Equol: A Microbiota Metabolite Able to Alleviate the Negative Effects of Zearalenone during In Vitro Culture of Ovine Preantral Follicles
Abstract
1. Introduction
2. Results
2.1. Morphology and Density of Preantral Follicles
2.2. Nitrite and Malondialdehyde Levels in Culture Medium
2.3. Oestradiol Levels in Culture Medium
2.4. Quantitative RT-PCR
2.5. Immunohistochemistry
2.5.1. ABCG2 Protein Expression
2.5.2. H2AX Protein Expression
2.5.3. PCNA Protein Expression
3. Discussion
4. Materials and Methods
4.1. Test Compounds
4.2. Animal Ethics Statement
4.3. Ovarian Tissue Collection
4.4. Experimental Design
4.5. In Vitro Culture
4.6. Histological Analysis
4.7. Density of Preantral Follicles
4.8. Nitrite and Malondialdehyde Quantification in Culture Medium
4.9. Estradiol Dosage
4.10. Immunohistochemistry
4.11. Ribonucleic Acid (RNA) Extraction and Quantitative Real-Time PCR
4.12. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABCG2/BCRP | ATP-binding cassette, subfamily G, member 2/breast cancer resistance protein |
| ACTB | Beta actin |
| ANOVA | Analysis of variance |
| BSA | Bovine serum albumin |
| cDNA | Complementary deoxyriboNucleic Acid |
| CX37 | Connexin 37 |
| CX43 | Connexin 43 |
| DAB | 3,3’-diaminobenzidine in chromogenic solution |
| DMSO | Dimethyl sulfoxide |
| DNA | DeoxyriboNucleic Acid |
| E2 | 17-β-oestradiol |
| ELFA | Enzyme-linked fluorescent assay |
| ER-α | Oestrogen receptor alpha |
| ER-β | Oestrogen receptor beta |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| H2AX | H2A histone family member X |
| HEPES | N-2-hydroxyethylpiperazine-N-ethanesulfonic acid |
| H2O2 | Hydrogen peroxide |
| H3PO4 | Phosphoric acid |
| IgG | Immunoglobulin G |
| IPEC-J2 | Intestinal Porcine Epithelial Cell line-J2 |
| LSD | Least significant difference |
| MA-10 Leydig cells | Mouse Leydig tumor cell line |
| MCF-7 | Breast cancer cells (Michigan Cancer Foundation-7) |
| MDA | Malondialdehyde |
| MEM | Minimum essential medium |
| PAS | Periodic Acid-Schiff |
| PCNA | Proliferating cell nuclear antigen |
| PHS | Prostaglandin H synthase |
| PI3K/Akt/mTOR | Phosphatidylinositol 3-kinase/serine-threonine protein kinase/mammalian target of rapamycin |
| P38/MAPK | p38 mitogen-activated protein kinase |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| RNA | Ribonucleic acid |
| TBARS | Thiobarbituric acid reactive substances |
| ZEN | Zearalenone |
References
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, L.; Waskiewicz, A.; Beszterda, M.; Kostecki, M.; Dabrowksi, M.; Obremski, K.; Golinski, P.; Gajecki, M. Zearalenone in the intestinal tissues of immature gilts exposed per os to mycotoxins. Toxins 2015, 7, 3210–3223. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risks for public health related to the presence of zearalenone in food. EFSA J. 2011, 9, 2197. [Google Scholar] [CrossRef]
- Mauro, T.; Hao, L.; Pop, L.C.; Buckley, B.; Schneider, S.H.; Bandera, E.V.; Shapses, S.A. Circulating zearalenone and its metabolites differ in women due to body mass index and food intake. Food Chem. Toxicol. 2018, 116, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; Dell’Aquila, M.E. Zearalenone and reproductive function in farm animals. Int. J. Mol. Sci. 2008, 9, 2570–2584. [Google Scholar] [CrossRef] [PubMed]
- Sambuu, R.; Takagi, M.; Namula, Z.; Otoi, T.; Shiga, S.; Rodrigues dos Santos, R.; Fink-Gremmels, J. Effects of exposure to zearalenone on porcine oocytes and sperm during maturation and fertilization in vitro. J. Reprod. Dev. 2011, 57, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Schoevers, E.J.; Santos, R.R.; Colenbrander, B.; Fink-Gremmels, J.; Roelen, B.A. Transgenerational toxicity of zearalenone in pigs. Reprod. Toxicol. 2012, 34, 110–119. [Google Scholar] [CrossRef]
- Sambuu, R.; Takagi, M.; Namula, Z.; Nii, M.; Taniguchi, M.; Uno, S.; Kokushi, E.; Tshering, C.; dos Santos, R.R.; Fink-Gremmels, J.; et al. Effects of long-term in vitro exposure of ejaculated boar sperm to zearalenone and α-zearalenol in sperm liquid storage medium. Anim. Sci. J. 2013, 84, 28–34. [Google Scholar] [CrossRef]
- Gallo, A.; Giuberti, G.; Frisvad, J.C.; Bertuzzi, T.; Nielsen, K.F. Review on mycotoxin issues in ruminants: Occurrence in forages, effects of mycotoxin ingestion on health status and animal performance and practical strategies to counteract their negative effects. Toxins 2015, 7, 3057–3111. [Google Scholar] [CrossRef]
- Santos, R.R.; Schoevers, E.J.; Roelen, B.A.J.; Fink-Gremmels, J. Mycotoxins and female reproduction: In vitro approaches. World Mycot. J. 2013, 6, 245–253. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, B.; Li, X.; Wang, T.; Zou, H.; Gu, J.; Yuan, Y.; Liu, X.; Bai, J.; Bian, J.; et al. Zearalenone promotes cell proliferation or causes cell death? Toxins 2018, 10, 184. [Google Scholar] [CrossRef] [PubMed]
- Filannino, A.; Sout, T.A.E.; Gadella, B.M.; Sostaric, E.; Pizzi, F.; Colenbrander, B.; Dell’Aquila, M.E.; Minervini, F. Dose-response effects of estrogenic mycotoxins (zearalenone, alpha- and beta-zearalenol) on motility, hyperactivation and the acrosome reaction of stallion sperm. Reprod. Biol. Endocrinol. 2001, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Malekinejad, H.; Schoevers, E.J.; Daemen, I.J.; Zijlstra, C.; Colenbrander, B.; Fink-Gremmels, J.; Roelen, B.A.J. Exposure of oocytes to the Fusarium toxins zearalenone and deoxynivalenol causes aneuploidy and abnormal embryo development in pigs. Biol. Reprod. 2007, 77, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, I.M.C.M.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Phasmacol. 2017, 174, 1263–1280. [Google Scholar] [CrossRef]
- Fleck, S.C.; Churchwell, M.I.; Doerge, D.R.; Teeguarden, J.G. Urine and serum biomonitoring of exposure to environmental estrogen II: Soy isoflavones and zearalenone in pregnant women. Food Chem. Toxicol. 2016, 95, 19–27. [Google Scholar] [CrossRef]
- Vejdovszky, K.; Schmidt, V.; Warth, B.; Marko, D. Combinatory estrogenic effects between the isoflavones genistein and the mycotoxins zearalenone and alternariol in vitro. Mol. Nutry. Food Res. 2017, 61, 1600526. [Google Scholar] [CrossRef]
- Mayo, B.; Vasquez, L.; Florez, A.B. Equol: A bacterial metabolite from the daidzein isoflavone and its presumed beneficial health effects. Nutrients 2019, 11, 2231. [Google Scholar] [CrossRef]
- Gu, L.; House, S.E.; Prior, R.L.; Fang, N.; Ronis, M.J.; Clarkson, T.B.; Wilson, M.E.; Badger, T.M. Metabolic phenotype of isoflavonces differ among female rats, pigs, monkeys, and women. J. Nutr. 2006, 136, 1215–1221. [Google Scholar] [CrossRef]
- Schwen, R.J.; Nguyen, L.; Jackson, R.L. Elucidation of the metabolic pathway of S-equol in rat, monkey and man. Food Chem. Toxicol. 2012, 50, 2074–2083. [Google Scholar] [CrossRef]
- Kuhn, G.; Hennig, U.; Kalbe, C.; Rehfeldt, C.; Ren, M.Q.; Moors, S.; Degen, G.H. Growth performance, carcass characteristics and bioavailability of isoflavones in pigs fed soy bean based diets. Arch. Anim. Nutr. 2004, 58, 265–276. [Google Scholar] [CrossRef]
- Farmer, C.; Robertson, P.; Xiao, C.; Rehfeldt, C.; Kalbe, C. Exogenous genistein in late gestation: Effects on fetal development and sow and piglet performance. Animal 2016, 10, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Schutt, D.A.; Braden, W.H. The significance of equol in relation to the oestrogenic responses in sheep ingesting clover with a high formononetin content. Aust. J. Agric. Res. 1968, 19, 545–553. [Google Scholar] [CrossRef]
- Van der Velpen, V.; Hollman, P.C.; van Nielen, M.; Schouten, E.G.; Mensink, M.; Van’t Veer, P.; Geelen, A. Large inter-individual variation in isoflavone plasma concentration limits use of isoflavone intake data for risk assessment. Eur. J. Clin. Nutr. 2014, 68, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Pazourekova, S.; Lucova, M.; Nosal, R.; Drabikova, K.; Harmatha, J.; Smidrkal, J.; Jancinova, V. Equol effectively inhibity toxic activity of human neutrophils without influencing their viability. Pharmacology 2016, 97, 138–145. [Google Scholar] [CrossRef]
- Degen, G.H.; Blaich, G.; Metzler, M. Multiple pathways for the oxitadive metabolism of estrogens in the Syrian hamster and rabbit kidney. J. Biochem. Toxicol. 1990, 5, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Rigalli, J.P.; Scholz, P.N.; Tocchetti, G.N.; Ruiz, M.L.; Weiss, J. The phytoestrogens daidzein and equol inhibit the drug transporter BCRP/ABCG2 in breast cancer cells: Potential chemosensitizing effect. Eur. J. Nutr. 2019, 58, 139–150. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, Q.; Bircsak, K.M.; Wen, X.; Aleksunes, L.M. In vitro screening of environmental chemicals identifies zearalenone as a novel substrate of the plancental BCRP/ABCG2 transporter. Toxicol. Res. 2015, 4, 695–706. [Google Scholar] [CrossRef]
- Cao, H.; Zhi, Y.; Xu, H.; Fang, H.; Jia, X. Zearalenone causes embryotoxicity and induces oxidative stress and apoptosis in differentiated human embryonic stem cells. Toxicol. In Vitro 2019, 54, 243–250. [Google Scholar] [CrossRef]
- Eze, U.A.; Huntriss, J.D.; Routledge, M.N.; Gong, Y.Y. In vitro effects of single and binary mixtures of regulated mycotoxins and persistent organochloride pesticides on steroid hormone production in MA-10 Leydig cell line. Toxicol. In Vitro 2019, 60, 272–280. [Google Scholar] [CrossRef]
- Cai, G.; Si, M.; Li, X.; Zou, H.; Gu, J.; Yuan, Y.; Liu, X.; Liu, Z.; Bian, J. Zearalenone induces apoptosis of rat Sertoli cells through Fas-Fas ligand and mitochondrial pathway. Environ. Toxicol. 2019, 34, 424–433. [Google Scholar] [CrossRef]
- Zhang, G.L.; Song, J.L.; Zhou, Y.; Zhang, R.Q.; Cheng, S.F.; Sun, X.F.; Qin, G.Q.; Shen, W.; Li, L. Differentiation of sow and mouse ovarian granulosa cells exposed to zearalenone in vitro using RNA-seq gene expression. Toxicol. Appl. Pharmacol. 2018, 350, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Takagi, M.; Mukai, S.; Kurivagawa, T.; Takagaki, K.; Uno, S.; Kokushi, E.; Otoi, T.; Budivanto, A.; Shirasuna, K.; Miyamoto, A.; et al. Detection of zearalenone and its metabolites in naturally contaminated follicular fluids by using LC/MS/MS and in in vitro effects of zearalenone on oocyte maturation in cattle. Reprod. Toxicol. 2008, 26, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, K.H.; Sun, M.H.; Lan, M.; Wan, X.; Zhang, Y.; Sun, S.C. Protective effects of melatonin against zearalenone toxicity on porcine embryos in vitro. Front. Pharmacol. 2019, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Pajewska, M.; Lojko, M.; Cendrowski, K.; Sawicki, W.; Kowalkowski, T.; Buszewski, B.; Gadzala-Kopciuch, R. The determination of zearalenone and ist major metabolites in endometrial cancer tissues. Anal. Bioanal. Chem. 2018, 410, 1571–1582. [Google Scholar] [CrossRef]
- Hennig-Pauka, S.; Koch, F.J.; Schaumberger, S.; Woechtl, B.; Novak, J.; Sulyok, M.; Nagl, V. Current challenges in the diagnosis of zearalenone toxicosis as illustrated by a field case of hyperestrogenism in suckling piglets. Porc. Health Manag. 2018, 4, 18. [Google Scholar] [CrossRef]
- Songsermsakul, P.; Bohm, J.; Aurich, C.; Zentek, J.; Razzazi-Fazeli, E. The levels of zearalenone and its metabolites in plasma, urine and faeces of horses fed with naturally, Fusarium toxin-contaminated oats. J. Anim. Physiol. Anim. Nutr. 2013, 97, 155–161. [Google Scholar] [CrossRef]
- Winkler, J.; Kersten, S.; Meyer, U.; Engelhardt, U.; Danicke, S. Residues of zearalenone (ZEN), deoxynivalenol (DON) and their metabolites in plasma of dairy cows fed Fusarium contaminated maize and their relationships to performance parameters. Food Chem. Toxicol. 2014, 65, 196–204. [Google Scholar] [CrossRef]
- Kuciel-Lisieska, G.; Obremski, K.; Stelmachow, J.; Gajecka, M.; Zielonka, U.; Jakimiuky, E.; Gajecki, M. Presence of zearalenone in blood plasma in women with neoplastic lesions in the mammary gland. Bull. Vet. Inst. Pulawy 2008, 52, 671–674. [Google Scholar]
- Sambuu, R.; Takagi, M.; Shiga, S.; Uno, S.; Kokushi, E.; Namula, Z.; Otoi, T.; Miyamoto, A.; Deguchi, E.; Fink-Gremmels, J. Detection of zearalenone and its metabolites in naturally contaminated porcine follicular fluid by using liquid chromatography-tandem mass spectrometry. J. Reprod. Dev. 2011, 57, 303–306. [Google Scholar] [CrossRef]
- Skorska-Wyszynska, E.; Jakimiuk, E.; Gajecka, M.; Mlynarczuk, J.; Obremski, K.; Gajecki, M. Preliminary evaluation of influence of zearalenone on cocultures of granulosa and internal theca cells of ovarian follicles in bitches in in vitro culture. Pol. J. Vet. Sci. 2004, 7, 305–309. [Google Scholar]
- Tiemann, U.; Brüssow, K.; Küchenmeister, U.; Jonas, L.; Kohlschein, P.; Pöhland, R.; Dänicke, S. Influence of diets with cereal grains contaminated by graded levels of two Fusarium toxins on selected enzymatic and histological parameters of liver in gilts. Food Chem. Toxicol. 2006, 44, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Miao, Y.; Ding, C.; Fan, W.; Liu, S.; Lv, Y.; Gao, X.; De Boevre, M.; Yan, L.; Okoth, S.; et al. Activation of the p38/MAPK pathway regulates autophagy in response to the CYPOR-dependent oxidative stress induced by zearalenone in porcine intestinal epithelial cells. Food Chem. Toxicol. 2019, 131, 110527. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.J.; Zheng, W.I.; Feng, N.N.; Wang, T.; Zou, H.; Gu, J.H.; Yuan, Y.; Liu, X.Z.; Liu, Z.P.; Bian, J.C. The effects of autophagy and pi3k/akt/m-tor signaling pathway on the cell-cycle arrest of rats primary sertoli cells induced by zearalenone. Toxins 2018, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hu, C.; Wu, X.; Li, Z. Equol elicits estrogenic activities via PI3K/akt pathway in the estrogen receptor-positive MCF-7 cells. Mol. Cell. Toxicol. 2014, 10, 285–291. [Google Scholar] [CrossRef]
- Mahalingam, S.; Gao, L.; Gonnering, M.; Helferich, W.; Flaws, J.A. Equol inhibits growth, induces atresia, and inhibits steroidogenesis of mouse antral follicles in vitro. Toxicol. Appl. Pharmacol. 2016, 295, 47–55. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, Y.; Yao, Y.; Li, J.; Wang, W.; Wu, X. Equol Induces Mitochondria-Dependent Apoptosis in Human Gastric Cancer Cells via the Sustained Activation of ERK1/2 Pathway. Mol. Cells 2016, 39, 742–749. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, X.; Yao, W. Individual difference in faecal and urine equol excretion and their correlation with intestinal microbiota in large white sows. Anim. Prod. Sci. 2016, 57, 262–270. [Google Scholar] [CrossRef]
- Stangroom, K.E.; Smith, T.K. Effect of whole and fractioniated dietary alfalfa meal on zearalenone toxicosis and metabolism in rats and swine. Can. J. Physiol. Pharmacol. 1984, 62, 1219–1224. [Google Scholar] [CrossRef]
- Del Fabbro, L.; Jesse, C.R.; de Gomes, M.G.; Borges Filho, C.; Donato, F.; Souza, L.C.; Goes, A.R.; Furian, A.F.; Boeira, S.P. The flavonoid chrysin protects against zearalenone induced reproductive toxicity in male mice. Toxicon 2019, 165, 13–21. [Google Scholar] [CrossRef]
- Kuiper, G.G.J.M.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; Van der Burg, B.; Gastafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4563. [Google Scholar] [CrossRef]
- Saunders, P.T.K.; Millar, M.R.; Williams, K.; Macpherson, S.; Harkiss, D.; Anderson, R.A.; Orr, B.; Groome, N.P.; Scobie, G.; Fraser, H.M. Differential expression of estrogen receptor-a and -b and androgen receptor in the ovaries of marmosets and humans. Biol. Reprod. 2000, 63, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Lenie, S.; Smitz, J. Estrogen receptor subtypes localization shifts in cultured mouse ovarian follicles. Histochem. Cell Biol. 2008, 129, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Habrowska-Gorcznska, D.E.; Urbanek, K.A.; Dominska, K.; Sakowicz, A.; Piastowska-Ciesjelska, A.W. Estrogen receptor β plays a protective role in zearalenone-induced oxidative stress in normal prostate epithelial cells. Ecotoxicol. Environ. Saf. 2019, 172, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Couse, J.F.; Yates, M.M.; Deroo, B.J.; Korach, K.S. Estrogen receptor-beta is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology 2005, 146, 3247–3262. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.E.; Fuller, P.J. The importance of ERb signalling in the ovary. J. Endocrinol. 2010, 205, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Henry, L.A.; Witt, D.M. Resveratrol: Phytoestrogen effects on reproductive physiology and behavior in female rats. Horm. Behav. 2002, 41, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Winship, A.L.; Stringer, J.M.; Liew, S.H.; Hutt, K.J. The importance of DNA repair for maintaining oocyte quality in response to anti-cancer treatments, environmental toxins and maternal ageing. Hum. Reprod. 2018, 24, 119–134. [Google Scholar] [CrossRef]
- Figueiredo, J.R.; Rodrigues, A.P.R.; Silva, J.R.V.; Santos, R.R. Cryopreservation and in vitro culture of caprine preantral follicles. Reprod. Fertil. Dev. 2011, 23, 40–47. [Google Scholar] [CrossRef]
- Solak, K.A.; Santos, R.R.; van den Berg, M.; Blaauboer, B.J.; Roelen, B.A.; van Duursen, M.B. Naringenin (NAR) and 8-prenylnaringenin (8-PN) reduce the developmental competence of porcine oocytes in vitro. Reprod. Toxicol. 2014, 49, 1–11. [Google Scholar] [CrossRef]
- Sales, A.D.; Duarte, A.B.; Santos, R.R.; Alves, K.A.; Lima, L.F.; Rodrigues, G.Q.; Brito, I.R.; Lobo, C.H.; Bruno, J.B.; Locatelli, Y.; et al. Modulation of aquaporins 3 and 9 after exposure of ovine ovarian tissue to cryoprotectants followed by in vitro culture. Cell Tissue Res. 2016, 365, 415–424. [Google Scholar] [CrossRef]
- Santos, R.R.; Rodrigues, A.P.; Costa, S.H.; Silva, J.R.; Matos, M.H.; Lucci, C.M.; Bao, S.N.; van den Hurk, R.; Figueiredo, J.R. Histological and ultrastructural analysis of cryopreserved sheep preantral follicles. Anim. Reprod. Sci. 2006, 91, 249–263. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Asadi-Azarbaijani, B.; Santos, R.R.; Jahnukainen, K.; Braber, S.; van Duursen, M.B.M.; Toppari, J.; Saugstad, O.D.; Nurmio, M.; Oskam, I.C. Developmentatl effects of imatinib mesylate on follicle assembly and early activation of primordial follicle pool in postnatal rat ovary. Reprod. Biol. 2017, 17, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Barbasz, A.; Rudolphi-Skorska, E.; Filek, M.; Janeczko, A. Exposure of human lymphoma cells (U-937) to the action of a single mycotoxin as well as in mixtures with the potential protectors 24-epibrassinolide and selenium ions. Mycotoxin Res. 2019, 35, 89–98. [Google Scholar] [CrossRef]
- Green, L.C.; Tannenbaum, S.R.; Goldman, P. Nitrite synthesis in the germfree and conventional rat. Science 1981, 212, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar]
- Liu, M.; Gao, R.; Meng, Q.; Zhang, Y.; Bi, C.; Shan, A. Toxic effects of maternal zearalenone exposure on intestinal oxidative stress, barrier function, immunological and morphological changes in rats. PLoS ONE 2014, 9, e106412. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
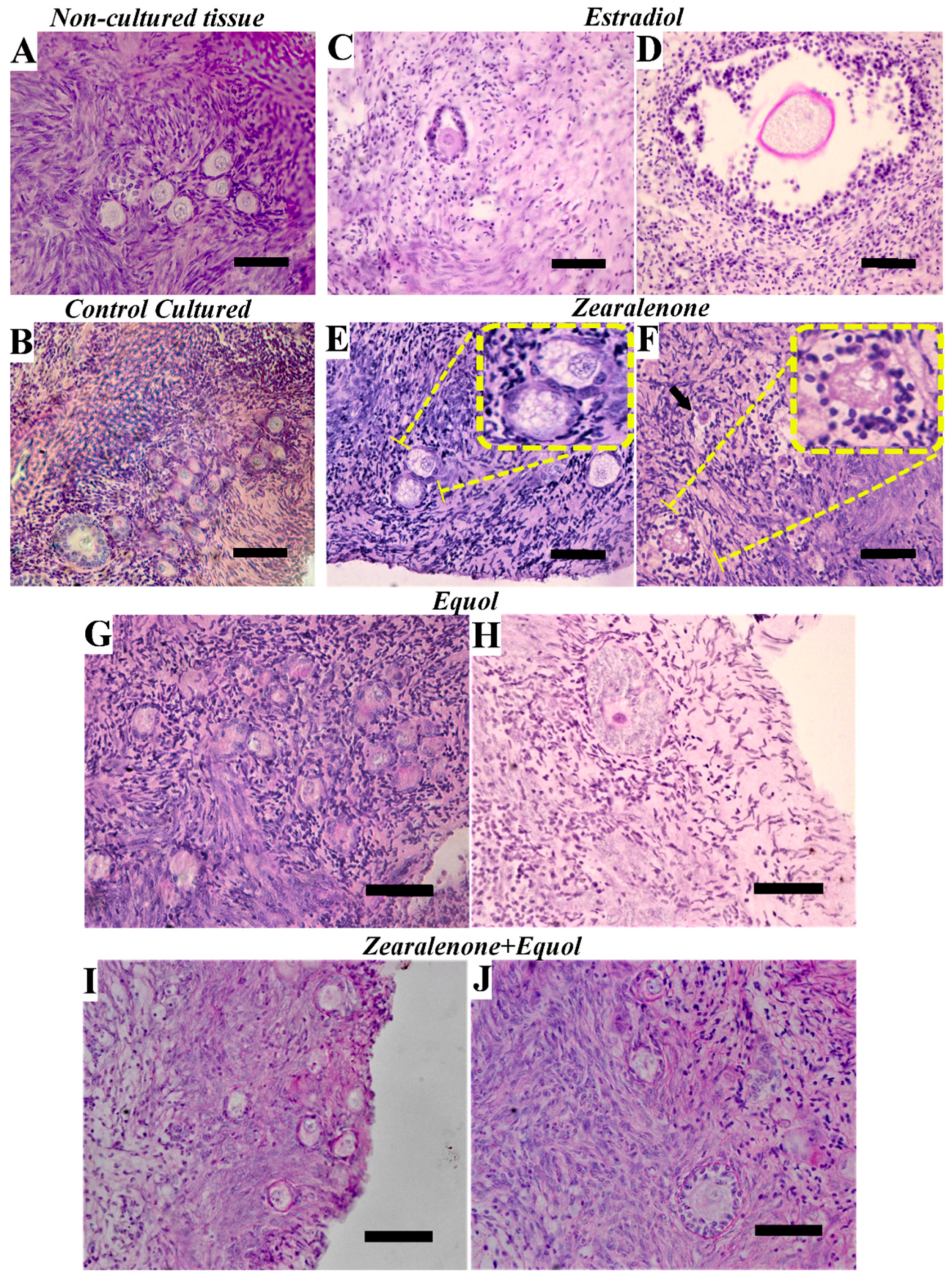
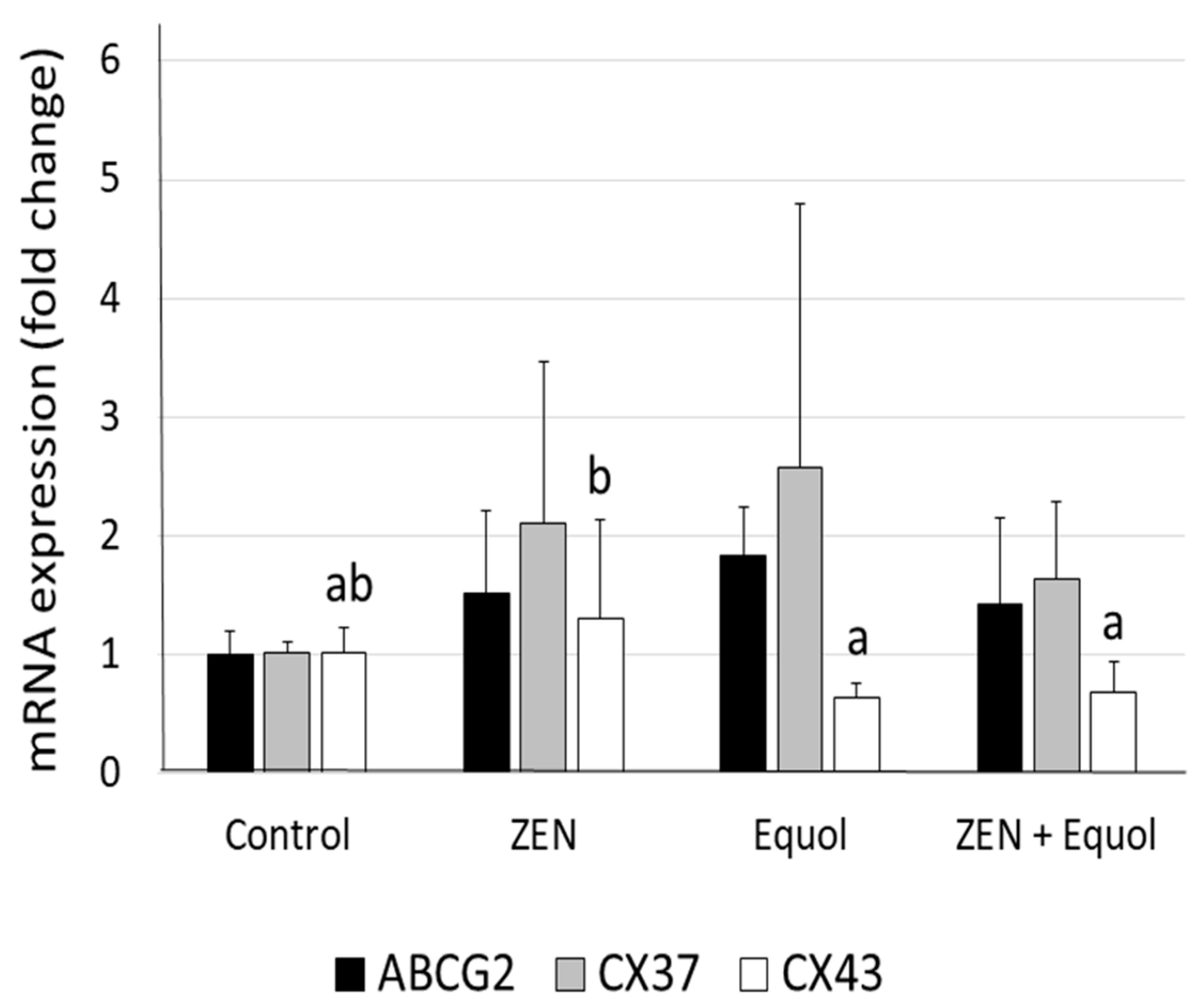
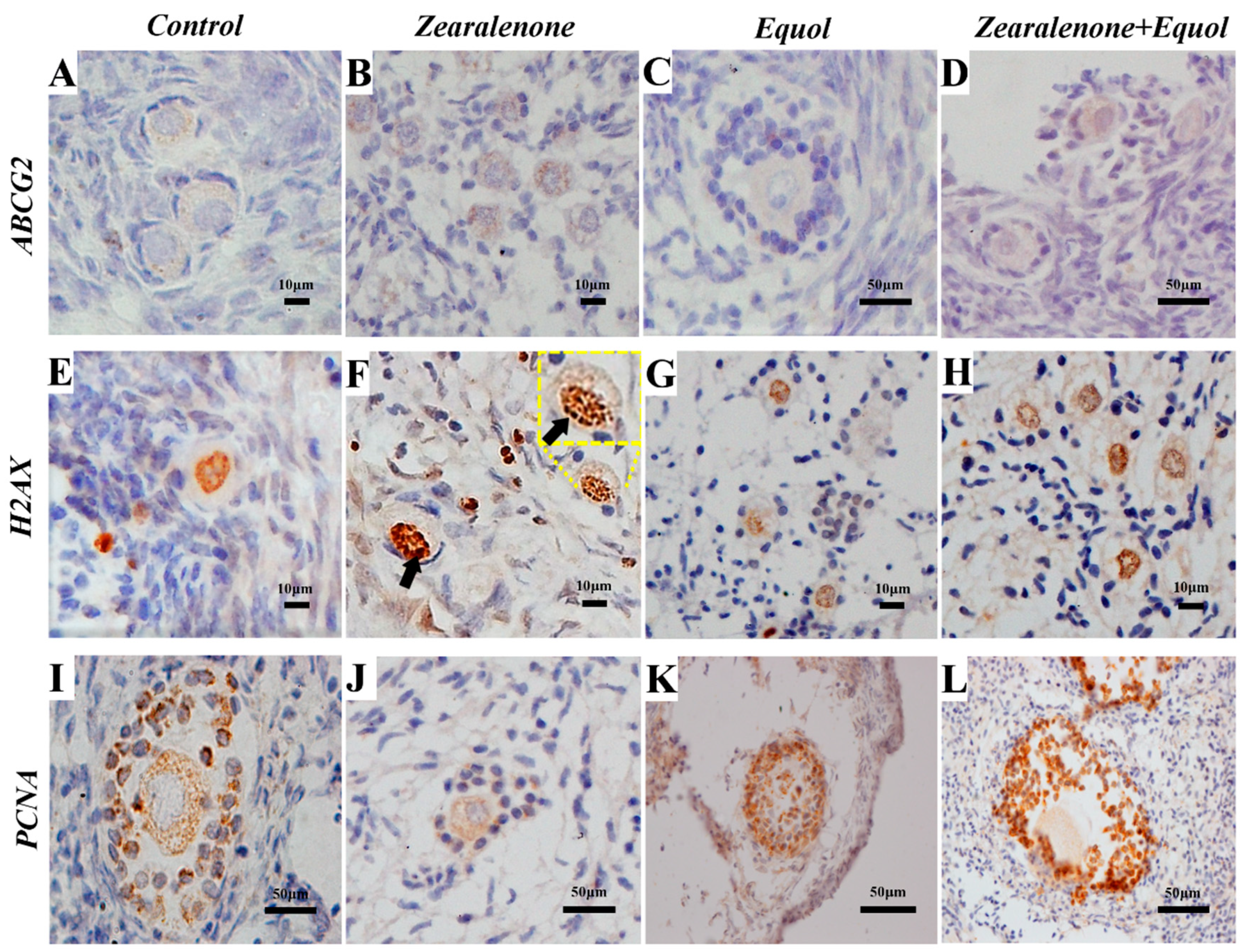
| Treatments | Morphologically Normal Preantral Follicles (%) | |||||
|---|---|---|---|---|---|---|
| Primordial | Primary | Secondary | ||||
| Non cultured control | 76.3 ± 12.6 | b | 78.0 ± 11.6 | c | 97.1 ± 6.39 | d |
| Cultured control | 64.4 ± 10.4 | b | 58.3 ± 16.5 | b | 74.7 ± 22.5 | c |
| Estradiol | 52.0 ± 12.3 | a | 36.2 ± 15.7 | a | 13.6 ± 19.3 | a |
| ZEN | 51.8 ± 11.2 | a | 45.1 ± 19.4 | ab | 50.6 ± 23.7 | b |
| Equol | 65.6 ± 14.6 | b | 48.1 ± 19.8 | ab | 53.6 ± 15.9 | bc |
| ZEN+Equol | 66.6 ± 14.2 | b | 60.2 ± 20.9 | b | 65.7 ± 25.4 | bc |
| p-values | 0.001 | <0.001 | <0.001 | |||
| Treatments | Density of Preantral Follicles (Follicles/mm3) | |||||
|---|---|---|---|---|---|---|
| Primordial | Primary | Secondary | ||||
| Non cultured control | 237 ± 53 | b | 53 ± 18 | a | 3.2 ± 2.1 | a |
| Cultured control | 154 ± 47 | a | 69 ± 40 | a | 10.9 ± 3.1 | bc |
| Estradiol | 93 ± 48 | a | 55 ± 27 | a | 7.6 ± 6.7 | ab |
| ZEN | 151 ± 98 | a | 127 ± 59 | b | 8.5 ± 7.7 | ab |
| Equol | 115 ± 78 | a | 58 ± 33 | a | 18.6 ± 10.8 | c |
| ZEN+Equol | 81 ± 48 | a | 81 ± 33 | a | 12.1 ± 8.3 | bc |
| p-values | 0.001 | 0.002 | 0.042 | |||
| Factors | Control n (% stained) | ZEN n (% stained) | Equol n (% stained) | ZEN + Equol n (mean % ± SEM) | p-Values |
|---|---|---|---|---|---|
| ABCG2 | |||||
| Primordial follicles | 251/319 | 365/436 | 232/309 | 162/194 | |
| Oocytes (cytoplasm) | 76.3 ± 6.0 | 83.2 ± 1.0 | 74.8 ± 1.0 | 83.3 ± 3.2 | 0.056 |
| Primary follicles | 80/101 | 96/110 | 46/58 | 52/65 | |
| Oocytes (cytoplasm) | 79.3 ± 3.7 | 84.0 ± 5.4 | 77.5 ± 8.7 | 80.6 ± 3.1 | 0.661 |
| Secondary follicles | 26/29 | 16/18 | 14/15 | 13/15 | |
| Oocytes (cytoplasm) | 86.0 ± 10.0 | 90.3 ± 5.8 | 88.9 ± 11.0 | 86.1 ± 7.4 | 0.937 |
| H2AX | |||||
| Primordial follicles | 132/321 | 141/228 | 72/162 | 108/210 | |
| Oocytes | 40.7 ± 5.5 a | 62.7 ± 4.2 c | 44.4 ± 1.2 a | 51.5 ± 9.4 b | p < 0.001 |
| Primary follicles | 89/223 | 75/171 | 53/123 | 43/114 | |
| Oocytes | 38.4 ± 0.9 | 42.8 ± 3.4 | 38.5 ± 4.6 | 42.5 ± 6.3 | 0.514 |
| Secondary follicles | 6/22 | 6/18 | 5/14 | 5/12 | |
| Oocytes | 31.3 ± 3.3 | 46.7 ± 3.5 | 33.3 ± 2.9 | 33.3 ± 3.4 | 0.477 |
| PCNA | |||||
| Primordial follicles | 97/235 | 144/336 | 69/204 | 58/168 | |
| Granulosa cells/follicle 1 | 9.3 ± 1.9 | 7.6 ± 1.8 | 13.1 ± 5.3 | 8.6 ± 3.4 | 0.248 |
| Primary follicles | 66/86 | 53/73 | 57/83 | 52/63 | |
| Granulosa cells/follicle 1 | 37.4 ± 6.4 a | 18.2 ± 1.8 b | 28.4 ± 13.2 ab | 36.2 ± 3.5 a | p < 0.01 |
| Secondary follicles | 17/17 | 15/15 | 18/18 | 13/13 | |
| Granulosa cells/follicle 1 | 75.5 ± 4.8 a | 55.1 ± 9.3 b | 81.7 ± 4.5 a | 77.2 ± 7.0 a | p < 0.001 |
| Antigen | Antibody | Dilution | Incubation |
|---|---|---|---|
| ABCG2 | Rabbit monoclonal primary antibody | 1:50 | 4° C O/N |
| H2AX | Mouse monoclonal gamma primary antibody | 1:100 | 4° C O/N |
| PCNA | Rabbit polyclonal primary antibody | 1:25 | 4° C O/N |
| Genes | GenBank Accession no. | Primer | Sequence |
|---|---|---|---|
| ACTB | XM_018039831.1 | Forward Reverse | CTTCCTTCTTGGGTATGGA ACGGATGTCAACGTCACACT |
| GAPDH | XM_018049688.1 | Forward Reverse | ATGCCTCCTGCACCACCA AGTCCCTCCCACGATGCCA |
| ABCG2 | XM_018049131 | Forward Reverse | CGGCATTCCAGAGACAACCT CGGCATTCCAGAGACAACCT |
| CX37 | AY745977.1 | Forward Reverse | CGACGAGCAGTCGGATTT AGATGACATGGCCCAGGTAG |
| CX43 | AY074716.1 | Forward Reverse | ATGAGCAGTCTGCCTTTCGT TCTGCTTCAAGTGCATGTCC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, T.E.S.; de Brito, D.C.C.; de Sá, N.A.R.; da Silva, R.F.; Ferreira, A.C.A.; da Silva, J.Y.G.; Guedes, M.I.F.; Rodrigues, A.P.R.; dos Santos, R.R.; de Figueiredo, J.R. Equol: A Microbiota Metabolite Able to Alleviate the Negative Effects of Zearalenone during In Vitro Culture of Ovine Preantral Follicles. Toxins 2019, 11, 652. https://doi.org/10.3390/toxins11110652
Silva TES, de Brito DCC, de Sá NAR, da Silva RF, Ferreira ACA, da Silva JYG, Guedes MIF, Rodrigues APR, dos Santos RR, de Figueiredo JR. Equol: A Microbiota Metabolite Able to Alleviate the Negative Effects of Zearalenone during In Vitro Culture of Ovine Preantral Follicles. Toxins. 2019; 11(11):652. https://doi.org/10.3390/toxins11110652
Chicago/Turabian StyleSilva, Talyne Emilia Santos, Danielle Cristina Calado de Brito, Naiza Arcângelo Ribeiro de Sá, Renato Felix da Silva, Anna Clara Accioly Ferreira, José Ytalo Gomes da Silva, Maria Izabel Florindo Guedes, Ana Paula Ribeiro Rodrigues, Regiane Rodrigues dos Santos, and José Ricardo de Figueiredo. 2019. "Equol: A Microbiota Metabolite Able to Alleviate the Negative Effects of Zearalenone during In Vitro Culture of Ovine Preantral Follicles" Toxins 11, no. 11: 652. https://doi.org/10.3390/toxins11110652
APA StyleSilva, T. E. S., de Brito, D. C. C., de Sá, N. A. R., da Silva, R. F., Ferreira, A. C. A., da Silva, J. Y. G., Guedes, M. I. F., Rodrigues, A. P. R., dos Santos, R. R., & de Figueiredo, J. R. (2019). Equol: A Microbiota Metabolite Able to Alleviate the Negative Effects of Zearalenone during In Vitro Culture of Ovine Preantral Follicles. Toxins, 11(11), 652. https://doi.org/10.3390/toxins11110652







