Pseudomonas putida Responds to the Toxin GraT by Inducing Ribosome Biogenesis Factors and Repressing TCA Cycle Enzymes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Whole-Cell Proteomic Response to GraT
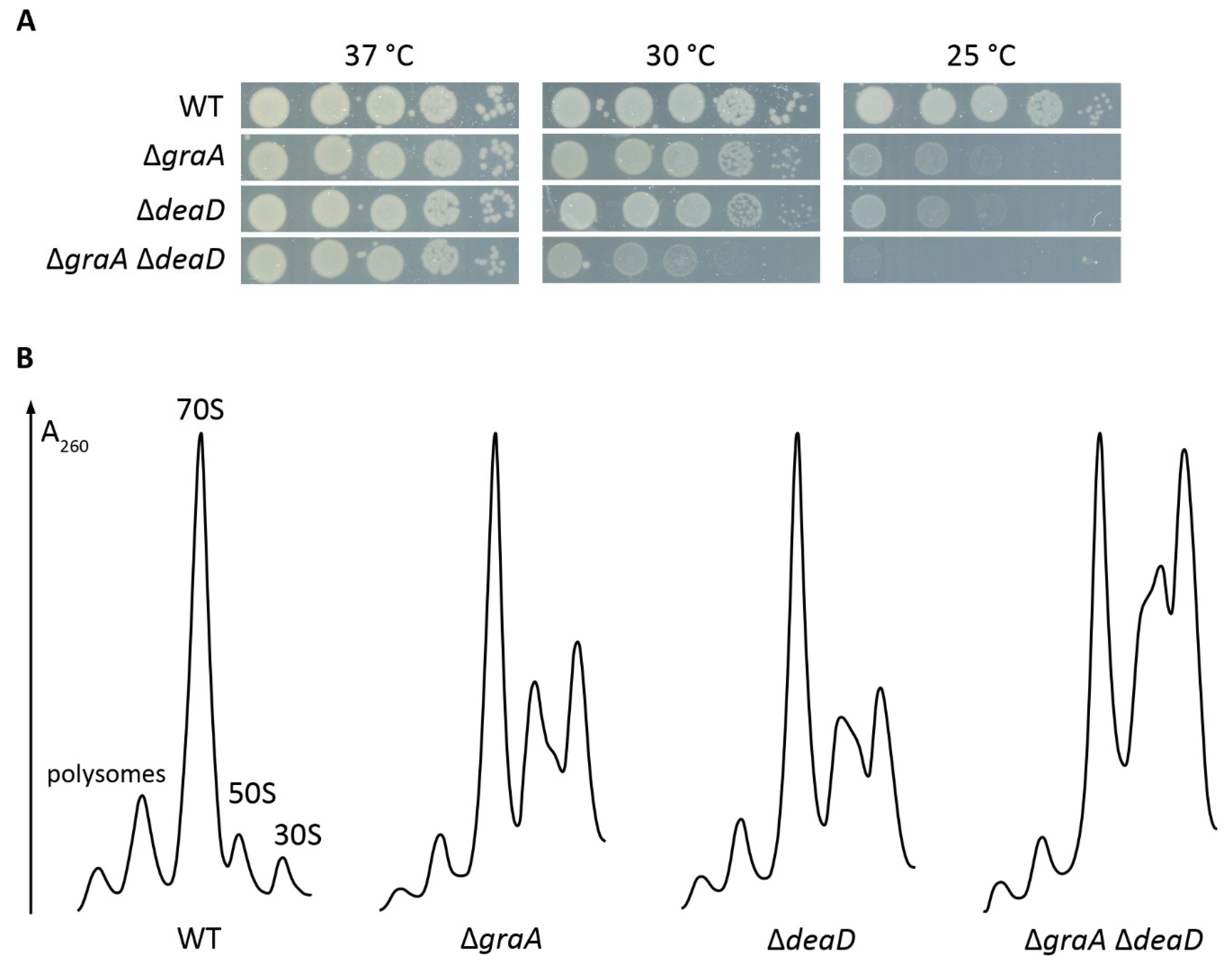
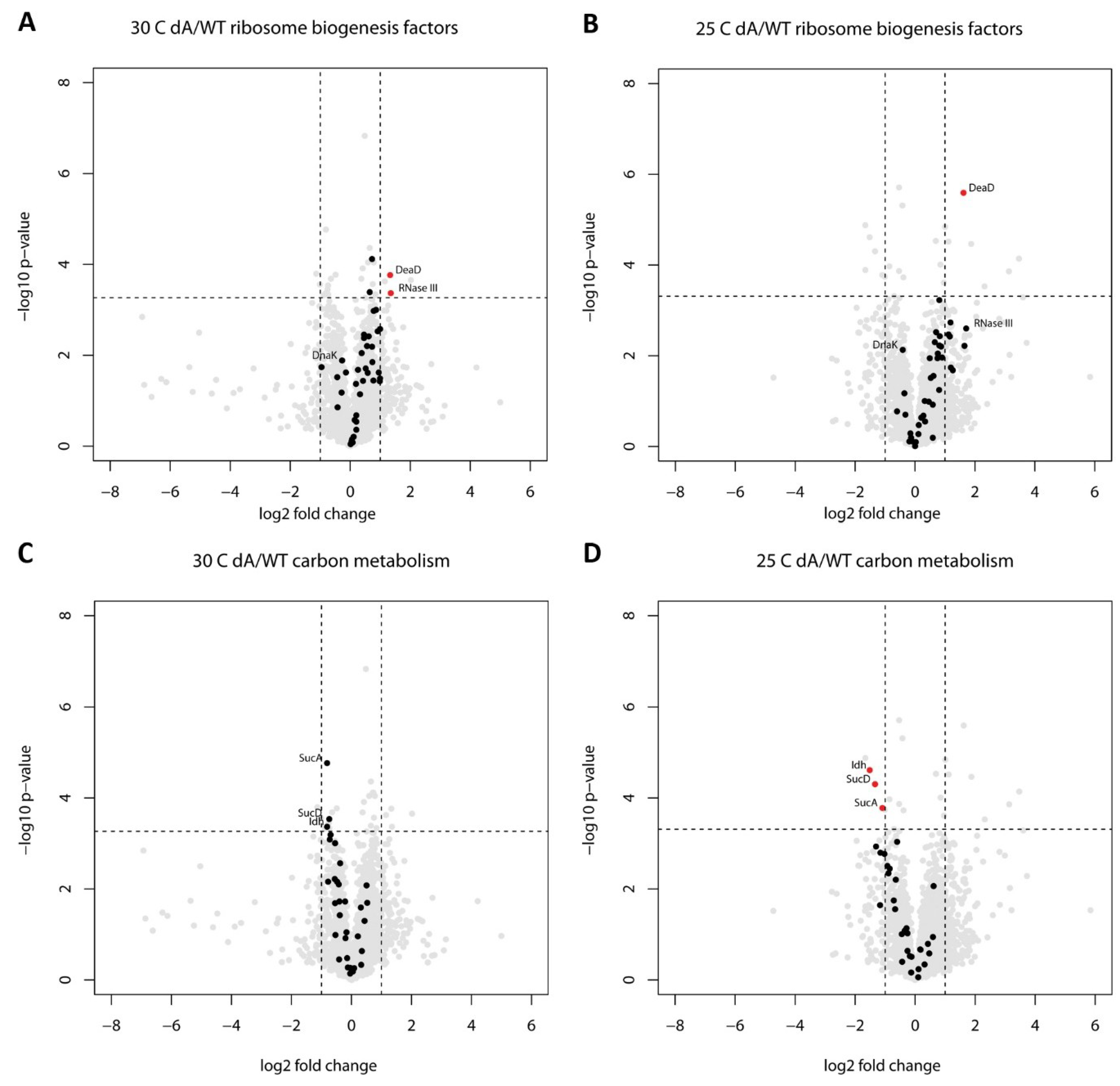
2.2. Proteome Changes Indicating the Cellular Response to the Ribosome Biogenesis Defect of the ΔgraA Strain
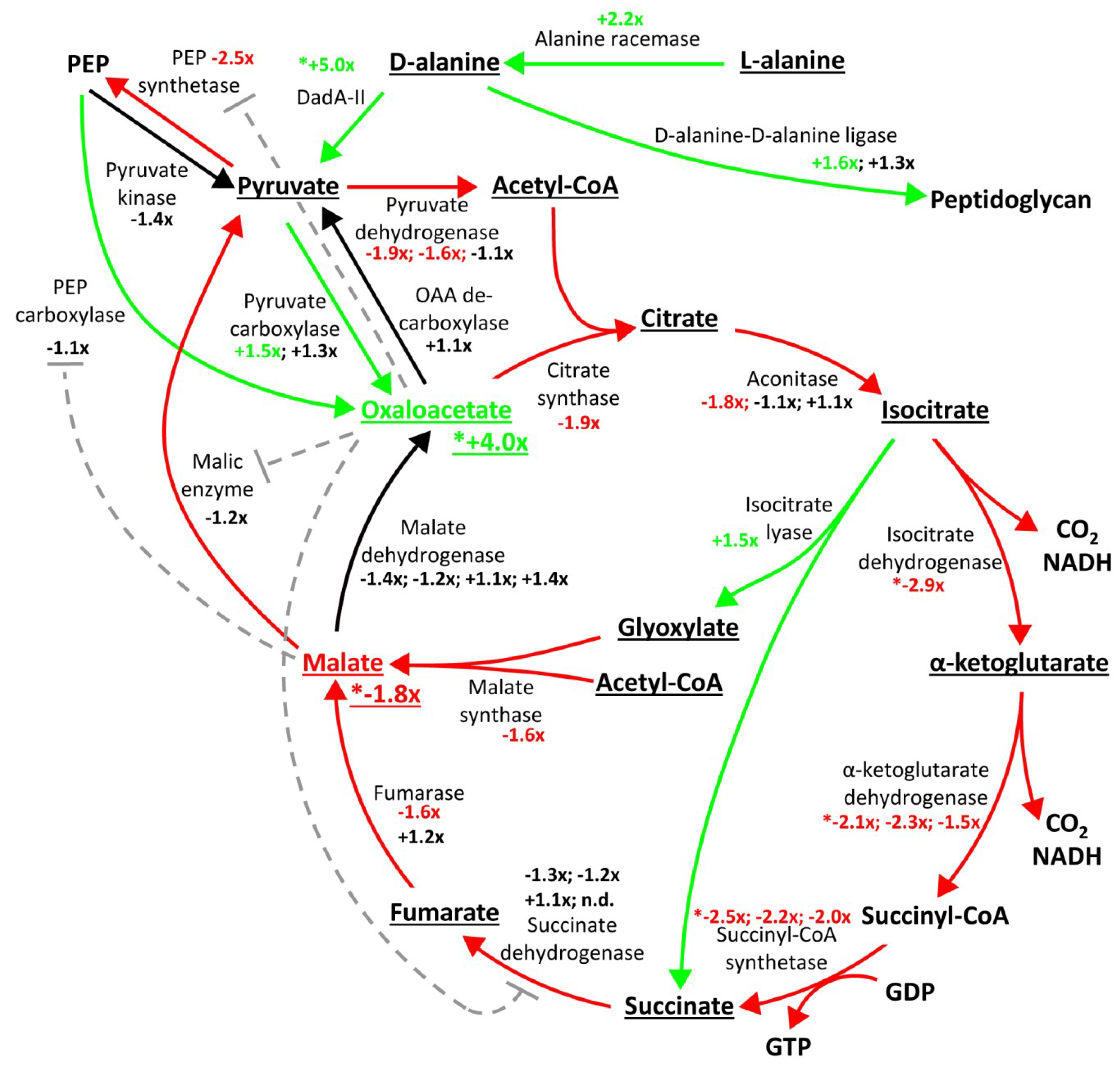
2.3. Proteome Changes Reveal Altered Carbon Metabolism of the ΔgraA Strain
2.4. Metabolomic Analysis Indicates Accumulation of Oxaloacetate in the ΔgraA Strain
3. Conclusions
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Construction of Plasmids and Strains
4.3. Proteomics
4.4. Metabolomics
4.5. Temperature Tolerance Plate Assay
4.6. Sucrose Gradient Profiles
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Song, S.; Wood, T.K. Post-segregational killing and phage inhibition are not mediated by cell death through toxin/antitoxin systems. Front. Microbiol. 2018, 9, 814. [Google Scholar] [CrossRef] [PubMed]
- Brzozowska, I.; Zielenkiewicz, U. Regulation of toxin-antitoxin systems by proteolysis. Plasmid 2013, 70, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.P.; Gerdes, K. Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res. 2005, 33, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Leplae, R.; Geeraerts, D.; Hallez, R.; Guglielmini, J.; Dreze, P.; Van Melderen, L. Diversity of bacterial type ii toxin-antitoxin systems: A comprehensive search and functional analysis of novel families. Nucleic Acids Res. 2011, 39, 5513–5525. [Google Scholar] [CrossRef] [PubMed]
- Van Melderen, L.; Saavedra De Bast, M. Bacterial toxin-antitoxin systems: More than selfish entities? PLoS Genet. 2009, 5, e1000437. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Bedzyk, L.A.; Thomas, S.M.; Ye, R.W.; Wood, T.K. Gene expression in Escherichia coli biofilms. Appl. Microbiol. Biotechnol. 2004, 64, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Kwan, B.W.; Lord, D.M.; Peti, W.; Page, R.; Benedik, M.J.; Wood, T.K. The Mqsr/Mqsa toxin/antitoxin system protects Escherichia coli during bile acid stress. Environ. Microbiol. 2015, 17, 3168–3181. [Google Scholar] [CrossRef]
- Wang, X.; Lord, D.M.; Hong, S.H.; Peti, W.; Benedik, M.J.; Page, R.; Wood, T.K. Type II toxin/antitoxin MqsR/MqsA controls type V toxin/antitoxin GhoT/GhoS. Environ. Microbiol. 2013, 15, 1734–1744. [Google Scholar] [CrossRef]
- Harms, A.; Fino, C.; Sørensen, M.A.; Semsey, S.; Gerdes, K. Prophages and growth dynamics confound experimental results with antibiotic-tolerant persister cells. mBio 2017, 8, e01964-17. [Google Scholar] [CrossRef]
- Goormaghtigh, F.; Fraikin, N.; Putrinš, M.; Hallaert, T.; Hauryliuk, V.; Garcia-Pino, A.; Sjödin, A.; Kasvandik, S.; Udekwu, K.; Tenson, T.; et al. Reassessing the role of type II toxin-antitoxin systems in formation of Escherichia coli type II persister cells. mBio 2018, 9, e00640-18. [Google Scholar] [CrossRef]
- Dörr, T.; Vulić, M.; Lewis, K. Ciprofloxacin causes persister formation by inducing the TisB toxin in Escherichia coli. PLOS Biol. 2010, 8, e1000317. [Google Scholar] [CrossRef] [PubMed]
- Helaine, S.; Cheverton, A.M.; Watson, K.G.; Faure, L.M.; Matthews, S.A.; Holden, D.W. Internalization of Salmonella by macrophages induces formation of nonreplicating persisters. Science 2014, 343, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, N.; Knapen, W.J.; Kint, C.I.; Liebens, V.; Van den Bergh, B.; Dewachter, L.; Michiels, J.E.; Fu, Q.; David, C.C.; Fierro, A.C.; et al. Obg and membrane depolarization are part of a microbial bet-hedging strategy that leads to antibiotic tolerance. Mol. Cell 2015, 59, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Rycroft, J.A.; Gollan, B.; Grabe, G.J.; Hall, A.; Cheverton, A.M.; Larrouy-Maumus, G.; Hare, S.A.; Helaine, S. Activity of acetyltransferase toxins involved in Salmonella persister formation during macrophage infection. Nat. Commun. 2018, 9, 1993. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Wood, T.K. Toxins Hha and CspD and small RNA regulator Hfq are involved in persister cell formation through MqsR in Escherichia coli. Biochem. Biophys. Res. Commun. 2010, 391, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Park, J.A.; Nagao, K.; Murayama, N.; Horiuchi, T. Control of segregation of chromosomal DNA by sex factor F in Escherichia coli: Mutants of DNA gyrase subunit a suppress letD (ccdB) product growth inhibition. J. Mol. Biol. 1992, 225, 39–52. [Google Scholar] [CrossRef]
- Yuan, J.; Sterckx, Y.; Mitchenall, L.A.; Maxwell, A.; Loris, R.; Waldor, M.K. Vibrio cholerae ParE2 poisons DNA gyrase via a mechanism distinct from other gyrase inhibitors. J. Biol. Chem. 2010, 285, 40397–40408. [Google Scholar] [CrossRef]
- Harms, A.; Stanger, F.V.; Scheu, P.D.; de Jong, I.G.; Goepfert, A.; Glatter, T.; Gerdes, K.; Schirmer, T.; Dehio, C. Adenylylation of gyrase and topo IV by FicT toxins disrupts bacterial DNA topology. Cell Rep. 2015, 12, 1497–1507. [Google Scholar] [CrossRef]
- Gerdes, K.; Bech, F.; Jørgensen, S.; Løbner-Olesen, A.; Rasmussen, P.; Atlung, T.; Boe, L.; Karlstrom, O.; Molin, S.; Meyenburg, K. Mechanism of postsegregational killing by the hok gene product of the parb system of plasmid R1 and its homology with the relF gene product of the E. coli relB operon. EMBO J. 1986, 5, 2023–2029. [Google Scholar] [CrossRef]
- Mutschler, H.; Gebhardt, M.; Shoeman, R.L.; Meinhart, A. A novel mechanism of programmed cell death in bacteria by toxin–antitoxin systems corrupts peptidoglycan synthesis. PLOS Biol. 2011, 9, e1001033. [Google Scholar] [CrossRef]
- Tan, Q.; Awano, N.; Inouye, M. YeeV is an Escherichia coli toxin that inhibits cell division by targeting the cytoskeleton proteins, FtsZ and MreB. Mol. Microbiol. 2011, 79, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Guglielmini, J.; Van Melderen, L. Bacterial toxin-antitoxin systems: Translation inhibitors everywhere. Mob. Genet. Elements 2011, 1, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Harms, A.; Brodersen, D.E.; Mitarai, N.; Gerdes, K. Toxins, targets, and triggers: An overview of toxin-antitoxin biology. Mol. Cell 2018, 70, 768–784. [Google Scholar] [CrossRef] [PubMed]
- Germain, E.; Castro-Roa, D.; Zenkin, N.; Gerdes, K. Molecular mechanism of bacterial persistence by HipA. Mol. Cell 2013, 52, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Kaspy, I.; Rotem, E.; Weiss, N.; Ronin, I.; Balaban, N.Q.; Glaser, G. HipA-mediated antibiotic persistence via phosphorylation of the glutamyl-tRNA-synthetase. Nat. Commun. 2013, 4, 3001. [Google Scholar] [CrossRef] [PubMed]
- Castro-Roa, D.; Garcia-Pino, A.; De Gieter, S.; van Nuland, N.A.; Loris, R.; Zenkin, N. The Fic protein Doc uses an inverted substrate to phosphorylate and inactivate EF-Tu. Nat. Chem. Biol. 2013, 9, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.W.; Rothenbacher, F.P.; Maehigashi, T.; Lane, W.S.; Dunham, C.M.; Woychik, N.A. Doc toxin is a kinase that inactivates elongation factor Tu. J. Biol. Chem. 2014, 289, 7788–7798. [Google Scholar] [CrossRef]
- Cheverton, A.M.; Gollan, B.; Przydacz, M.; Wong, C.T.; Mylona, A.; Hare, S.A.; Helaine, S. A Salmonella toxin promotes persister formation through acetylation of tRNA. Mol. Cell 2016, 63, 86–96. [Google Scholar] [CrossRef]
- Jurėnas, D.; Chatterjee, S.; Konijnenberg, A.; Sobott, F.; Droogmans, L.; Garcia-Pino, A.; Van Melderen, L. AtaT blocks translation initiation by N-acetylation of the initiator tRNA fMet. Nat. Chem. Biol. 2017, 13, 640. [Google Scholar] [CrossRef]
- Winther, K.S.; Gerdes, K. Enteric virulence associated protein VapC inhibits translation by cleavage of initiator tRNA. Proc. Natl. Acad. Sci. USA 2011, 108, 7403–7407. [Google Scholar] [CrossRef]
- Schifano, J.M.; Cruz, J.W.; Vvedenskaya, I.O.; Edifor, R.; Ouyang, M.; Husson, R.N.; Nickels, B.E.; Woychik, N.A. tRNA is a new target for cleavage by a MazF toxin. Nucleic Acids Res. 2016, 44, 1256–1270. [Google Scholar] [CrossRef] [PubMed]
- Mets, T.; Lippus, M.; Schryer, D.; Liiv, A.; Kasari, V.; Paier, A.; Maiväli, Ü.; Remme, J.; Tenson, T.; Kaldalu, N. Toxins MazF and MqsR cleave Escherichia coli rRNA precursors at multiple sites. RNA Biol. 2017, 14, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Culviner, P.H.; Laub, M.T. Global analysis of the E. coli toxin MazF reveals widespread cleavage of mRNA and the inhibition of rRNA maturation and ribosome biogenesis. Mol. Cell 2018. [Google Scholar] [CrossRef] [PubMed]
- Schifano, J.M.; Edifor, R.; Sharp, J.D.; Ouyang, M.; Konkimalla, A.; Husson, R.N.; Woychik, N.A. Mycobacterial toxin MazF-mt6 inhibits translation through cleavage of 23s rRNA at the ribosomal A site. Proc. Natl. Acad. Sci. USA 2013, 110, 8501–8506. [Google Scholar] [CrossRef] [PubMed]
- Winther, K.S.; Brodersen, D.E.; Brown, A.K.; Gerdes, K. VapC20 of Mycobacterium tuberculosis cleaves the sarcin-ricin loop of 23s rRNA. Nat. Commun. 2013, 4, 2796. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Hoeflich, K.P.; Ikura, M.; Qing, G.; Inouye, M. MazF cleaves cellular mRNAs specifically at ACA to block protein synthesis in Escherichia coli. Mol. Cell 2003, 12, 913–923. [Google Scholar] [CrossRef]
- Kawano, M.; Aravind, L.; Storz, G. An antisense RNA controls synthesis of an SOS-induced toxin evolved from an antitoxin. Mol. Microbiol. 2007, 64, 738–754. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Park, J.H.; Inouye, M. MqsR, a crucial regulator for quorum sensing and biofilm formation, is a GCU-specific mRNA interferase in Escherichia coli. J. Biol. Chem. 2009, 284, 28746–28753. [Google Scholar] [CrossRef]
- Short, F.L.; Pei, X.Y.; Blower, T.R.; Ong, S.-L.; Fineran, P.C.; Luisi, B.F.; Salmond, G.P.C. Selectivity and self-assembly in the control of a bacterial toxin by an antitoxic noncoding RNA pseudoknot. Proc. Natl. Acad. Sci. USA 2013, 110, E241. [Google Scholar] [CrossRef]
- Bibi-Triki, S.; Li de la Sierra-Gallay, I.; Lazar, N.; Leroy, A.; Van Tilbeurgh, H.; Sebbane, F.; Pradel, E. Functional and structural analysis of HicA3-HicB3, a novel toxin-antitoxin system of Yersinia pestis. J. Bacteriol. 2014, 196, 3712–3723. [Google Scholar] [CrossRef]
- Pedersen, K.; Zavialov, A.V.; Pavlov, M.Y.; Elf, J.; Gerdes, K.; Ehrenberg, M. The bacterial toxin RelE displays codon-specific cleavage of mRNAs in the ribosomal A site. Cell 2003, 112, 131–140. [Google Scholar] [CrossRef]
- Christensen-Dalsgaard, M.; Gerdes, K. Two higBA loci in the Vibrio cholerae superintegron encode mRNA cleaving enzymes and can stabilize plasmids. Mol. Microbiol. 2006, 62, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Christensen-Dalsgaard, M.; Gerdes, K. Translation affects YoeB and MazF messenger RNA interferase activities by different mechanisms. Nucleic Acids Res. 2008, 36, 6472–6481. [Google Scholar] [CrossRef] [PubMed]
- Prysak, M.H.; Mozdzierz, C.J.; Cook, A.M.; Zhu, L.; Zhang, Y.; Inouye, M.; Woychik, N.A. Bacterial toxin YafQ is an endoribonuclease that associates with the ribosome and blocks translation elongation through sequence-specific and frame-dependent mRNA cleavage. Mol. Microbiol. 2009, 71, 1071–1087. [Google Scholar] [CrossRef] [PubMed]
- Christensen-Dalsgaard, M.; Jorgensen, M.G.; Gerdes, K. Three new RelE-homologous mRNA interferases of Escherichia coli differentially induced by environmental stresses. Mol. Microbiol. 2010, 75, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Tamman, H.; Ainelo, A.; Ainsaar, K.; Hõrak, R. A moderate toxin, GraT, modulates growth rate and stress tolerance of Pseudomonas putida. J. Bacteriol. 2014, 196, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.M.; Woychik, N.A. Bacterial toxin HigB associates with ribosomes and mediates translation-dependent mRNA cleavage at A-rich sites. J. Biol. Chem. 2009, 284, 18605–18613. [Google Scholar] [CrossRef] [PubMed]
- Talavera, A.; Tamman, H.; Ainelo, A.; Konijnenberg, A.; Hadži, S.; Sobott, F.; Garcia-Pino, A.; Hõrak, R.; Loris, R. A dual role in regulation and toxicity for the disordered N-terminus of the toxin GraT. Nat. Commun. 2019. Accepted for publication. [Google Scholar]
- Ainelo, A.; Tamman, H.; Leppik, M.; Remme, J.; Hõrak, R. The toxin GraT inhibits ribosome biogenesis. Mol. Microbiol. 2016, 100, 719–734. [Google Scholar] [CrossRef]
- Schureck, M.A.; Maehigashi, T.; Miles, S.J.; Marquez, J.; Cho, S.E.; Erdman, R.; Dunham, C.M. Structure of the Proteus vulgaris HigB-(HigA)2-HigB toxin-antitoxin complex. J. Biol. Chem. 2014, 289, 1060–1070. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, K.; Liu, P.; Dong, Y.; Gao, Z.; Zhang, J.; Liu, Q. Structural insight into the E. coli HigBA complex. Biochem. Biophys. Res. Commun. 2016, 478, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Hadži, S.; Garcia-Pino, A.; Haesaerts, S.; Jurėnas, D.; Gerdes, K.; Lah, J.; Loris, R. Ribosome-dependent Vibrio cholerae mRNAse HigB2 is regulated by a β-strand sliding mechanism. Nucleic Acids Res. 2017, 45, 4972–4983. [Google Scholar] [CrossRef] [PubMed]
- Jahn, N.; Preis, H.; Wiedemann, C.; Brantl, S. BsrG/SR4 from Bacillus subtilis—The first temperature-dependent type I toxin–antitoxin system. Mol. Microbiol. 2012, 83, 579–598. [Google Scholar] [CrossRef] [PubMed]
- Rocker, A.; Meinhart, A. A cis-acting antitoxin domain within the chromosomal toxin-antitoxin module EzeT of Escherichia coli quenches toxin activity. Mol. Microbiol. 2015, 97, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Janssen, B.D.; Garza-Sánchez, F.; Hayes, C.S. YoeB toxin is activated during thermal stress. MicrobiologyOpen 2015, 4, 682–697. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; McAnulty, M.J.; Wood, T.K. Toxin YafQ reduces Escherichia coli growth at low temperatures. PLOS ONE 2016, 11, e0161577. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Ma, Q.; Zhou, C.; Chen, X.; Zhang, H.; Yang, J.; Mao, F.; Lai, W.; Xu, Y. DOOR 2.0: Presenting operons and their functions through dynamic and integrated views. Nucleic Acids Res. 2013, 42, D654–D659. [Google Scholar] [CrossRef]
- Peil, L.; Virumäe, K.; Remme, J. Ribosome assembly in Escherichia coli strains lacking the RNA helicase DeaD/CsdA or DbpA. FEBS J. 2008, 275, 3772–3782. [Google Scholar] [CrossRef]
- Jones, P.G.; Mitta, M.; Kim, Y.; Jiang, W.; Inouye, M. Cold shock induces a major ribosomal-associated protein that unwinds double-stranded RNA in Escherichia coli. Proc. Natl. Acad. Sci. USA 1996, 93, 76–80. [Google Scholar] [CrossRef]
- Charollais, J.; Dreyfus, M.; Iost, I. CsdA, a cold-shock RNA helicase from Escherichia coli, is involved in the biogenesis of 50S ribosomal subunit. Nucleic Acids Res. 2004, 32, 2751–2759. [Google Scholar] [CrossRef]
- Ginsburg, D.; Steitz, J.A. The 30 S ribosomal precursor RNA from Escherichia coli. A primary transcript containing 23 S, 16 S, and 5 S sequences. J. Biol. Chem. 1975, 250, 5647–5654. [Google Scholar] [PubMed]
- Inoue, K.; Alsina, J.; Chen, J.; Inouye, M. Suppression of defective ribosome assembly in a rbfa deletion mutant by overexpression of Era, an essential GTPase in Escherichia coli. Mol. Microbiol. 2003, 48, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.I.; Köhrer, C.; Davies, B.W.; RajBhandary, U.L.; Walker, G.C. Conserved bacterial rRNase YbeY plays key roles in 70S ribosome quality control and 16S rRNA maturation. Mol. Cell 2013, 49, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.A.; Gupta, N.; Denny, K.; Culver, G.M. Characterization of 16S rRNA processing with pre-30S subunit assembly intermediates from E. coli. J. Mol. Biol. 2018, 430, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, M.; Köhrer, C.; Shen, Y.; Proulx, S.; Ghosal, A.; Davies, B.W.; RajBhandary, U.L.; Walker, G.C. Identification of YbeY-protein interactions involved in 16S rRNA maturation and stress regulation in Escherichia coli. mBio 2016, 7, e01785-16. [Google Scholar] [CrossRef] [PubMed]
- Butland, G.; Peregrín-Alvarez, J.M.; Li, J.; Yang, W.; Yang, X.; Canadien, V.; Starostine, A.; Richards, D.; Beattie, B.; Krogan, N.; et al. Interaction network containing conserved and essential protein complexes in Escherichia coli. Nature 2005, 433, 531. [Google Scholar] [CrossRef] [PubMed]
- Shajani, Z.; Sykes, M.T.; Williamson, J.R. Assembly of bacterial ribosomes. Annu. Rev. Biochem. 2011, 80, 501–526. [Google Scholar] [CrossRef] [PubMed]
- Siibak, T.; Remme, J. Subribosomal particle analysis reveals the stages of bacterial ribosome assembly at which rRNA nucleotides are modified. RNA 2010, 16, 2023–2032. [Google Scholar] [CrossRef]
- Gibbs, M.R.; Fredrick, K. Roles of elusive translational GTPases come to light and inform on the process of ribosome biogenesis in bacteria. Mol. Microbiol. 2017, 107, 445–454. [Google Scholar] [CrossRef]
- Kikuchi, G.; Motokawa, Y.; Yoshida, T.; Hiraga, K. Glycine cleavage system: Reaction mechanism, physiological significance, and hyperglycinemia. Proc. Jpn. Acad. Ser. B 2008, 84, 246–263. [Google Scholar] [CrossRef]
- Ghrist, A.C.; Heil, G.; Stauffer, G.V. GcvR interacts with GcvA to inhibit activation of the Escherichia coli glycine cleavage operon. Microbiology 2001, 147, 2215–2221. [Google Scholar] [CrossRef] [PubMed]
- Heil, G.; Stauffer, L.T.; Stauffer, G.V. Glycine binds the transcriptional accessory protein GcvR to disrupt a GcvA/GcvR interaction and allow GcvA-mediated activation of the Escherichia coli gcvTHP operon. Microbiology 2002, 148, 2203–2214. [Google Scholar] [CrossRef] [PubMed]
- Rocchetta, H.L.; Pacan, J.C.; Lam, J.S. Synthesis of the A-band polysaccharide sugar D-rhamnose requires Rmd and WbpW: Identification of multiple AlgA homologues, WbpW and ORF488, in Pseudomonas aeruginosa. Mol. Microbiol. 1998, 29, 1419–1434. [Google Scholar] [CrossRef]
- King, J.D.; Poon, K.K.H.; Webb, N.A.; Anderson, E.M.; McNally, D.J.; Brisson, J.-R.; Messner, P.; Garavito, R.M.; Lam, J.S. The structural basis for catalytic function of GMD and RMD, two closely related enzymes from the GDP-d-rhamnose biosynthesis pathway. FEBS J. 2009, 276, 2686–2700. [Google Scholar] [CrossRef] [PubMed]
- Boulette, M.L.; Baynham, P.J.; Jorth, P.A.; Kukavica-Ibrulj, I.; Longoria, A.; Barrera, K.; Levesque, R.C.; Whiteley, M. Characterization of alanine catabolism in Pseudomonas aeruginosa and its importance for proliferation in vivo. J. Bacteriol. 2009, 191, 6329. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Li, C.; Lu, C.-D. Regulation and characterization of the dadRAX locus for D-amino acid catabolism in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2011, 193, 2107. [Google Scholar] [CrossRef]
- Walsh, C.T. Enzymes in the D-alanine branch of bacterial cell wall peptidoglycan assembly. J. Biol. Chem. 1989, 264, 2393–2396. [Google Scholar]
- Marschall, C.; Labrousse, V.; Kreimer, M.; Weichart, D.; Kolb, A.; Hengge-Aronis, R. Molecular analysis of the regulation of csiD, a carbon starvation-inducible gene in Escherichia coli that is exclusively dependent on σS and requires activation by cAMP-CR.P.1. J. Mol. Biol. 1998, 276, 339–353. [Google Scholar] [CrossRef]
- Chance, M.R.; Bresnick, A.R.; Burley, S.K.; Jiang, J.S.; Lima, C.D.; Sali, A.; Almo, S.C.; Bonanno, J.B.; Buglino, J.A.; Boulton, S.; et al. Structural genomics: A pipeline for providing structures for the biologist. Protein Sci. 2002, 11, 723–738. [Google Scholar] [CrossRef]
- Kalliri, E.; Mulrooney, S.B.; Hausinger, R.P. Identification of Escherichia coli YgaF as an L-2-hydroxyglutarate oxidase. J. Bacteriol. 2008, 190, 3793–3798. [Google Scholar] [CrossRef]
- Metzner, M.; Germer, J.; Hengge, R. Multiple stress signal integration in the regulation of the complex σS-dependent csiD-ygaF-gabDTP operon in Escherichia coli. Mol. Microbiol. 2004, 51, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, M.; Izui, K.; Taguchi, M.; Katsuki, H. Regulation of Escherichia coli phosphoenolpyruvate carboxylase by multiple effectors in vivo. J. Biochem. 1980, 87, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Sanwal, B.D.; Smando, R. Malic enzyme of Escherichia coli. Diversity of the effectors controlling enzyme activity. J. Biol. Chem. 1969, 244, 1817–1823. [Google Scholar] [PubMed]
- Chulavatnatol, M.; Atkinson, D.E. Phosphoenolpyruvate synthetase from Escherichia coli effects of adenylate energy charge and modifier concentrations. J. Biol. Chem. 1973, 248, 2712–2715. [Google Scholar] [PubMed]
- Molenaar, D.; van der Rest, M.E.; Drysch, A.; Yücel, R. Functions of the membrane-associated and cytoplasmic malate dehydrogenases in the citric acid cycle of Corynebacterium glutamicum. J. Bacteriol. 2000, 182, 6884–6891. [Google Scholar] [CrossRef] [PubMed]
- Riemer, S.A.; Rex, R.; Schomburg, D. A metabolite-centric view on flux distributions in genome-scale metabolic models. BMC Syst. Biol. 2013, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Bayley, S.A.; Duggleby, C.J.; Worsey, M.J.; Williams, P.A.; Hardy, K.G.; Broda, P. Two modes of loss of the Tol function from Pseudomonas putida mt-2. Mol. Gen. Genet. 1977, 154, 203–204. [Google Scholar] [CrossRef]
- Regenhardt, D.; Heuer, H.; Heim, S.; Fernandez, D.U.; Strompl, C.; Moore, E.R.; Timmis, K.N. Pedigree and taxonomic credentials of Pseudomonas putida strain KT2440. Environ. Microbiol. 2002, 4, 912–915. [Google Scholar] [CrossRef]
- Sharma, R.C.; Schimke, R.T. Preparation of electrocompetent E. coli using salt-free growth medium. Biotechniques 1996, 20, 42–44. [Google Scholar] [CrossRef]
- Martinez-Garcia, E.; de Lorenzo, V. Engineering multiple genomic deletions in gram-negative bacteria: Analysis of the multi-resistant antibiotic profile of Pseudomonas putida KT2440. Environ. Microbiol. 2011, 13, 2702–2716. [Google Scholar] [CrossRef]
- Wong, S.M.; Mekalanos, J.J. Genetic footprinting with mariner-based transposition in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2000, 97, 10191–10196. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Porosk, R.; Terasmaa, A.; Mahlapuu, R.; Soomets, U.; Kilk, K. Metabolomics of the wolfram syndrome 1 gene (Wfs1) deficient mice. OMICS 2017, 21, 721–732. [Google Scholar] [CrossRef] [PubMed]
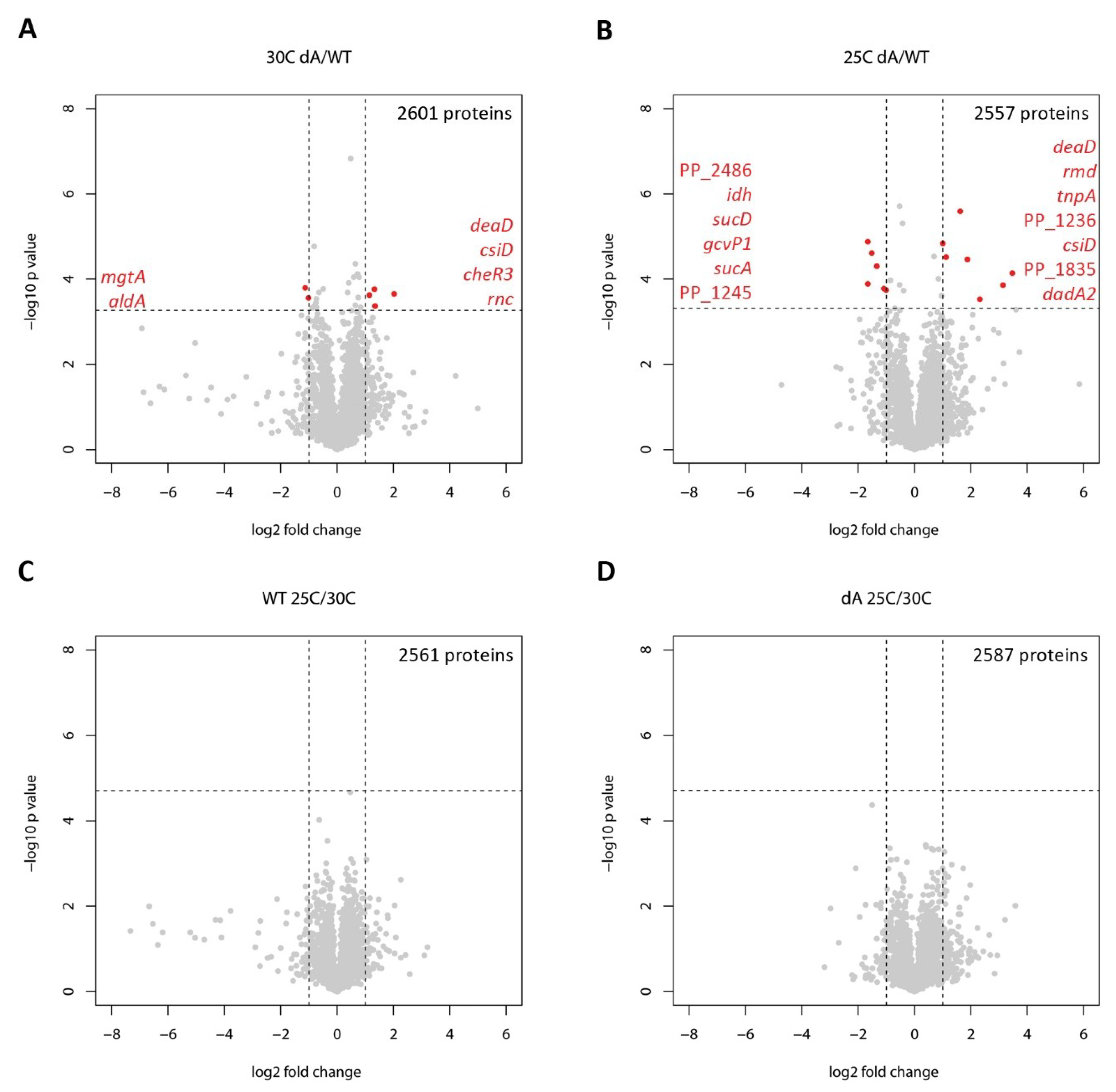
| ΔgraA vs. WT, 30 °C | ||||
|---|---|---|---|---|
| Locus | Gene | Protein | Fold Change * | p-Value |
| PP_2645 | mgtA | Magnesium transporter, ATP-dependent | −2.19 | 0.00016 |
| PP_2487 | aldA | Putative aldehyde dehydrogenase | −2.01 | 0.00028 |
| PP_3760 | cheR3 | Putative methyltransferase CheR3 | 2.23 | 0.00024 |
| PP_1868 | deaD | ATP-dependent RNA helicase DeaD | 2.52 | 0.00017 |
| PP_1433 | rnc | Ribonuclease 3 (RNase III) | 2.55 | 0.00043 |
| PP_2909 | csiD | Protein CsiD | 4.06 | 0.00022 |
| ΔgraA vs. WT, 25 °C | ||||
| PP_2486 | NADH-dependent flavin oxidoreductase, Oye family | −3.16 | 0.00001 | |
| PP_0988 | gcvP1 | Glycine dehydrogenase (decarboxylating) 1 | −3.16 | 0.00013 |
| PP_4012 | idh | Isocitrate dehydrogenase | −2.87 | 0.00002 |
| PP_4185 | sucD | Succinate--CoA ligase [ADP-forming] subunit alpha | −2.52 | 0.00005 |
| PP_4189 | sucA | 2-oxoglutarate decarboxylase, thiamine-requiring E1 subunit | −2.12 | 0.00017 |
| PP_1245 | Uncharacterized protein | −2.01 | 0.00018 | |
| PP_1800 | rmd | Oxidoreductase Rmd | 2.01 | 0.00001 |
| PP_0637 PP_4025 PP_4745 | tnpA | Transposase | 2.17 | 0.00003 |
| PP_1868 | deaD | ATP-dependent RNA helicase DeaD | 3.08 | 2.57E-06 |
| PP_1236 | Putative Glycine cleavage system transcriptional repressor | 3.68 | 0.00003 | |
| PP_5270 | dadA2 | D-amino acid dehydrogenase 2 | 5.01 | 0.0003 |
| PP_1835 | Uncharacterized protein | 8.79 | 0.00014 | |
| PP_2909 | csiD | Protein CsiD | 11.08 | 0.00007 |
| Strain or Plasmid | Genotype or Characteristics | Source or Reference |
|---|---|---|
| E. coli strains | ||
| DH5α λpir | λpir lysogen of DH5α | [90] |
| P. putida strains | ||
| PaW85 | Wild-type, isogenic to KT2440 | [87] |
| ΔgraA | graA is deleted from PaW85 | [46] |
| ΔdeaD | deaD (PP_1868) is deleted from PaW85 | This study |
| ΔgraA ΔdeaD | deaD is deleted from ΔgraA strain | This study |
| ΔcsiD | csiD (PP_2909) is deleted from PaW85 | This study |
| ΔgraA ΔcsiD | csiD is deleted from ΔgraA strain | This study |
| ΔlhgO | lhgO (PP_2910) is deleted from PaW85 | This study |
| ΔgraA ΔlhgO | lhgO is deleted from ΔgraA strain | This study |
| Plasmids | ||
| pEMG | Suicide plasmid containing lacZα with two flanking I-SceI sites (Kmr) | [90] |
| pSW(I-SceI) | For I-SceI endonuclease expression (Apr) | [91] |
| pEMG-ΔdeaD | pEMG with a PCR-designed 1180 bp KpnI-SacI insert for deleting the deaD gene (Kmr) | This study |
| pEMG-ΔcsiD | pEMG with a PCR-designed 1200 bp EcoRI-BamHI insert for deleting the csiD gene (Kmr) | This study |
| pEMG-ΔlhgO | pEMG with a PCR-designed 1158 bp EcoRI-BamHI insert for deleting the lhgO gene (Kmr) | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ainelo, A.; Porosk, R.; Kilk, K.; Rosendahl, S.; Remme, J.; Hõrak, R. Pseudomonas putida Responds to the Toxin GraT by Inducing Ribosome Biogenesis Factors and Repressing TCA Cycle Enzymes. Toxins 2019, 11, 103. https://doi.org/10.3390/toxins11020103
Ainelo A, Porosk R, Kilk K, Rosendahl S, Remme J, Hõrak R. Pseudomonas putida Responds to the Toxin GraT by Inducing Ribosome Biogenesis Factors and Repressing TCA Cycle Enzymes. Toxins. 2019; 11(2):103. https://doi.org/10.3390/toxins11020103
Chicago/Turabian StyleAinelo, Andres, Rando Porosk, Kalle Kilk, Sirli Rosendahl, Jaanus Remme, and Rita Hõrak. 2019. "Pseudomonas putida Responds to the Toxin GraT by Inducing Ribosome Biogenesis Factors and Repressing TCA Cycle Enzymes" Toxins 11, no. 2: 103. https://doi.org/10.3390/toxins11020103
APA StyleAinelo, A., Porosk, R., Kilk, K., Rosendahl, S., Remme, J., & Hõrak, R. (2019). Pseudomonas putida Responds to the Toxin GraT by Inducing Ribosome Biogenesis Factors and Repressing TCA Cycle Enzymes. Toxins, 11(2), 103. https://doi.org/10.3390/toxins11020103





