Ribosome-Inactivating Protein α-Momorcharin Derived from Edible Plant Momordica charantia Induces Inflammatory Responses by Activating the NF-kappaB and JNK Pathways
Abstract
1. Introduction
2. Results
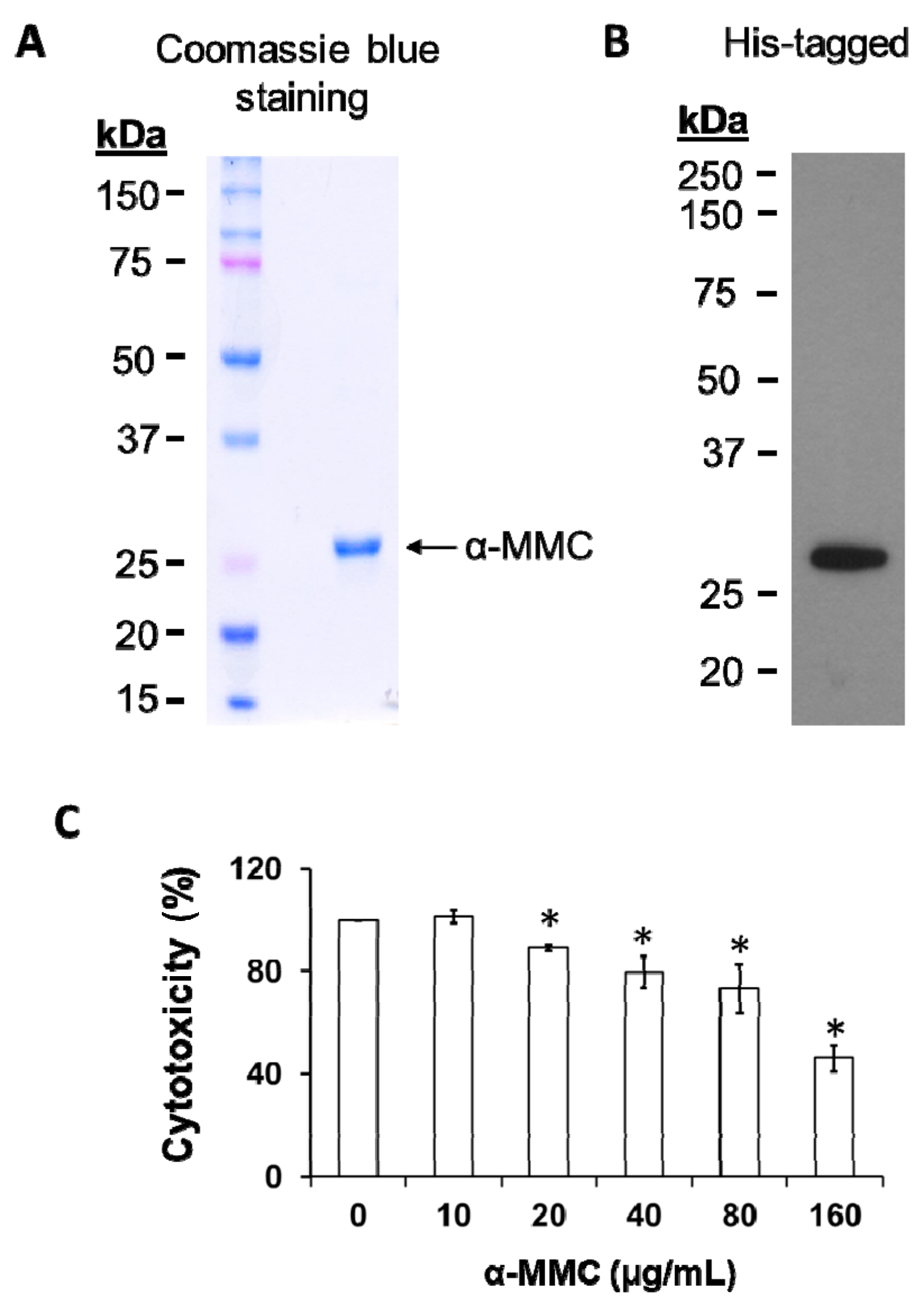
2.1. Heterologous Expression and Cytotoxicity of the Recombinant α-MMC
2.2. Microarray Analyses of α-MMC-Induced Inflammatory Responses
2.3. Recombinant α-MMC Induced Cytokine mRNAs Production and Cytokine Secretion in THP-1 Cells
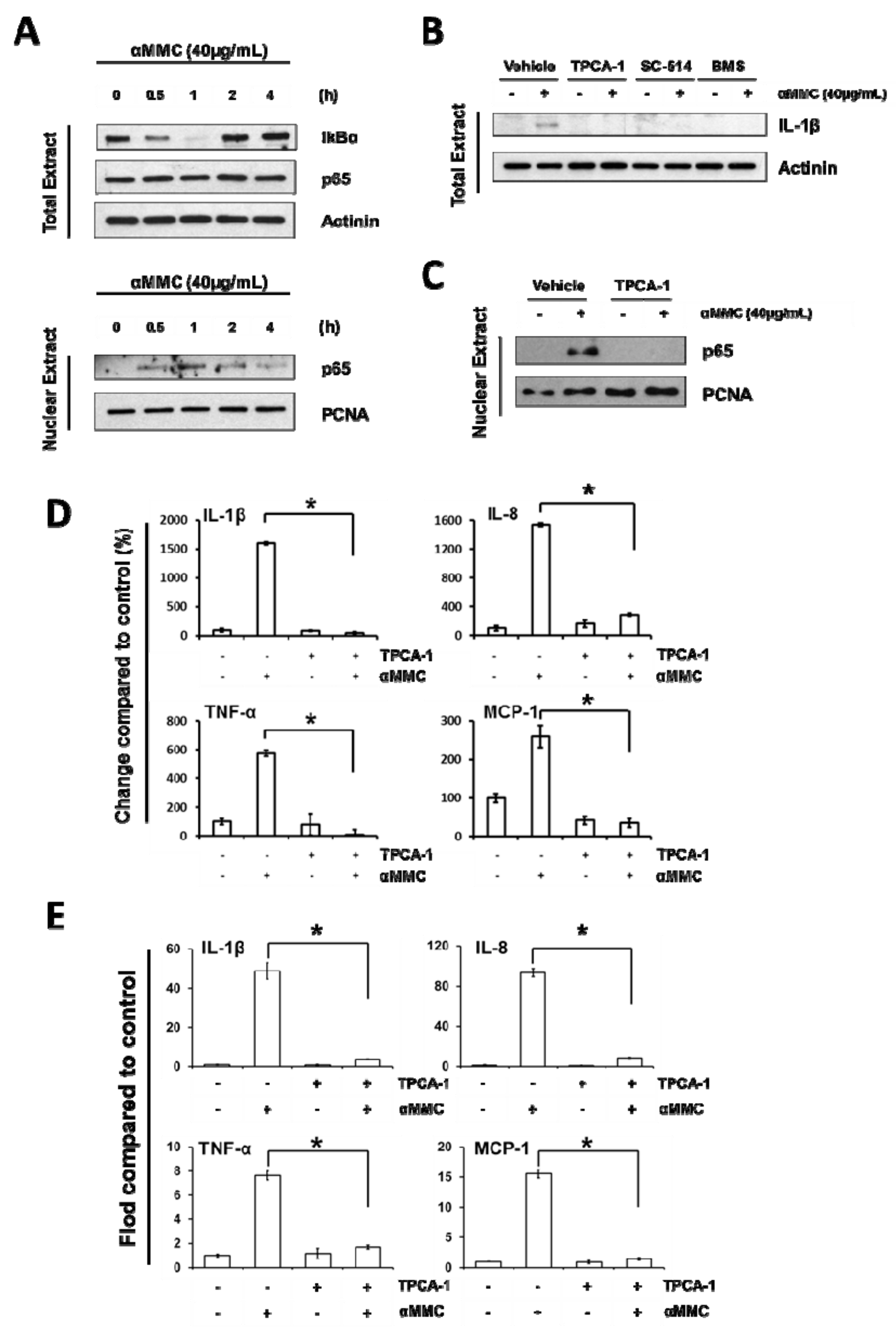
2.4. α-MMC-Induced Inflammatory Responses through NF-kappaB Pathway
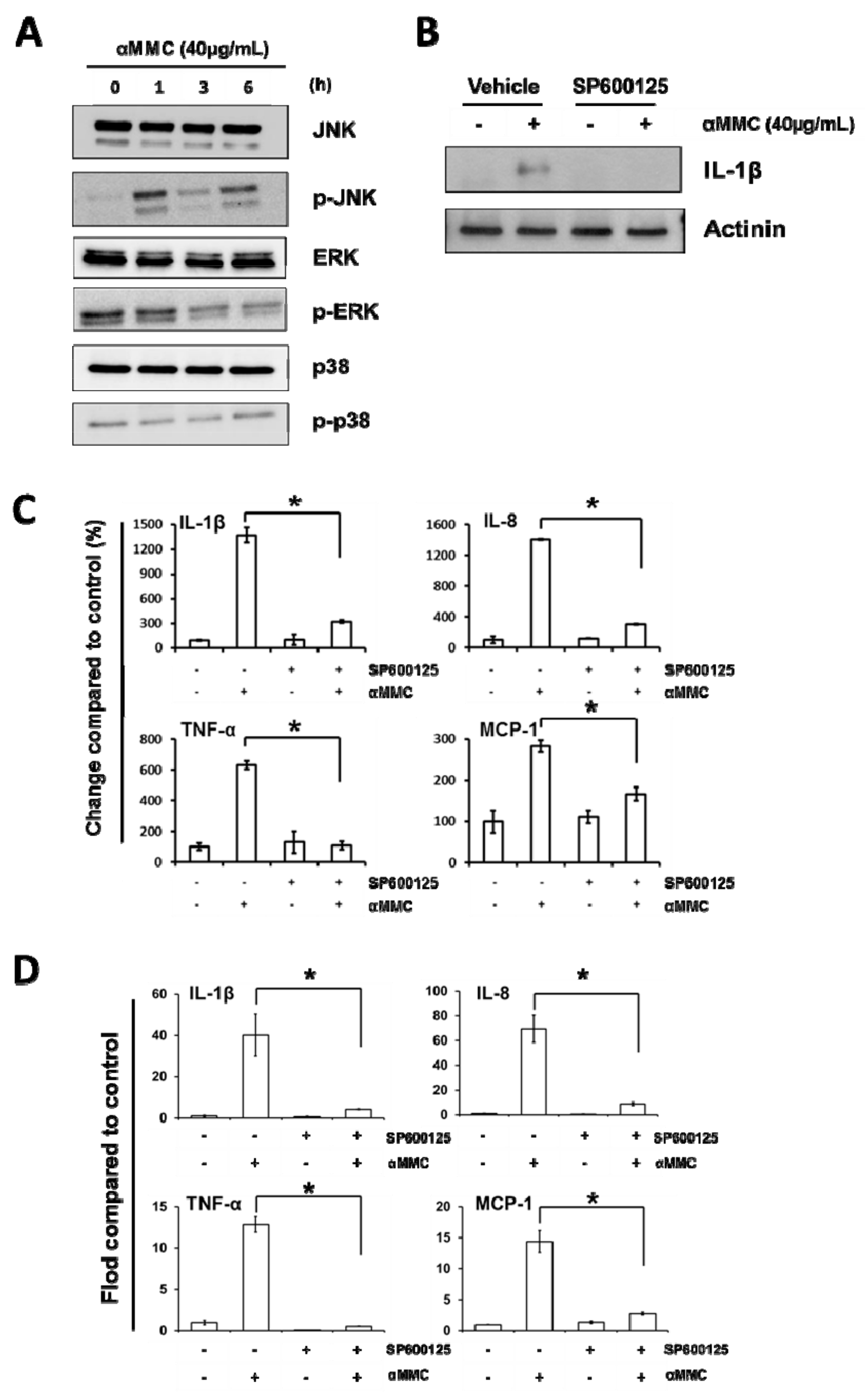
2.5. α-MMC-Induced Inflammatory Responses via JNK Pathway
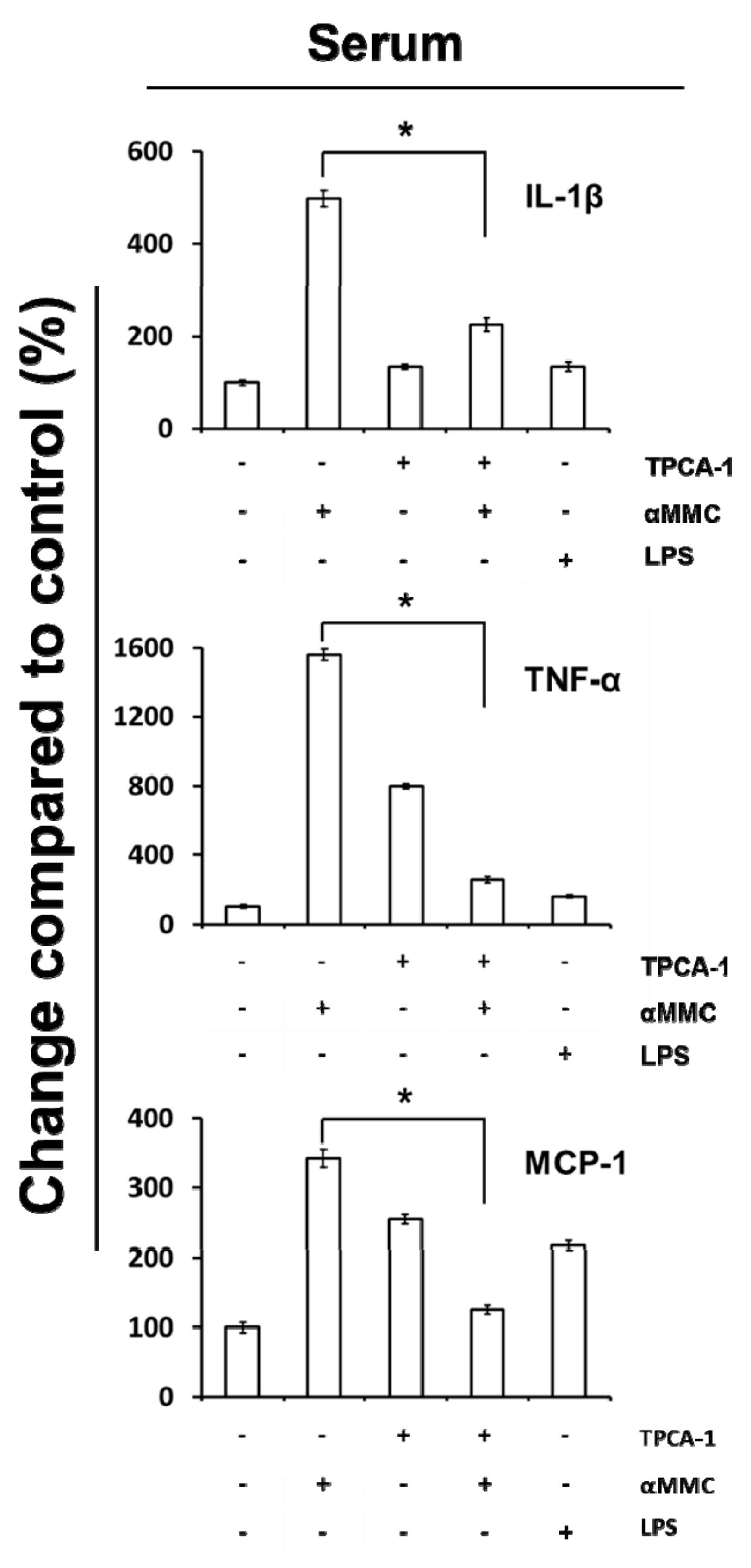
2.6. Secretion of IL-1β, TNF-α, and MCP-1 in the Serum of α-MMC-Administrated Mice
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Expression and Purification of Recombinant α-MMC
5.3. Cell Cultures
5.4. Cytotoxicity Assay
5.5. Enzyme-Linked Immunosorbent Assay (ELISA)
5.6. Reverse Transcription-PCR and Real-Time PCR (RT-PCR) Analysis
5.7. Western Blot Analysis
5.8. Animal Treatments
5.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Puri, M.; Kaur, I.; Perugini, M.A.; Gupta, R.C. Ribosome-inactivating proteins: Current status and biomedical applications. Drug Discov. Today 2012, 17, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Stirpe, F.; Battelli, M.G. Ribosome-inactivating proteins: Progress and problems. Cell. Mol. Life Sci. 2006, 63, 1850–1866. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, A.; Bortolotti, M.; Maiello, S.; Battelli, M.G.; Polito, L. Ribosome-Inactivating Proteins from Plants: A Historical Overview. Molecules 2016, 21, 1627. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, E.; Di Maro, A. A new age for biomedical applications of Ribosome Inactivating Proteins (RIPs): From bioconjugate to nanoconstructs. J. Biomed. Sci. 2016, 23, 54. [Google Scholar] [CrossRef]
- Fabbrini, M.S.; Katayama, M.; Nakase, I.; Vago, R. Plant Ribosome-Inactivating Proteins: Progesses, Challenges and Biotechnological Applications (and a Few Digressions). Toxins 2017, 9, 314. [Google Scholar] [CrossRef]
- Barbieri, L.; Battelli, M.G.; Stirpe, F. Ribosome-inactivating proteins from plants. Biochim. Biophys. Acta 1993, 1154, 237–282. [Google Scholar] [CrossRef]
- Hajto, T.; Hostanska, K.; Frei, K.; Rordorf, C.; Gabius, H.J. Increased secretion of tumor necrosis factors alpha, interleukin 1, and interleukin 6 by human mononuclear cells exposed to beta-galactoside-specific lectin from clinically applied mistletoe extract. Cancer Res. 1990, 50, 3322–3336. [Google Scholar]
- Licastro, F.; Morini, M.C.; Bolognesi, A.; Stirpe, F. Ricin induces the production of tumour necrosis factor-alpha and interleukin-1 beta by human peripheral-blood mononuclear cells. Biochem. J. 1993, 294, 517–520. [Google Scholar] [CrossRef]
- Yamasaki, C.; Nishikawa, K.; Zeng, X.T.; Katayama, Y.; Natori, Y.; Komatsu, N.; Oda, T.; Natori, Y. Induction of cytokines by toxins that have an identical RNA N-glycosidase activity: Shiga toxin, ricin, and modeccin. Biochim. Biophys. Acta 2004, 1671, 44–50. [Google Scholar] [CrossRef]
- Kim, D.; Yamasaki, Y.; Jiang, Z.; Nakayama, Y.; Yamanishi, T.; Yamaguchi, K.; Oda, T. Comparative study on modeccin- and phytohemagglutinin (PHA)-induced secretion of cytokines and nitric oxide (NO) in RAW264.7 cells. Acta Biochim. Biophys. Sin. (Shanghai) 2011, 43, 52–60. [Google Scholar] [CrossRef]
- Santana, S.S.; Gennari-Cardoso, M.L.; Carvalho, F.C.; Roque-Barreira, M.C.; Santiago Ada, S.; Alvim, F.C.; Pirovani, C.P. Eutirucallin, a RIP-2 type lectin from the latex of Euphorbia tirucalli L. presents proinflammatory properties. PLoS ONE 2014, 9, e88422. [Google Scholar] [CrossRef] [PubMed]
- Korcheva, V.; Wong, J.; Corless, C.; Iordanov, M.; Magun, B. Administration of ricin induces a severe inflammatory response via nonredundant stimulation of ERK, JNK, and P38 MAPK and provides a mouse model of hemolytic uremic syndrome. Am. J. Pathol. 2005, 166, 323–339. [Google Scholar] [CrossRef]
- Xu, W.; Hou, W.; Yao, G.; Ji, Y.; Yeh, M.; Sun, B. Inhibition of Th1- and enhancement of Th2-initiating cytokines and chemokines in trichosanthin- treated macrophages. Biochem. Biophys. Res. Commun. 2001, 284, 168–172. [Google Scholar] [CrossRef]
- Yang, C.H.; Ji, Y.Y.; Yeh, M. The kinetics of IL-4 and IFN-gamma gene expression in mice after Trichosansin immunization. Cell Res. 1998, 8, 295–302. [Google Scholar] [CrossRef]
- Huber, R.; Rostock, M.; Goedl, R.; Lüdtke, R.; Urech, K.; Buck, S.; Klein, R. Mistletoe treatment induces GM-CSF- and IL-5 production by PBMC and increases blood granulocyte- and eosinophil counts: A placebo controlled randomized study in healthy subjects. Eur. J. Med. Res. 2005, 10, 411–418. [Google Scholar]
- Liu, F.; Xia, Y.; Parker, A.S.; Verma, I.M. IKK biology. Immunol. Rev. 2012, 246, 239–253. [Google Scholar] [CrossRef]
- Gasperi-Campani, A.; Barbieri, L.; Battelli, M.G.; Stirpe, F. On the distribution of ribosome-inactivating proteins amongst plants. J. Nat. Prod. 1985, 48, 446–454. [Google Scholar] [CrossRef]
- Prestle, J.; Schönfelder, M.; Adam, G.; Mundry, K.W. Type 1 ribosome-inactivating proteins depurinate plant 25S rRNA without species specificity. Nucleic Acids Res. 1992, 20, 3179–3182. [Google Scholar] [CrossRef]
- Basch, E.; Gabardi, S.; Ulbricht, C. Bitter melon (Momordica charantia): A review of efficacy and safety. Am. J. Health Syst. Pharm. 2003, 60, 356–359. [Google Scholar] [CrossRef]
- Meng, Y.; Liu, B.; Lei, N.; Zheng, J.; He, Q.; Li, D.; Zhao, X.; Shen, F. Alpha-momorcharin possessing high immunogenicity, immunotoxicity and hepatotoxicity in SD rats. J. Ethnopharmacol. 2012, 139, 590–598. [Google Scholar] [CrossRef]
- Tsao, S.W.; Ng, T.B.; Yeung, H.W. Toxicities of trichosanthin and alpha-momorcharin, abortifacient proteins from Chinese medicinal plants, on cultured tumor cell lines. Toxicon 1990, 28, 1183–1192. [Google Scholar] [CrossRef]
- Ren, J.; Wang, Y.; Dong, Y.; Stuart, D.I. The N-glycosidase mechanism of ribosome-inactivating proteins implied by crystal structures of alpha-momorcharin. Structure 1994, 2, 7–16. [Google Scholar] [CrossRef]
- Mock, J.W.; Ng, T.B.; Wong, R.N.; Yao, Q.Z.; Yeung, H.W.; Fong, W.P. Demonstration of ribonuclease activity in the plant ribosome-inactivating proteins alpha- and beta-momorcharins. Life Sci. 1996, 59, 1853–1859. [Google Scholar] [CrossRef]
- Bian, X.; Shen, F.; Chen, Y.; Wang, B.; Deng, M.; Meng, Y. PEGylation of alpha-momorcharin: Synthesis and characterization of novel anti-tumor conjugates with therapeutic potential. Biotechnol. Lett. 2010, 32, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.C.; Lei, N.; He, Q.C.; Hu, W.; Jin, J.G.; Meng, Y.; Deng, N.H.; Meng, Y.F.; Zhang, C.J.; Shen, F.B. PEGylation is effective in reducing immunogenicity, immunotoxicity, and hepatotoxicity of α-momorcharin in vivo. Immunopharmacol. Immunotoxicol. 2012, 34, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Velloso, L.A.; Folli, F.; Saad, M.J. TLR4 at the crossroads of nutrients, gut microbiota, and metabolic inflammation. Endocr. Rev. 2015, 36, 245–271. [Google Scholar] [CrossRef]
- Li, M.; Chen, F.; Liu, C.P.; Li, D.M.; Li, X.; Wang, C.; Li, J.C. Dexamethasone enhances trichosanthin-induced apoptosis in the HepG2 hepatoma cell line. Life Sci. 2010, 86, 10–16. [Google Scholar] [CrossRef]
- Wang, Y.M.; Lu, T.L.; Hsu, P.N.; Tang, C.H.; Chen, J.H.; Liu, K.C.; Kao, J.T.; Tzen, J.T.; Wu, Y.Y. Ribosome inactivating protein B-chain induces osteoclast differentiation from monocyte/macrophage lineage precursor cells. Bone 2011, 48, 1336–1345. [Google Scholar] [CrossRef]
- Gonzalez, T.V.; Farrant, S.A.; Mantis, N.J. Ricin induces IL-8 secretion from human monocyte/macrophages by activating the p38 MAP kinase pathway. Mol. Immunol. 2006, 43, 1920–1923. [Google Scholar] [CrossRef]
- Higuchi, S.; Tamura, T.; Oda, T. Cross-talk between the pathways leading to the induction of apoptosis and the secretion of tumor necrosis factor-alpha in ricin-treated RAW 264.7 cells. J. Biochem. 2003, 134, 927–933. [Google Scholar] [CrossRef]
- Wang, L.; Shen, F.; Zhang, M.; He, Q.; Zhao, H.; Yu, X.; Yang, S.; Liu, Y.; Deng, N.; Zheng, J.; et al. Cytotoxicity mechanism of α-MMC in normal liver cells through LRP1 mediated endocytosis and JNK activation. Toxicology 2016, 357–358, 33–43. [Google Scholar] [CrossRef] [PubMed]
- De-la-Peña, C.; Badri, D.V.; Vivanco, J.M. Novel role for pectin methylesterase in Arabidopsis: A new function showing ribosome-inactivating protein (RIP) activity. Biochim. Biophys. Acta 2008, 1780, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Liu, H.; He, Y.; Yan, J.; Wu, Z.; Ding, Y. Molecular cloning and functional analysis of a recombinant ribosome-inactivating protein (alpha-momorcharin) from Momordica charantia. Appl. Microbiol. Biotechnol. 2012, 96, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, Y.; Yan, J.; Zhu, Z.; Wu, Z.; Ding, Y. Alpha-momorcharin: A ribosome-inactivating protein from Momordica charantia, possessing DNA cleavage properties. Protein Pept. Lett. 2013, 20, 1257–1263. [Google Scholar] [CrossRef]
- Qian, Q.; Huang, L.; Yi, R.; Wang, S.; Ding, Y. Enhanced resistance to blast fungus in rice (Oryza sativa L.) by expressing the ribosome-inactivating protein α-momorcharin. Plant Sci. 2014, 217–218, 1–7. [Google Scholar] [CrossRef]
- Pan, W.L.; Wong, J.H.; Fang, E.F.; Chan, Y.S.; Ng, T.B.; Cheung, R.C. Preferential cytotoxicity of the type I ribosome inactivating protein alpha-momorcharin on human nasopharyngeal carcinoma cells under normoxia and hypoxia. Biochem. Pharmacol. 2014, 89, 329–339. [Google Scholar] [CrossRef]
- Cao, D.; Sun, Y.; Wang, L.; He, Q.; Zheng, J.; Deng, F.; Deng, S.; Chang, S.; Yu, X.; Li, M.; et al. Alpha-momorcharin (α-MMC) exerts effective anti-human breast tumor activities but has a narrow therapeutic window in vivo. Fitoterapia 2015, 100, 139–149. [Google Scholar] [CrossRef]
- Byers, V.S.; Levin, A.S.; Malvino, A.; Waites, L.; Robins, R.A.; Baldwin, R.W. A phase II study of effect of addition of trichosanthin to zidovudine in patients with HIV disease and failing antiretroviral agents. AIDS Res. Hum. Retrovirus 1994, 10, 413–420. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.P.; Kahn, J.N.; Tumer, N.E. Functional Assays for Measuring the Catalytic Activity of Ribosome Inactivating Proteins. Toxins 2018, 10, 240. [Google Scholar] [CrossRef]
- Huber, R.; Rostock, M.; Goedl, R.; Lüdtke, R.; Urech, K.; Klein, R. Immunologic effects of mistletoe lectins: A placebo-controlled study in healthy subjects. J. Soc. Integr. Oncol. 2006, 4, 3–7. [Google Scholar]
- Aashaq, S.; Batool, A.; Andrabi, K.I. TAK1 mediates convergence of cellular signals for death and survival. Apoptosis 2019, 24, 3–20. [Google Scholar] [CrossRef]
- Fuchs, H. Dianthin and Its Potential in Targeted Tumor Therapies. Toxins 2019, 11, 592. [Google Scholar] [CrossRef]
- Deng, N.; Sun, Y.; Liu, M.; He, Q.; Wang, L.; Zhang, Y.; Sun, W.; Lei, N.; Liu, Y.; Luo, Y.; et al. Alpha-momorcharin regulates cytokine expression and induces apoptosis in monocytes. Immunopharmacol. Immunotoxicol. 2019, 41, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; Li, M.; Shen, D.; He, Q.; Sun, W.; Liu, M.; Liu, Y.; Zhou, Y.; Zheng, J.; Shen, F. LRP1 receptor-mediated immunosuppression of α-MMC on monocytes. Int. Immunopharmacol. 2019, 70, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Tse, A.K.; Cao, H.H.; Cheng, C.Y.; Kwan, H.Y.; Yu, H.; Fong, W.F.; Yu, Z.L. Indomethacin sensitizes TRAIL-resistant melanoma cells to TRAIL-induced apoptosis through ROS-mediated upregulation of death receptor 5 and downregulation of survivin. J. Investig. Dermatol. 2014, 134, 1397–1407. [Google Scholar] [CrossRef]
- Tse, A.K.; Wan, C.K.; Shen, X.L.; Yang, M.; Fong, W.F. Honokiol inhibits TNF-alpha-stimulated NF-kappaB activation and NF-kappaB-regulated gene expression through suppression of IKK activation. Biochem. Pharmacol. 2005, 70, 1443–1457. [Google Scholar] [CrossRef] [PubMed]

| Symbol | Upregulated Gene Expression | ||
|---|---|---|---|
| Gene Name | Fold Change (>2) | ||
| αMMC | LPS | ||
| BCL6 | B-cell CLL/lymphoma 6 | 4.42 | 8.02 |
| C3 | complement C3 | 13.58 | 15.79 |
| C3AR1 | complement C3a receptor 1 | 6.58 | 14.09 |
| CCL11 | C-C motif chemokine ligand 11 | N/A | 3.91 |
| CCL13 | C-C motif chemokine ligand 13 | 14.79 | 35.68 |
| CCL16 | C-C motif chemokine ligand 16 | N/A | 4.73 |
| CCL19 | C-C motif chemokine ligand 19 | N/A | 8.44 |
| CCL2 | C-C motif chemokine ligand 2 | 79.86 | 435.64 |
| CCL3 | C-C motif chemokine ligand 3 | 36.62 | 188.18 |
| CCL4 | C-C motif chemokine ligand 4 | 112.39 | 806.76 |
| CCL5 | C-C motif chemokine ligand 5 | N/A | 2.78 |
| CCL7 | C-C motif chemokine ligand 7 | 9.93 | 18.16 |
| CCL8 | C-C motif chemokine ligand 8 | 8.58 | 23.23 |
| CCR4 | C-C motif chemokine receptor 4 | N/A | 2.99 |
| CCR7 | C-C motif chemokine receptor 7 | 11.47 | 106.82 |
| CD14 | CD14 molecule | 3.98 | 22.67 |
| CD40 | CD40 molecule | 8.89 | 9.11 |
| CSF1 | colony stimulating factor 1 | N/A | 2.09 |
| CXCL1 | C-X-C motif chemokine ligand 1 | 27.92 | 55.1 |
| CXCL10 | C-X-C motif chemokine ligand 10 | 100.52 | 172.33 |
| CXCL2 | C-X-C motif chemokine ligand 2 | 29.89 | 57.36 |
| CXCL3 | C-X-C motif chemokine ligand 3 | 17.37 | 24.9 |
| CXCL6 | C-X-C motif chemokine ligand 6 | 6.41 | 9.84 |
| CXCL9 | C-X-C motif chemokine ligand 9 | 14.82 | 76.48 |
| FASLG | Fas ligand | 3.32 | N/A |
| IFNG | interferon gamma | 5.77 | 2.45 |
| IL10 | interleukin 10 | N/A | 7.81 |
| IL15 | interleukin 15 | 2.74 | 5.62 |
| IL17A | interleukin 17A | N/A | 2.45 |
| IL1A | interleukin 1 alpha | 4.14 | 5.8 |
| IL1B | interleukin 1 beta | 60.4 | 314.3 |
| IL1R1 | interleukin 1 receptor type 1 | N/A | 2.76 |
| IL1RN | interleukin 1 receptor antagonist | 3.37 | 8.5 |
| IL22 | interleukin 22 | 3.18 | 5.36 |
| IL23A | interleukin 23 subunit alpha | 8.46 | 39.64 |
| IL6 | interleukin 6 | 187.71 | 3140.98 |
| CXCL8 | C-X-C motif chemokine ligand 8 | 135.62 | 186.63 |
| IL9 | interleukin 9 | N/A | 4.19 |
| LTA | lymphotoxin alpha | 7.14 | 3.55 |
| LTB | lymphotoxin beta | 2.57 | 6.95 |
| LY96 | lymphocyte antigen 96 | 5.56 | 13.53 |
| MYD88 | myeloid differentiation primary response 88 | 2.79 | 3.14 |
| NFKB1 | nuclear factor kappa B subunit 1 | 4.65 | 4.14 |
| PTGS2 | prostaglandin-endoperoxide synthase 2 | 3.06 | 5.77 |
| RIPK2 | receptor interacting serine/threonine kinase 2 | N/A | 2.01 |
| SELE | selectin E | N/A | 8.25 |
| TLR1 | toll like receptor 1 | 5.38 | 5.75 |
| TLR3 | toll like receptor 3 | 4.24 | 8.76 |
| TLR6 | toll like receptor 6 | 3.81 | 3.34 |
| TLR7 | toll like receptor 7 | 4.6 | 6.17 |
| TNF | tumor necrosis factor | 3.73 | 12.58 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-J.; Zhu, J.-Q.; Fu, X.-Q.; Su, T.; Li, T.; Guo, H.; Zhu, P.-L.; Lee, S.K.-W.; Yu, H.; Tse, A.K.-W.; et al. Ribosome-Inactivating Protein α-Momorcharin Derived from Edible Plant Momordica charantia Induces Inflammatory Responses by Activating the NF-kappaB and JNK Pathways. Toxins 2019, 11, 694. https://doi.org/10.3390/toxins11120694
Chen Y-J, Zhu J-Q, Fu X-Q, Su T, Li T, Guo H, Zhu P-L, Lee SK-W, Yu H, Tse AK-W, et al. Ribosome-Inactivating Protein α-Momorcharin Derived from Edible Plant Momordica charantia Induces Inflammatory Responses by Activating the NF-kappaB and JNK Pathways. Toxins. 2019; 11(12):694. https://doi.org/10.3390/toxins11120694
Chicago/Turabian StyleChen, Ying-Jie, Jia-Qian Zhu, Xiu-Qiong Fu, Tao Su, Ting Li, Hui Guo, Pei-Li Zhu, Sally Kin-Wah Lee, Hua Yu, Anfernee Kai-Wing Tse, and et al. 2019. "Ribosome-Inactivating Protein α-Momorcharin Derived from Edible Plant Momordica charantia Induces Inflammatory Responses by Activating the NF-kappaB and JNK Pathways" Toxins 11, no. 12: 694. https://doi.org/10.3390/toxins11120694
APA StyleChen, Y.-J., Zhu, J.-Q., Fu, X.-Q., Su, T., Li, T., Guo, H., Zhu, P.-L., Lee, S. K.-W., Yu, H., Tse, A. K.-W., & Yu, Z.-L. (2019). Ribosome-Inactivating Protein α-Momorcharin Derived from Edible Plant Momordica charantia Induces Inflammatory Responses by Activating the NF-kappaB and JNK Pathways. Toxins, 11(12), 694. https://doi.org/10.3390/toxins11120694






