Serotherapy against Voltage-Gated Sodium Channel-Targeting α-Toxins from Androctonus Scorpion Venom
Abstract
:1. Introduction
2. Immediate Envenomation Symptoms
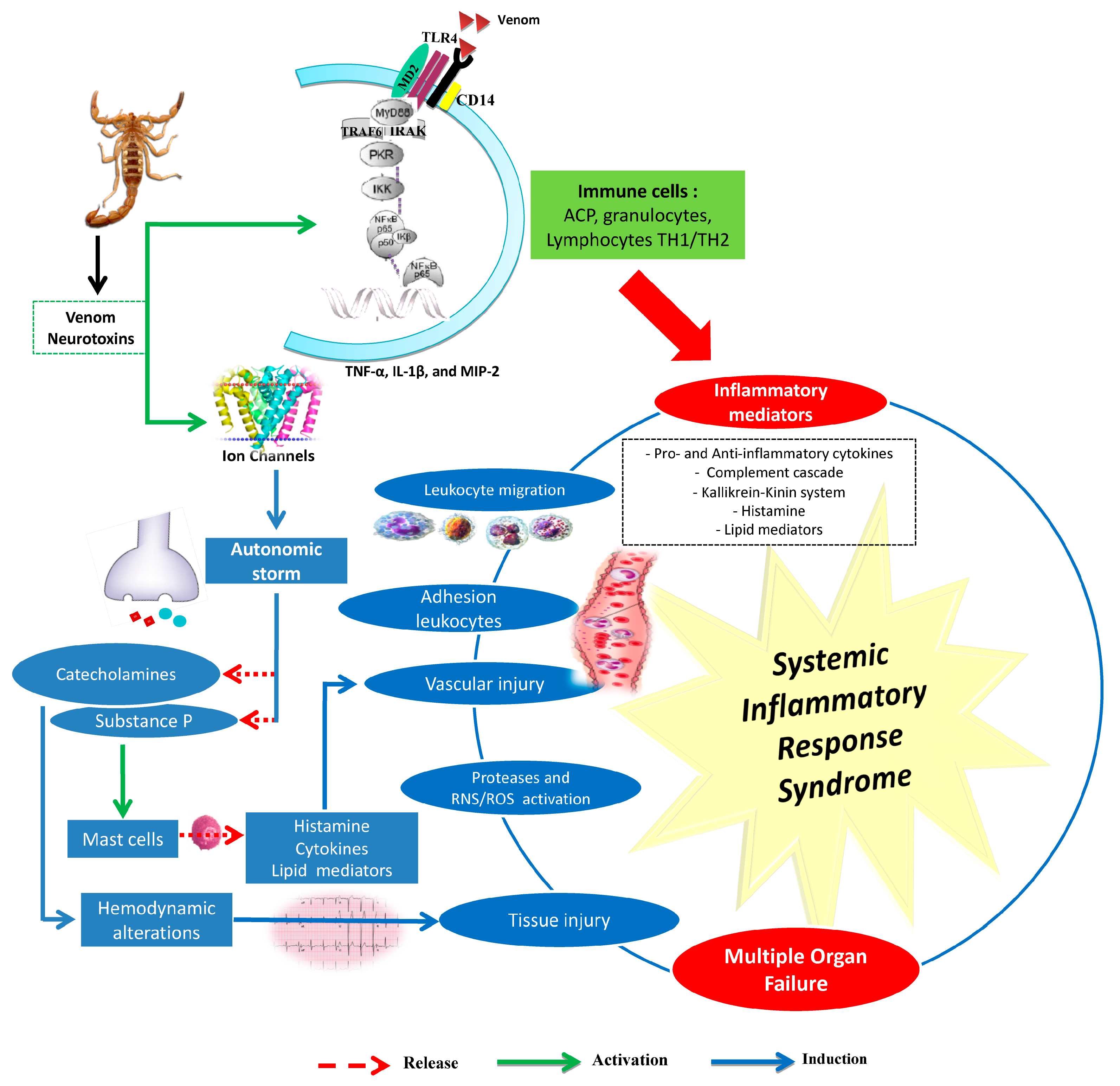
3. Androctonus Genus Venoms Involved in Complex Systemic Inflammatory Response Syndrome
3.1. Scorpion Venom and Inflammatory Response
3.2. Involvement of the Innate Immune System
3.3. Oxidative Stress and Scorpion Pathogenesis
4. Antivenoms and Immunological Properties of Scorpion Toxins
4.1. Antivenoms
4.2. Characterization of Toxins at The Pharmacological, Structural, and Immunological Level
4.2.1. Pharmacology of Androctonus α-Toxins Targeting Nav Channels
4.2.2. Structure of Androctonus α-Toxins Targeting Nav Channels
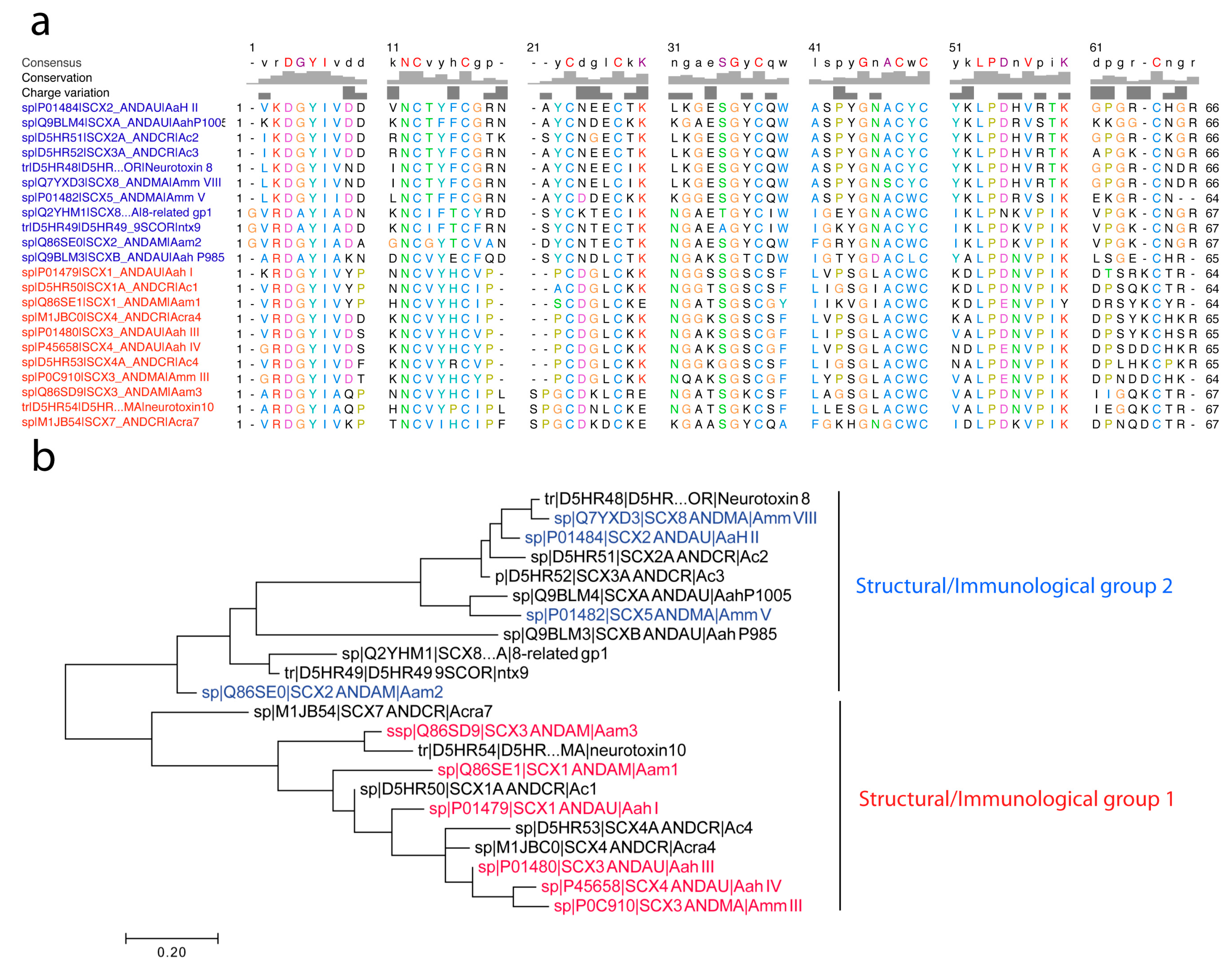
4.2.3. First Immunological Characterization of Androctonus α-Toxins: Definition of the Structural and Immunological Groups
- (1)
- Group I, exemplified by Aah I, which also contains Aah III and Aah IV from Androctonus australis hector, Amm III from Androctonus mauretanicus mauretanicus, as well as Aam H1 and Aam H3 from Androctonus amoreuxi;
- (2)
- Group II, with Aah II from Androctonus australis hector as protoype toxin, also contains Bot III from Buthus occitanus tunetanus, LqqV from Leiurus quinquestriatus quinquestriatus, AmmV and AmmVIII from Androctonus mauretanicus mauretanicus, and Aam H2 from Androctonus amoreuxi;
- (3)
- Group III, which contains Bot I and Bot II from Buthus occitanus tunetanus, is the largest, because it contains almost all the α-like toxins from Buthus, and the α-toxins against insects, like Lqh αIT from Leiurus quinquestriatus hebraeus;
- (4)
- Group IV, containing toxins similar to Lqq IV from Leiurus quinquestriatus quinquestriatus.
5. Epitope Mapping of Androctonus Toxins
5.1. First Attempts to Characterize Toxin Antigenic Sites
5.2. Use of Synthetic Peptides to Define Conformational Epitopes
5.3. The Pepscan Method
5.4. Use of Monoclonal Antibodies to Characterize Androctonus Toxins Epitopes
6. Research Studies to Improve Antibody Preparation
6.1. Detoxification by Chemical Modification
6.2. Venom Trapped in Liposomes
6.3. Use of Synthetic Peptides or Synthetic Aah II to Generate Neutralizing Antibodies
6.4. Chemically Synthesized Aah II Variant Devoid of Cysteines Bridges
6.5. Use of Native Anatoxin to Generate Antiserum
6.6. Androctonus Toxins as Fusion Proteins Expressed in Escherichia coli
6.7. Phage Display
6.8. Monoclonal Antibodies
6.9. Camelid
7. Advanced Scorpion Envenomation Therapies
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharma, P.P.; Fernandez, R.; Esposito, L.A.; Gonzalez-Santillan, E.; Monod, L. Phylogenomic resolution of scorpions reveals multilevel discordance with morphological phylogenetic signal. Proc. Biol. Sci. 2015, 282, 20142953. [Google Scholar] [CrossRef]
- Dunlop, J.A.; Selden, P.A. Scorpion fragments from the Silurian of Powys, Wales. Arachnology 2013, 16, 27–32. [Google Scholar] [CrossRef]
- Chippaux, J.P.; Goyffon, M. Epidemiology of scorpionism: a global appraisal. Acta Trop. 2008, 107, 71–79. [Google Scholar] [CrossRef]
- Ward, M.J.; Ellsworth, S.A.; Nystrom, G.S. A global accounting of medically significant scorpions: Epidemiology, major toxins, and comparative resources in harmless counterparts. Toxicon 2018, 151, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Bücherl, W. Classification, biology and venom extraction of scorpions. In Venomous Animals and their Venoms; Bücherl, W., Buckley, E., Eds.; Academic Press: New York, NY, USA, 1971; Volume 3, pp. 317–348. [Google Scholar]
- Koch, C.L. Buthidae. Taxonomic Serial No. 82718; 1837. Available online: https://www.itis.gov/ (accessed on 21 January 2019).
- Benguedda, A.C.; Laraba-Djebari, F.; Ouahdi, M.; Hellal, H.; Griene, L.; Guerenik, M.; Laid, Y.; Comité national de lutte contre l’envenimation scorpionique (CNLES). Fifteen years’ experience in scorpion envenomation control in Algeria. Bull. Soc. Pathol. Exot. 2002, 95, 205–208. [Google Scholar] [PubMed]
- Martin-Eauclaire, M.F.; Abbas, N.; Ceard, B.; Rosso, J.P.; Bougis, P.E. Androctonus toxins targeting voltage-gated sodium channels. In Scorpion Venoms; Gopalakrishnakone, P., Ferroni Schwartz, E., Possani, L.D., Rodríguez de la Vega, R.C., Eds.; Springer: Amsterdam, The Netherlands, 2014; pp. 1–25. [Google Scholar]
- Van der Meijden, A.; Koch, B.; van der Valk, T.; Vargas-Munoz, L.J.; Estrada-Gomez, S. Target-Specificity in Scorpions; Comparing Lethality of Scorpion Venoms across Arthropods and Vertebrates. Toxins 2017, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Hidan, M.A.E.; Touloun, O.; Bouazza, A.; Laaradia, M.A.; Boumezzough, A. Androctonus genus species in arid regions: Ecological niche models, geographical distributions, and envenomation risk. Vet. World 2018, 11, 286–292. [Google Scholar] [CrossRef]
- Laid, Y.; Boutekdjiret, L.; Oudjehane, R.; Laraba-Djebari, F.; Hellal, H.; Guerinik, M.; Griene, L.; Alamir, B.; Merad, R.; Chippaux, J.P. Incidence and severity of scorpion stings in Algeria. J. Venom. Anim. Toxins Incl. Trop. Dis. 2011, 18, 399–410. [Google Scholar] [CrossRef]
- Bouaziz, M.; Bahloul, M.; Kallel, H.; Samet, M.; Ksibi, H.; Dammak, H.; Ahmed, M.N.; Chtara, K.; Chelly, H.; Hamida, C.B.; et al. Epidemiological, clinical characteristics and outcome of severe scorpion envenomation in South Tunisia: multivariate analysis of 951 cases. Toxicon 2008, 52, 918–926. [Google Scholar] [CrossRef]
- Blumenthal, K.M.; Seibert, A.L. Voltage-gated sodium channel toxins: poisons, probes, and future promise. Cell Biochem. Biophys. 2003, 38, 215–238. [Google Scholar] [CrossRef]
- Martin-Eauclaire, M.F.; Couraud, F. Scorpion toxins: Effects and mechanisms. In Neurotoxinology Handbook II: Effects and Mechanisms; Chang Dyer: New York, NY, USA, 1995; Volume 22, pp. 683–716. [Google Scholar]
- Rodríguez de la Vega, R.C.; Possani, L.D. Overview of scorpion toxins specific for Na+ channels and related peptides: biodiversity, structure-function relationships and evolution. Toxicon 2005, 46, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Cestele, S.; Catterall, W.A. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie 2000, 82, 883–892. [Google Scholar] [CrossRef]
- Whittemore, F.W., Jr.; Keegan, H.L.; Borowitz, J.L. Studies of scorpion antivenins. 1. Paraspecificity. Bull. World Health Organ. 1961, 25, 185–188. [Google Scholar] [PubMed]
- Espino-Solis, G.P.; Riano-Umbarila, L.; Becerril, B.; Possani, L.D. Antidotes against venomous animals: state of the art and prospectives. J. Proteom. 2009, 72, 183–199. [Google Scholar] [CrossRef] [PubMed]
- De-Matos, I.; Talvani, A.; Rocha, O.; Freire-Maia, L.; Teixeira, M. Evidence for a role of mast cells in the lung edema induced by Tityus serrulatus venom in rats. Toxicon 2001, 39, 863–867. [Google Scholar] [CrossRef]
- Adi-Bessalem, S.; Hammoudi-Triki, D.; Laraba-Djebari, F. Pathophysiological effects of Androctonus australis hector scorpion venom: tissue damages and inflammatory response. Exp. Toxicol. Pathol. 2008, 60, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, J.P.; Freitas, T.A.; Conceição, I.M.; Kwasniewski, F.H. Tityus serrulatus venom increases vascular permeability in selected airway tissues in a mast cell-independent way. Exp. Toxicol. Pathol. 2013, 65, 229–234. [Google Scholar] [CrossRef]
- Magalhães, M.M.; Pereira, M.E.S.; Amaral, C.F.; Rezende, N.A.; Campolina, D.; Bucaretchi, F.; Gazzinelli, R.T.; Cunha-Melo, J.R. Serum levels of cytokines in patients envenomed by Tityus serrulatus scorpion sting. Toxicon 1999, 37, 1155–1164. [Google Scholar] [CrossRef]
- Petricevich, V.L. Cytokine and nitric oxide production following severe envenomation. Curr. Drug Targets-Inflamm. Allergy 2004, 3, 325–332. [Google Scholar] [CrossRef]
- Petricevich, V.L. Scorpion venom and the inflammatory response. Med. Inflamm. 2010, 2010, 903295. [Google Scholar] [CrossRef]
- Sami-Merah, S.; Hammoudi-Triki, D.; Martin-Eauclaire, M.-F.; Laraba-Djebari, F. Combination of two antibody fragments F (ab′) 2/Fab: An alternative for scorpion envenoming treatment. Int. Immunopharmacol. 2008, 8, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Raouraoua-Boukari, R.; Sami-Merah, S.; Hammoudi-Triki, D.; Martin-Eauclaire, M.-F.; Laraba-Djebari, F. Immunomodulation of the inflammatory response induced by Androctonus australis hector neurotoxins: biomarker interactions. Neuroimmunomodulation 2012, 19, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Ait-Lounis, A.; Laraba-Djebari, F. TNF-α involvement in insulin resistance induced by experimental scorpion envenomation. PLoS Negl. Trop. Dis. 2012, 6, e1740. [Google Scholar] [CrossRef] [PubMed]
- Saidi, H.; Adi-Bessalem, S.; Hammoudi-Triki, D.; Laraba-Djebari, F. Effects of atropine and propranolol on lung inflammation in experimental envenomation: comparison of two buthidae venoms. J. Venom. Anim. Toxins Incl. Trop. Dis. 2013, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Lamraoui, A.; Adi-Bessalem, S.; Laraba-Djebari, F. Modulation of tissue inflammatory response by histamine receptors in scorpion envenomation pathogenesis: involvement of H4 receptor. Inflammation 2014, 37, 1689–1704. [Google Scholar] [CrossRef] [PubMed]
- Taibi-Djennah, Z.; Laraba-Djebari, F. Effect of cytokine antibodies in the immunomodulation of inflammatory response and metabolic disorders induced by scorpion venom. Int. Immunopharmacol. 2015, 27, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Chaïr-Yousfi, I.; Laraba-Djebari, F.; Hammoudi-Triki, D. Androctonus australis hector venom contributes to the interaction between neuropeptides and mast cells in pulmonary hyperresponsiveness. Int. Immunopharmacol. 2015, 25, 19–29. [Google Scholar] [CrossRef]
- Medjadba, W.; Martin-Eauclaire, M.-F.; Laraba-Djebari, F. Involvement of kallikrein-Kinin system on cardiopulmonary alterations and inflammatory response induced by purified Aah I toxin from scorpion venom. Inflammation 2016, 39, 290–302. [Google Scholar] [CrossRef]
- Hammoudi-Triki, D.; Lefort, J.; Rougeot, C.; Robbe-Vincent, A.; Bon, C.; Laraba-Djebari, F.; Choumet, V. Toxicokinetic and toxicodynamic analyses of Androctonus australis hector venom in rats: optimization of antivenom therapy. Toxicol. Appl. Pharmacol. 2007, 218, 205–214. [Google Scholar] [CrossRef]
- Adi-Bessalem, S.; Mendil, A.; Hammoudi-Triki, D.; Laraba-Djebari, F. Lung immunoreactivity and airway inflammation: their assessment after scorpion envenomation. Inflammation 2012, 35, 501–508. [Google Scholar] [CrossRef]
- Bekkari, N.; Martin-Eauclaire, M.-F.; Laraba-Djebari, F. Complement system and immunological mediators: their involvements in the induced inflammatory process by Androctonus australis hector venom and its toxic components. Exp. Toxicol. Pathol. 2015, 67, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Hammoudi-Triki, D.; Ferquel, E.; Robbe-Vincent, A.; Bon, C.; Choumet, V.; Laraba-Djebari, F. Epidemiological data, clinical admission gradation and biological quantification by ELISA of scorpion envenomations in Algeria: Effect of immunotherapy. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 240–250. [Google Scholar] [CrossRef]
- Kaddache, A.; Hassan, M.; Laraba-Djebari, F.; Hammoudi-Triki, D. Switch of steady-state to an accelerated granulopoiesis in response to Androctonus australis hector venom. Inflammation 2017, 40, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Saidi, H.; Bérubé, J.; Laraba-Djebari, F.; Hammoudi-Triki, D. Involvement of Alveolar Macrophages and Neutrophils in Acute Lung Injury After Scorpion Envenomation: New Pharmacological Targets. Inflammation 2018, 41, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Sami-Merah, S.; Hammoudi-Triki, D.; Adi-Bessalem, S.; Mendil, A.; Martin-Eauclaire, M.; Laraba-Djebari, F. L’augmentation de la permeabilité vasculaire serait-elle un facteur déclenchant de l’oedeme pulmonaire induit par le venin du scorpion Androctonus australis hector. Toxine Signalisation-Rencontre Toxinologie Editions SFET 2009, 161–163. [Google Scholar]
- Sifi, A.; Adi-Bessalem, S.; Laraba-Djebari, F. Role of angiotensin II and angiotensin type-1 receptor in scorpion venom-induced cardiac and aortic tissue inflammation. Exp. Mol. Pathol. 2017, 102, 32–40. [Google Scholar] [CrossRef]
- Bhandari, V.; Elias, J.A. Cytokines in tolerance to hyperoxia-induced injury in the developing and adult lung. Free Radic. Biol. Med. 2006, 41, 4–18. [Google Scholar] [CrossRef]
- D’suze, G.; Moncada, S.; González, C.; Sevcik, C.; Aguilar, V.; Alagón, A. Relationship between plasmatic levels of various cytokines, tumour necrosis factor, enzymes, glucose and venom concentration following Tityus scorpion sting. Toxicon 2003, 41, 367–375. [Google Scholar] [CrossRef]
- Fukuhara, Y.; Reis, M.; Dellalibera-Joviliano, R.; Cunha, F.; Donadi, E. Increased plasma levels of IL-1β, IL-6, IL-8, IL-10 and TNF-α in patients moderately or severely envenomed by Tityus serrulatus scorpion sting. Toxicon 2003, 41, 49–55. [Google Scholar] [CrossRef]
- Abdel-Haleem, A.-H.A.; Meki, A.-R.M.; Noaman, H.A.; Mohamed, Z.T. Serum levels of IL-6 and its soluble receptor, TNF-α and chemokine RANTES in scorpion envenomed children: their relation to scorpion envenomation outcome. Toxicon 2006, 47, 437–444. [Google Scholar] [CrossRef]
- Nencioni, A.L.A.; Lourenço, G.A.; Lebrun, I.; Florio, J.C.; Dorce, V.A. Central effects of Tityus serrulatus and Tityus bahiensis scorpion venoms after intraperitoneal injection in rats. Neurosci. Lett. 2009, 463, 234–238. [Google Scholar] [CrossRef]
- Zoccal, K.F.; da Silva Bitencourt, C.; Sorgi, C.A.; Bordon, K.d.C.F.; Sampaio, S.V.; Arantes, E.C.; Faccioli, L.H. Ts6 and Ts2 from Tityus serrulatus venom induce inflammation by mechanisms dependent on lipid mediators and cytokine production. Toxicon 2013, 61, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Costal-Oliveira, F.; Guerra-Duarte, C.; Castro, K.; Tintaya, B.; Bonilla, C.; Silva, W.; Yarlequé, A.; Fujiwara, R.; Melo, M.; Chávez-Olórtegui, C. Serological, biochemical and enzymatic alterations in rodents after experimental envenomation with Hadruroides lunatus scorpion venom. Toxicon 2015, 103, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Zoccal, K.F.; Paula-Silva, F.W.G.; da Silva Bitencourt, C.; Sorgi, C.A.; Bordon, K.d.C.F.; Arantes, E.C.; Faccioli, L.H. PPAR-γ activation by Tityus serrulatus venom regulates lipid body formation and lipid mediator production. Toxicon 2015, 93, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Lamraoui, A.; Adi-Bessalem, S.; Laraba-Djebari, F. Immunopathologic effects of scorpion venom on hepato-renal tissues: Involvement of lipid derived inflammatory mediators. Exp. Mol. Pathol. 2015, 99, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.; Alves, E.; Henriques, O. Peptide T, a novel bradykinin potentiator isolated from Tityus serrulatus scorpion venom. Toxicon 1993, 31, 941–947. [Google Scholar] [CrossRef]
- Teixeira, C.; Galante, F.; Manzoli, S.; Steil, A.; Jancar, S. Inflammatory reaction induced by Tityus serrulatus crude venom (TsV) in the lung of rats. J. Venom. Anim. Toxins 1997, 3, 111–115. [Google Scholar]
- Zoccal, K.F.; da Silva Bitencourt, C.; Paula-Silva, F.W.G.; Sorgi, C.A.; Bordon, K.d.C.F.; Arantes, E.C.; Faccioli, L.H. TLR2, TLR4 and CD14 recognize venom-associated molecular patterns from Tityus serrulatus to induce macrophage-derived inflammatory mediators. PLoS ONE 2014, 9, e88174. [Google Scholar] [CrossRef]
- Kaddache, A.; Laraba-Djebari, F.; Hammoudi-Triki, D. Androctonus australis hector venom triggers accelerated granulopoiesis through cytokines secretion. Toxicon 2018, 149, 106. [Google Scholar] [CrossRef]
- Dousset, E.; Carrega, L.; Steinberg, J.; Clot-Faybesse, O.; Jouirou, B.; Sauze, N.; Devaux, C.; Autier, Y.; Jammes, Y.; Martin-Eauclaire, M. Evidence that free radical generation occurs during scorpion envenomation. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005, 140, 221–226. [Google Scholar] [CrossRef]
- Taibi-Djennah, Z.; Matin-Eauclaire, M.-F.; Laraba-Djebari, F. Systemic responses following brain injuries and inflammatory process activation induced by a neurotoxin of Androctonus scorpion venom. Neuroimmunomodulation 2015, 22, 347–357. [Google Scholar] [CrossRef]
- Meki, A.-R.; El-Dean, Z.M. Serum interleukin-1β, interleukin-6, nitric oxide and α1-antitrypsin in scorpion envenomed children. Toxicon 1998, 36, 1851–1859. [Google Scholar] [CrossRef]
- Meki, A.-R.A.; Mohamed, Z.M.; El-deen, H.M.M. Significance of assessment of serum cardiac troponin I and interleukin-8 in scorpion envenomed children. Toxicon 2003, 41, 129–137. [Google Scholar] [CrossRef]
- Petricevich, V.L.; Lebrun, I. Immunomodulatory effects of the Tityus serrulatus venom on murine macrophage functions in vitro. Med. Inflamm. 2005, 2005, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Khorchid, A.; Fragoso, G.; Shore, G.; Almazan, G. Catecholamine-induced oligodendrocyte cell death in culture is developmentally regulated and involves free radical generation and differential activation of caspase-3. Glia 2002, 40, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Zoccal, K.F.; Sorgi, C.A.; Hori, J.I.; Paula-Silva, F.W.; Arantes, E.C.; Serezani, C.H.; Zamboni, D.S.; Faccioli, L.H. Opposing roles of LTB 4 and PGE 2 in regulating the inflammasome-dependent scorpion venom-induced mortality. Nat. Commun. 2016, 7, 10760. [Google Scholar] [CrossRef] [PubMed]
- Saari, H.; Sorsa, T.; Konttinen, Y.T. Reactive oxygen species and hyaluronate in serum and synovial fluid in arthritis. Int. J. Tissue React. 1990, 12, 81–89. [Google Scholar] [PubMed]
- Hmila, I.; Abdallah, R.B.; Saerens, D.; Benlasfar, Z.; Conrath, K.; Ayeb, M.E.; Muyldermans, S.; Bouhaouala-Zahar, B. VHH, bivalent domains and chimeric Heavy chain-only antibodies with high neutralizing efficacy for scorpion toxin AahI’. Mol. Immunol. 2008, 45, 3847–3856. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.M.; Leon, G.; Lomonte, B. Pharmacokinetic-pharmacodynamic relationships of immunoglobulin therapy for envenomation. Clin. Pharmacokinet. 2003, 42, 721–741. [Google Scholar] [CrossRef] [PubMed]
- Kalia, J.; Milescu, M.; Salvatierra, J.; Wagner, J.; Klint, J.K.; King, G.F.; Olivera, B.M.; Bosmans, F. From foe to friend: using animal toxins to investigate ion channel function. J. Mol. Biol. 2015, 427, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500–544. [Google Scholar] [CrossRef] [PubMed]
- Bosmans, F.; Martin-Eauclaire, M.F.; Swartz, K.J. Deconstructing voltage sensor function and pharmacology in sodium channels. Nature 2008, 456, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Activation of the action potential Na+ ionophore by neurotoxins. An allosteric model. J. Biol. Chem. 1977, 252, 8669–8676. [Google Scholar] [PubMed]
- Catterall, W.A. Membrane potential-dependent binding of scorpion toxin to the action potential Na+ ionophore. Studies with a toxin derivative prepared by lactoperoxidase-catalyzed iodination. J. Biol. Chem. 1977, 252, 8660–8668. [Google Scholar]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992, 8, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Bougis, P.E.; Rochat, H.; Smith, L.A. Precursors of Androctonus australis scorpion neurotoxins. Structures of precursors, processing outcomes, and expression of a functional recombinant toxin II. J. Biol. Chem. 1989, 264, 19259–19265. [Google Scholar] [PubMed]
- Fontecilla-Camps, J.C.; Habersetzer-Rochat, C.; Rochat, H. Orthorhombic crystals and three-dimensional structure of the potent toxin II from the scorpion Androctonus australis Hector. Proc. Natl. Acad. Sci. USA 1988, 85, 7443–7447. [Google Scholar] [CrossRef]
- Housset, D.; Habersetzer-Rochat, C.; Astier, J.P.; Fontecilla-Camps, J.C. Crystal structure of toxin II from the scorpion Androctonus australis Hector refined at 1.3 A resolution. J. Mol. Biol. 1994, 238, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Blessing, R.H.; Ealick, S.E.; Fontecilla-Camps, J.C.; Hauptman, H.A.; Housset, D.; Langs, D.A.; Miller, R. Ab initio structure determination and refinement of a scorpion protein toxin. Acta Crystallogr. D Biol. Crystallogr. 1997, 53, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Krimm, I.; Gilles, N.; Sautiere, P.; Stankiewicz, M.; Pelhate, M.; Gordon, D.; Lancelin, J.M. NMR structures and activity of a novel alpha-like toxin from the scorpion Leiurus quinquestriatus hebraeus. J. Mol. Biol. 1999, 285, 1749–1763. [Google Scholar] [CrossRef]
- Rochat, H.; Bernard, P.; Couraud, F. Scorpion toxins: chemistry and mode of action. Adv. Cytopharmacol. 1979, 3, 325–334. [Google Scholar] [PubMed]
- Delori, P.; Van Rietschoten, J.; Rochat, H. Scorpion venoms and neurotoxins: an immunological study. Toxicon 1981, 19, 393–407. [Google Scholar] [CrossRef]
- Martin-Eauclaire, M.F.; Salvatierra, J.; Bosmans, F.; Bougis, P.E. The scorpion toxin Bot IX is a potent member of the alpha-like family and has a unique N-terminal sequence extension. FEBS Lett. 2016, 590, 3221–3232. [Google Scholar] [CrossRef] [PubMed]
- El Ayeb, M.; Delori, P.; Rochat, H. Immunochemistry of scorpion alpha-toxins: antigenic homologies checked with radioimmunoassays (RIA). Toxicon 1983, 21, 709–716. [Google Scholar] [CrossRef]
- El Ayeb, M.; Martin, M.F.; Delori, P.; Bechis, G.; Rochat, H. Immunochemistry of scorpion alpha-neurotoxins. Determination of the antigenic site number and isolation of a highly enriched antibody specific to a single antigenic site of toxin II of Androctonus australis Hector. Mol. Immunol. 1983, 20, 697–708. [Google Scholar] [CrossRef]
- Novotny, J.; Haber, E. Static accessibility model of protein antigenicity: the case of scorpion neurotoxin. Biochemistry 1986, 25, 6748–6754. [Google Scholar] [CrossRef] [PubMed]
- Granier, C.; Novotny, J.; Fontecilla-Camps, J.C.; Fourquet, P.; el Ayeb, M.; Bahraoui, E. The antigenic structure of a scorpion toxin. Mol. Immunol. 1989, 26, 503–513. [Google Scholar] [CrossRef]
- El Ayeb, M.; Bahraoui, E.M.; Granier, C.; Delori, P.; Van Rietschoten, J.; Rochat, H. Immunochemistry of scorpion alpha-toxins: purification and characterization of two functionally independent IgG populations raised against toxin II of Androctonus australis Hector. Mol. Immunol. 1984, 21, 223–232. [Google Scholar] [CrossRef]
- Granier, C.; Bahraoui, E.; Van Rietschoten, J.; Rochat, H.; El Ayeb, M. Synthesis and immunological characterization of two peptides which are models for two of the four major antigenic sites of a scorpion toxin. Int. J. Pept. Protein Res. 1984, 23, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Fontecilla-Camps, J.C.; Genovesio-Traverne, J.C.; Habersetzer-Rochat, C. Three-Dimensional Structures and Structure-Function Relationships of Scorpion Neurotoxins. In Natural Toxins: Characterization, Pharmacology and Therapeutics; Ownby, C.L., Odell, G.V., Eds.; Perganon Press: Amsterdam, The Netherlands, 1989; pp. 123–129. [Google Scholar]
- Bahraoui, E.M.; Granier, C.; Van Rietschoten, J.; Rochat, H.; el Ayeb, M. Specificity and neutralizing capacity of antibodies elicited by a synthetic peptide of scorpion toxin. J. Immunol. 1986, 136, 3371–3377. [Google Scholar]
- Bahraoui, E.; el Ayeb, M.; Granier, C.; Van Rietschoten, J.; Rochat, H. Antigenicity of peptide 19–28 of toxin II from the scorpion Androctonus australis as measured by different solid-phase tests and characterization of specific antibodies purified by immunoaffinity on the peptide or the toxin. Toxicon 1987, 25, 957–964. [Google Scholar] [CrossRef]
- Bahraoui, E.; el Ayeb, M.; Granier, C.; Rochat, H. Immunochemistry of scorpion toxins. Immunogenicity of peptide 19–28 a model of an accessible and relatively rigid region. Eur. J. Biochem. 1987, 167, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Bahraoui, E.; el Ayeb, M.; Van Rietschoten, J.; Rochat, H.; Granier, C. Immunochemistry of scorpion alpha-toxins: study with synthetic peptides of the antigenicity of four regions of toxin II of Androctonus australis Hector. Mol. Immunol. 1986, 23, 357–366. [Google Scholar] [CrossRef]
- El Ayeb, M.; Bahraoui, E.M.; Granier, C.; Rochat, H. Use of antibodies specific to defined regions of scorpion alpha-toxin to study its interaction with its receptor site on the sodium channel. Biochemistry 1986, 25, 6671–6678. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Fabrichny, I.P.; Mondielli, G.; Conrod, S.; Martin-Eauclaire, M.F.; Bourne, Y.; Marchot, P. Structural insights into antibody sequestering and neutralizing of Na+ channel alpha-type modulator from old world scorpion venom. J. Biol. Chem. 2012, 287, 14136–14148. [Google Scholar] [CrossRef] [PubMed]
- Molina, F.; Laune, D.; Gougat, C.; Pau, B.; Granier, C. Improved performances of spot multiple peptide synthesis. Pept. Res. 1996, 9, 151–155. [Google Scholar]
- Devaux, C.; Juin, M.; Mansuelle, P.; Granier, C. Fine molecular analysis of the antigenicity of the Androctonus australis hector scorpion neurotoxin II: a new antigenic epitope disclosed by the Pepscan method. Mol. Immunol. 1993, 30, 1061–1068. [Google Scholar] [CrossRef]
- Garcia y Perez, G.; Martin, M.F.; Rochat, H. Preparation of a polyvalent antivenom against various Mexican scorpion Centruroides species. Toxicon 1988, 26, 1102–1106. [Google Scholar] [CrossRef]
- Chavez-Olortegui, C.; Amara, D.A.; Rochat, H.; Diniz, C.; Granier, C. In vivo protection against scorpion toxins by liposomal immunization. Vaccine 1991, 9, 907–910. [Google Scholar] [CrossRef]
- Ait-Amara, D.; Chavez-Olortegui, C.; Romi, R.; Mery, J.; Brugidou, J.; Albericio, F.; Devaux, C.; Granier, C. Antibodies cross-reactive with the scorpion-toxin II from Androctonus australis Hector elicited in mice by a synthetic peptide. Nat. Toxins 1993, 1, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Zenouaki, I.; Kharrat, R.; Sabatier, J.M.; Devaux, C.; Karoui, H.; Van Rietschoten, J.; el Ayeb, M.; Rochat, H. In vivo protection against Androctonus australis hector scorpion toxin and venom by immunization with a synthetic analog of toxin II. Vaccine 1997, 15, 187–194. [Google Scholar] [CrossRef]
- Martin-Eauclaire, M.F.; Alami, M.; Giamarchi, A.; Missimilli, V.; Rosso, J.P.; Bougis, P.E. A natural anatoxin, Amm VIII, induces neutralizing antibodies against the potent scorpion alpha-toxins. Vaccine 2006, 24, 1990–1996. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, L.; Moreau, V.; Felicori, L.; Nguyen, C.; Duarte, C.; Chavez-Olortegui, C.; Molina, F.; Martin-Eauclaire, M.F.; Granier, C. Design of antibody-reactive peptides from discontinuous parts of scorpion toxins. Vaccine 2010, 28, 970–980. [Google Scholar] [CrossRef] [PubMed]
- Legros, C.; Kaabi, H.; El Ayeb, M.; Ceard, B.; Vacher, H.; Bougis, P.E.; Martin-Eauclaire, M.F. Use of fusion protein constructs to generate potent immunotherapy and protection against scorpion toxins. Vaccine 2001, 20, 934–942. [Google Scholar] [CrossRef]
- Gazarian, K.G.; Gazarian, T.; Hernandez, R.; Possani, L.D. Immunology of scorpion toxins and perspectives for generation of anti-venom vaccines. Vaccine 2005, 23, 3357–3368. [Google Scholar] [CrossRef] [PubMed]
- Ledsgaard, L.; Kilstrup, M.; Karatt-Vellatt, A.; McCafferty, J.; Laustsen, A.H. Basics of Antibody Phage Display Technology. Toxins 2018, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- Bahraoui, E.; Pichon, J.; Muller, J.M.; Darbon, H.; Elayeb, M.; Granier, C.; Marvaldi, J.; Rochat, H. Monoclonal antibodies to scorpion toxins. Characterization and molecular mechanisms of neutralization. J. Immunol. 1988, 141, 214–220. [Google Scholar]
- Yahi, N.; Devaux, C.; Mansuelle, P.; Defendini, M.L.; Granier, C. Monoclonal antibodies to toxin II from the scorpion Androctonus australis Hector: further characterization of epitope specificities and neutralizing capacities. Toxicon 1992, 30, 723–731. [Google Scholar] [CrossRef]
- Devaux, C.; Clot-Faybesse, O.; Juin, M.; Mabrouk, K.; Sabatier, J.M.; Rochat, H. Monoclonal antibodies neutralizing the toxin II from Androctonus australis hector scorpion venom: usefulness of a synthetic, non-toxic analog. FEBS Lett. 1997, 412, 456–460. [Google Scholar] [CrossRef]
- Clot-Faybesse, O.; Juin, M.; Rochat, H.; Devaux, C. Monoclonal antibodies against the Androctonus australis hector scorpion neurotoxin I: characterisation and use for venom neutralisation. FEBS Lett. 1999, 458, 313–318. [Google Scholar] [CrossRef]
- Devaux, C.; Clot-Faybesse, O.; Pugniere, M.; Mani, J.C.; Rochat, H.; Granier, C. A strategy for inducing an immune response against Androctonus australis scorpion venom toxin I in mice. Production of high-affinity monoclonal antibodies and their use in a sensitive two-site immunometric assay. J. Immunol. Methods 2002, 271, 37–46. [Google Scholar] [CrossRef]
- Mousli, M.; Devaux, C.; Rochat, H.; Goyffon, M.; Billiald, P. A recombinant single-chain antibody fragment that neutralizes toxin II from the venom of the scorpion Androctonus australis hector. FEBS Lett. 1999, 442, 183–188. [Google Scholar] [CrossRef]
- Aubrey, N.; Devaux, C.; Sizaret, P.Y.; Rochat, H.; Goyffon, M.; Billiald, P. Design and evaluation of a diabody to improve protection against a potent scorpion neurotoxin. Cell Mol. Life Sci. 2003, 60, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Juste, M.; Martin-Eauclaire, M.F.; Devaux, C.; Billiald, P.; Aubrey, N. Using a recombinant bispecific antibody to block Na+ -channel toxins protects against experimental scorpion envenoming. Cell Mol. Life Sci. 2007, 64, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Di Tommaso, A.; Juste, M.O.; Martin-Eauclaire, M.F.; Dimier-Poisson, I.; Billiald, P.; Aubrey, N. Diabody mixture providing full protection against experimental scorpion envenoming with crude Androctonus australis venom. J. Biol. Chem. 2012, 287, 14149–14156. [Google Scholar] [CrossRef] [PubMed]
- Meddeb-Mouelhi, F.; Bouhaouala-Zahar, B.; Benlasfar, Z.; Hammadi, M.; Mejri, T.; Moslah, M.; Karoui, H.; Khorchani, T.; El Ayeb, M. Immunized camel sera and derived immunoglobulin subclasses neutralizing Androctonus australis hector scorpion toxins. Toxicon 2003, 42, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Osteen, J.D.; Herzig, V.; Gilchrist, J.; Emrick, J.J.; Zhang, C.; Wang, X.; Castro, J.; Garcia-Caraballo, S.; Grundy, L.; Rychkov, G.Y.; et al. Selective spider toxins reveal a role for the Nav1.1 channel in mechanical pain. Nature 2016, 534, 494–499. [Google Scholar] [CrossRef]
- Abderrazek, R.B.; Hmila, I.; Vincke, C.; Benlasfar, Z.; Pellis, M.; Dabbek, H.; Saerens, D.; El Ayeb, M.; Muyldermans, S.; Bouhaouala-Zahar, B. Identification of potent nanobodies to neutralize the most poisonous polypeptide from scorpion venom. Biochem. J. 2009, 424, 263–272. [Google Scholar] [CrossRef]
- Ben Abderrazek, R.; Vincke, C.; Hmila, I.; Saerens, D.; Abidi, N.; El Ayeb, M.; Muyldermans, S.; Bouhaouala-Zahar, B. Development of Cys38 knock-out and humanized version of NbAahII10 nanobody with improved neutralization of AahII scorpion toxin. Protein Eng. Des. Sel. 2011, 24, 727–735. [Google Scholar] [CrossRef]
- Ksouri, A.; Ghedira, K.; Ben Abderrazek, R.; Shankar, B.A.G.; Benkahla, A.; Bishop, O.T.; Bouhaouala-Zahar, B. Homology modeling and docking of AahII-Nanobody complexes reveal the epitope binding site on AahII scorpion toxin. Biochem. Biophys. Res. Commun. 2018, 496, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Weitzner, B.D.; Jeliazkov, J.R.; Lyskov, S.; Marze, N.; Kuroda, D.; Frick, R.; Adolf-Bryfogle, J.; Biswas, N.; Dunbrack, R.L., Jr.; Gray, J.J. Modeling and docking of antibody structures with Rosetta. Nat. Protoc. 2017, 12, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Pierce, B.G.; Wiehe, K.; Hwang, H.; Kim, B.H.; Vreven, T.; Weng, Z. ZDOCK server: interactive docking prediction of protein-protein complexes and symmetric multimers. Bioinformatics 2014, 30, 1771–1773. [Google Scholar] [CrossRef] [PubMed]
- Hmila, I.; Saerens, D.; Ben Abderrazek, R.; Vincke, C.; Abidi, N.; Benlasfar, Z.; Govaert, J.; El Ayeb, M.; Bouhaouala-Zahar, B.; Muyldermans, S. A bispecific nanobody to provide full protection against lethal scorpion envenoming. FASEB J. 2010, 24, 3479–3489. [Google Scholar] [CrossRef] [PubMed]
- Hmila, I.; Cosyns, B.; Tounsi, H.; Roosens, B.; Caveliers, V.; Abderrazek, R.B.; Boubaker, S.; Muyldermans, S.; El Ayeb, M.; Bouhaouala-Zahar, B. Pre-clinical studies of toxin-specific Nanobodies: Evidence of in vivo efficacy to prevent fatal disturbances provoked by scorpion envenoming. Toxicol. Appl. Pharmacol. 2012, 264, 222–231. [Google Scholar] [CrossRef]
- Utkin, Y.N. Animal venom studies: Current benefits and future developments. World J. Biol. Chem. 2015, 6, 28. [Google Scholar] [CrossRef]
- Darvish, M.; Ebrahimi, S.A.; Shahbazzadeh, D.; Bagheri, K.-P.; Behdani, M.; Shokrgozar, M.A. Camelid antivenom development and potential in vivo neutralization of Hottentotta saulcyi scorpion venom. Toxicon 2016, 113, 70–75. [Google Scholar] [CrossRef]
- León, G.; Monge, M.; Rojas, E.; Lomonte, B.; Gutiérrez, J.M. Comparison between IgG and F (ab′) 2 polyvalent antivenoms: neutralization of systemic effects induced by Bothrops asper venom in mice, extravasation to muscle tissue, and potential for induction of adverse reactions. Toxicon 2001, 39, 793–801. [Google Scholar] [CrossRef]
- Alvarez-Rueda, N.; Behar, G.; Ferré, V.; Pugniere, M.; Roquet, F.; Gastinel, L.; Jacquot, C.; Aubry, J.; Baty, D.; Barbet, J. Generation of llama single-domain antibodies against methotrexate, a prototypical hapten. Mol. Immunol. 2007, 44, 1680–1690. [Google Scholar] [CrossRef]
- Bagheri, M.; Babaei, E.; Shahbazzadeh, D.; Habibi-Anbouhi, M.; Alirahimi, E.; Kazemi-Lomedasht, F.; Behdani, M. Development of a recombinant camelid specific diabody against the heminecrolysin fraction of Hemiscorpius lepturus scorpion. Toxin Rev. 2017, 36, 7–11. [Google Scholar] [CrossRef]
- Thalley, B.S.; Carroll, S.B. Rattlesnake and scorpion antivenoms from the egg yolks of immunized hens. Nat. Biotechnol. 1990, 8, 934. [Google Scholar] [CrossRef]
- Carroll, S.B.; Thalley, B.S.; Theakston, R.; Laing, G. Comparison of the purity and efficacy of affinity purified avian antivenoms with commercial equine crotalid antivenoms. Toxicon 1992, 30, 1017–1025. [Google Scholar] [CrossRef]
- Kiem, T. The production of Calloselasma rhodostoma antivenom (CRAV) from egg yolk of hens immunized with venom: its application for treatment of snake bite patients in Vietnam. In Proceedings of Proceedings of the XIIIth World Congress of the International Society of Toxinology, Paris, France, 18–22 September 2000. [Google Scholar]
- Narat, M. Production of antibodies in chickens. Food Technol. Biotechnol. 2003, 41, 259–267. [Google Scholar]
- Kovacs-Nolan, J.; Mine, Y. Egg yolk antibodies for passive immunity. Annu. Rev. Food Sci. Technol. 2012, 3, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Sifi, A.; Adi-Bessalem, S.; Laraba-Djebari, F. Development of a new approach of immunotherapy against scorpion envenoming: Avian IgYs an alternative to equine IgGs. Int. Immunopharmacol. 2018, 61, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Nouri, A.; Laraba-Djebari, F. Enhancement of long-lasting immunoprotective effect against Androctonus australis hector envenomation using safe antigens: Comparative role of MF59 and Alum adjuvants. Vaccine 2015, 33, 5756–5763. [Google Scholar] [CrossRef]
- Bachsais, N.; Boussag-Abib, L.; Laraba-Djebari, F. Assessment of Inflammatory response of developed vaccine against scorpion envenomation using attenuated venom. Med. Technol. J. 2018, 2, 224–225. [Google Scholar]
- Lila, B.-A.; Laraba-Djebari, F. Enhanced immune sera and vaccine: safe approach to treat scorpion envenoming. Vaccine 2011, 29, 8951–8959. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; O’Hagan, D. Advances in vaccine adjuvants. Nat. Biotechnol. 1999, 17, 1075. [Google Scholar] [CrossRef]
- Mohamed, F.A.N.; Laraba-Djebari, F. Development and characterization of a new carrier for vaccine delivery based on calcium-alginate nanoparticles: Safe immunoprotective approach against scorpion envenoming. Vaccine 2016, 34, 2692–2699. [Google Scholar] [CrossRef]
- Nait Mohamed, F.A.; Nouri, A.; Laraba-Djebari, F. Reactogenicity and safety assessment of an attenuated nanovaccine against scorpion envenomation: Preclinical study. Vaccine 2017, 35, 6657–6663. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin-Eauclaire, M.-F.; Adi-Bessalem, S.; Hammoudi-Triki, D.; Laraba-Djebari, F.; Bougis, P.E. Serotherapy against Voltage-Gated Sodium Channel-Targeting α-Toxins from Androctonus Scorpion Venom. Toxins 2019, 11, 63. https://doi.org/10.3390/toxins11020063
Martin-Eauclaire M-F, Adi-Bessalem S, Hammoudi-Triki D, Laraba-Djebari F, Bougis PE. Serotherapy against Voltage-Gated Sodium Channel-Targeting α-Toxins from Androctonus Scorpion Venom. Toxins. 2019; 11(2):63. https://doi.org/10.3390/toxins11020063
Chicago/Turabian StyleMartin-Eauclaire, Marie-France, Sonia Adi-Bessalem, Djelila Hammoudi-Triki, Fatima Laraba-Djebari, and Pierre E. Bougis. 2019. "Serotherapy against Voltage-Gated Sodium Channel-Targeting α-Toxins from Androctonus Scorpion Venom" Toxins 11, no. 2: 63. https://doi.org/10.3390/toxins11020063
APA StyleMartin-Eauclaire, M.-F., Adi-Bessalem, S., Hammoudi-Triki, D., Laraba-Djebari, F., & Bougis, P. E. (2019). Serotherapy against Voltage-Gated Sodium Channel-Targeting α-Toxins from Androctonus Scorpion Venom. Toxins, 11(2), 63. https://doi.org/10.3390/toxins11020063




