10.3390/toxins11040197






