The Effect of Botulinum Toxin Injections on Gross Motor Function for Lower Limb Spasticity in Children with Cerebral Palsy
Abstract
1. Introduction
2. Results
2.1. Demographic Data
2.2. Injection Profile
2.3. Reduction of Tone
2.4. Combined Short Leg Cast Application
2.5. GMFM-88 Change
2.6. Factor Analysis of Changes in GMFM-88 Scores
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Design
5.2. Intervention
5.3. Assessment
5.4. Statistical Analyses
Author Contributions
Funding
Conflicts of Interest
References
- Bakheit, A.M.; Severa, S.; Cosgrove, A.; Morton, R.; Roussounis, S.H.; Doderlein, L.; Lin, J.P. Safety profile and efficacy of botulinum toxin A (Dysport) in children with muscle spasticity. Dev. Med. Child Neurol. 2001, 43, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Thorley, M.; Donaghey, S.; Edwards, P.; Copeland, L.; Kentish, M.; McLennan, K.; Lindsley, J.; Gascoigne-Pees, L.; Sakzewski, L.; Boyd, R.N. Evaluation of the effects of botulinum toxin A injections when used to improve ease of care and comfort in children with cerebral palsy whom are non-ambulant: A double blind randomized controlled trial. BMC Pediatrics 2012, 12, 120. [Google Scholar] [CrossRef] [PubMed]
- Linden, O.; Hagglund, G.; Rodby-Bousquet, E.; Wagner, P. The development of spasticity with age in 4,162 children with cerebral palsy: A register-based prospective cohort study. Acta Orthop. 2019, 90, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Franzen, M.; Hagglund, G.; Alriksson-Schmidt, A. Treatment with Botulinum toxin A in a total population of children with cerebral palsy—A retrospective cohort registry study. BMC Musculoskelet. Disord. 2017, 18. [Google Scholar] [CrossRef]
- Glanzman, A.M.; Kim, H.; Swaminathan, K.; Beck, T. Efficacy of botulinum toxin A, serial casting, and combined treatment for spastic equinus: A retrospective analysis. Dev. Med. Child Neurol. 2004, 46, 807–811. [Google Scholar] [CrossRef]
- Love, S.C.; Novak, I.; Kentish, M.; Desloovere, K.; Heinen, F.; Molenaers, G.; O’Flaherty, S.; Graham, H.K. Botulinum toxin assessment, intervention and after-care for lower limb spasticity in children with cerebral palsy: International consensus statement. Eur. J. Neurol. 2010, 17 (Suppl. 2), 9–37. [Google Scholar] [CrossRef]
- Sheean, G.L. Botulinum treatment of spasticity: Why is it so difficult to show a functional benefit? Curr. Opin. Neurol. 2001, 14, 771–776. [Google Scholar] [CrossRef]
- Juneja, M.; Jain, R.; Gautam, A.; Khanna, R.; Narang, K. Effect of multilevel lower-limb botulinum injections & intensive physical therapy on children with cerebral palsy. Indian J. Med. Res. 2017, 146, S8–S14. [Google Scholar]
- Unlu, E.; Cevikol, A.; Bal, B.; Gonen, E.; Celik, O.; Kose, G. Multilevel botulinum toxin type a as a treatment for spasticity in children with cerebral palsy: A retrospective study. Clinics 2010, 65, 613–619. [Google Scholar]
- Molenaers, G.; Fagard, K.; Van Campenhout, A.; Desloovere, K. Botulinum toxin A treatment of the lower extremities in children with cerebral palsy. J. Child Orthop. 2013, 7, 383–387. [Google Scholar] [CrossRef]
- El, O.; Peker, O.; Kosay, C.; Iyilikci, L.; Bozan, O.; Berk, H. Botulinum toxin A injection for spasticity in diplegic-type cerebral palsy. J. Child Neurol. 2006, 21, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Balbaloglu, O.; Basaran, A.; Ayoglu, H. Functional outcomes of multilevel botulinum toxin and comprehensive rehabilitation in cerebral palsy. J. Child Neurol. 2011, 26, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Hagglund, G.; Wagner, P. Development of spasticity with age in a total population of children with cerebral palsy. BMC Musculoskelet. Disord. 2008, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Blackmore, A.M.; Boettcher-Hunt, E.; Jordan, M.; Chan, M.D. A systematic review of the effects of casting on equinus in children with cerebral palsy: An evidence report of the AACPDM. Dev. Med. Child Neurol. 2007, 49, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Hagglund, G.; Wagner, P. Spasticity of the gastrosoleus muscle is related to the development of reduced passive dorsiflexion of the ankle in children with cerebral palsy: A registry analysis of 2,796 examinations in 355 children. Acta Orthop. 2011, 82, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Tedroff, K.; Lowing, K.; Haglund-Akerlind, Y.; Gutierrez-Farewik, E.; Forssberg, H. Botulinum toxin A treatment in toddlers with cerebral palsy. Acta Paediatrica 2010, 99, 1156–1162. [Google Scholar] [CrossRef]
- Liu, J.J.; Ji, S.R.; Wu, W.H.; Zhang, Y.; Zeng, F.Y.; Li, N.L. The relief effect of botulinum toxin-A for spastic iliopsoas of cerebral palsy on children. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3223–3228. [Google Scholar]
- Flemban, A.; Elsayed, W. Effect of combined rehabilitation program with botulinum toxin type A injections on gross motor function scores in children with spastic cerebral palsy. J. Phys. Ther. Sci. 2018, 30, 902–905. [Google Scholar] [CrossRef][Green Version]
- Fonseca, P.R., Jr.; Calhes Franco de Moura, R.; Galli, M.; Santos Oliveira, C. Effect of physiotherapeutic intervention on the gait after the application of botulinum toxin in children with cerebral palsy: Systematic review. Eur. J. Phys. Rehabil. Med. 2018, 54, 757–765. [Google Scholar] [CrossRef]
- Chaturvedi, S.K.; Rai, Y.; Chourasia, A.; Goel, P.; Paliwal, V.K.; Garg, R.K.; Rathore, R.K.; Pandey, C.M.; Gupta, R.K. Comparative assessment of therapeutic response to physiotherapy with or without botulinum toxin injection using diffusion tensor tractography and clinical scores in term diplegic cerebral palsy children. Brain Dev. 2013, 35, 647–653. [Google Scholar] [CrossRef]
- Xu, K.S.; Yan, T.B.; Mai, J.N. Effects of botulinum toxin guided by electric stimulation on spasticity in ankle plantar flexor of children with cerebral palsy: A randomized trial. Zhonghua Er Ke Za Zhi Chin. J. Pediatrics 2006, 44, 913–917. [Google Scholar]
- Linder, M.; Schindler, G.; Michaelis, U.; Stein, S.; Kirschner, J.; Mall, V.; Berweck, S.; Korinthenberg, R.; Heinen, F. Medium-term functional benefits in children with cerebral palsy treated with botulinum toxin type A: 1-year follow-up using gross motor function measure. Eur. J. Neurol. 2001, 8 (Suppl. 5), 120–126. [Google Scholar] [CrossRef]
- Yana, M.; Tutuola, F.; Westwater-Wood, S.; Kavlak, E. The efficacy of botulinum toxin A lower limb injections in addition to physiotherapy approaches in children with cerebral palsy: A systematic review. NeuroRehabilitation 2019, 44, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Fattal-Valevski, A.; Domenievitz, D.; Giladi, N.; Wientroub, S.; Hayek, S. Long-term effect of repeated injections of botulinum toxin in children with cerebral palsy: A prospective study. J. Child Orthop. 2008, 2, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Scholtes, V.A.; Dallmeijer, A.J.; Knol, D.L.; Speth, L.A.; Maathuis, C.G.; Jongerius, P.H.; Becher, J.G. The combined effect of lower-limb multilevel botulinum toxin type a and comprehensive rehabilitation on mobility in children with cerebral palsy: A randomized clinical trial. Arch. Phys. Med. Rehabil. 2006, 87, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Scholtes, V.A.; Dallmeijer, A.J.; Knol, D.L.; Speth, L.A.; Maathuis, C.G.; Jongerius, P.H.; Becher, J.G. Effect of multilevel botulinum toxin a and comprehensive rehabilitation on gait in cerebral palsy. Pediatric Neurol. 2007, 36, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, A.; Seyhan, K.; Deger, U.; Kutluturk, S.; Mutlu, A. Should botulinum toxin A injections be repeated in children with cerebral palsy? A systematic review. Dev. Med. Child Neurol. 2016, 58, 910–917. [Google Scholar] [CrossRef]
- Rosenbaum, P.L.; Walter, S.D.; Hanna, S.E.; Palisano, R.J.; Russell, D.J.; Raina, P.; Wood, E.; Bartlett, D.J.; Galuppi, B.E. Prognosis for gross motor function in cerebral palsy: Creation of motor development curves. JAMA 2002, 288, 1357–1363. [Google Scholar] [CrossRef]
- Scholtes, V.A.; Dallmeijer, A.J.; Becher, J.G. Can we identify predictors of multilevel botulinum toxin A injections in children with cerebral palsy who walk with a flexed knee pattern? J. Child Neurol. 2008, 23, 628–634. [Google Scholar] [CrossRef]
- Koog, Y.H.; Min, B.I. Effects of botulinum toxin A on calf muscles in children with cerebral palsy: A systematic review. Clin. Rehabil. 2010, 24, 685–700. [Google Scholar] [CrossRef]
- Ryll, U.; Bastiaenen, C.; De Bie, R.; Staal, B. Effects of leg muscle botulinum toxin A injections on walking in children with spasticity-related cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2011, 53, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Pin, T.W.; Elmasry, J.; Lewis, J. Efficacy of botulinum toxin A in children with cerebral palsy in Gross Motor Function Classification System levels IV and V: A systematic review. Dev. Med. Child Neurol. 2013, 55, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Mall, V.; Heinen, F.; Kirschner, J.; Linder, M.; Stein, S.; Michaelis, U.; Bernius, P.; Lane, M.; Korinthenberg, R. Evaluation of botulinum toxin A therapy in children with adductor spasm by gross motor function measure. J. Child Neurol. 2000, 15, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Engelen, V.; Ketelaar, M.; Gorter, J.W. Selecting the appropriate outcome in paediatric physical therapy: How individual treatment goals of children with cerebral palsy are reflected in GMFM-88 and PEDI. J. Rehabil. Med. 2007, 39, 225–231. [Google Scholar] [CrossRef] [PubMed][Green Version]
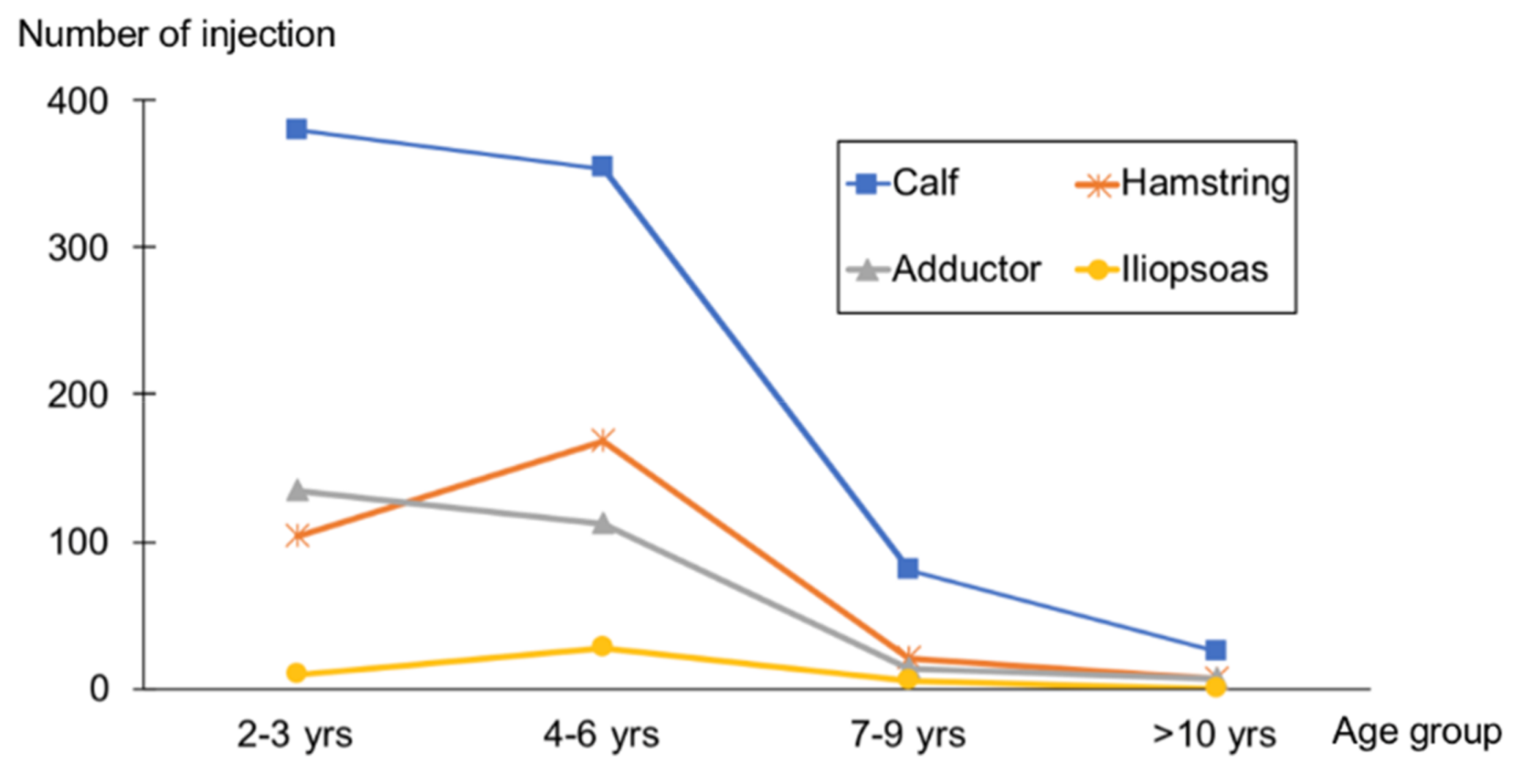
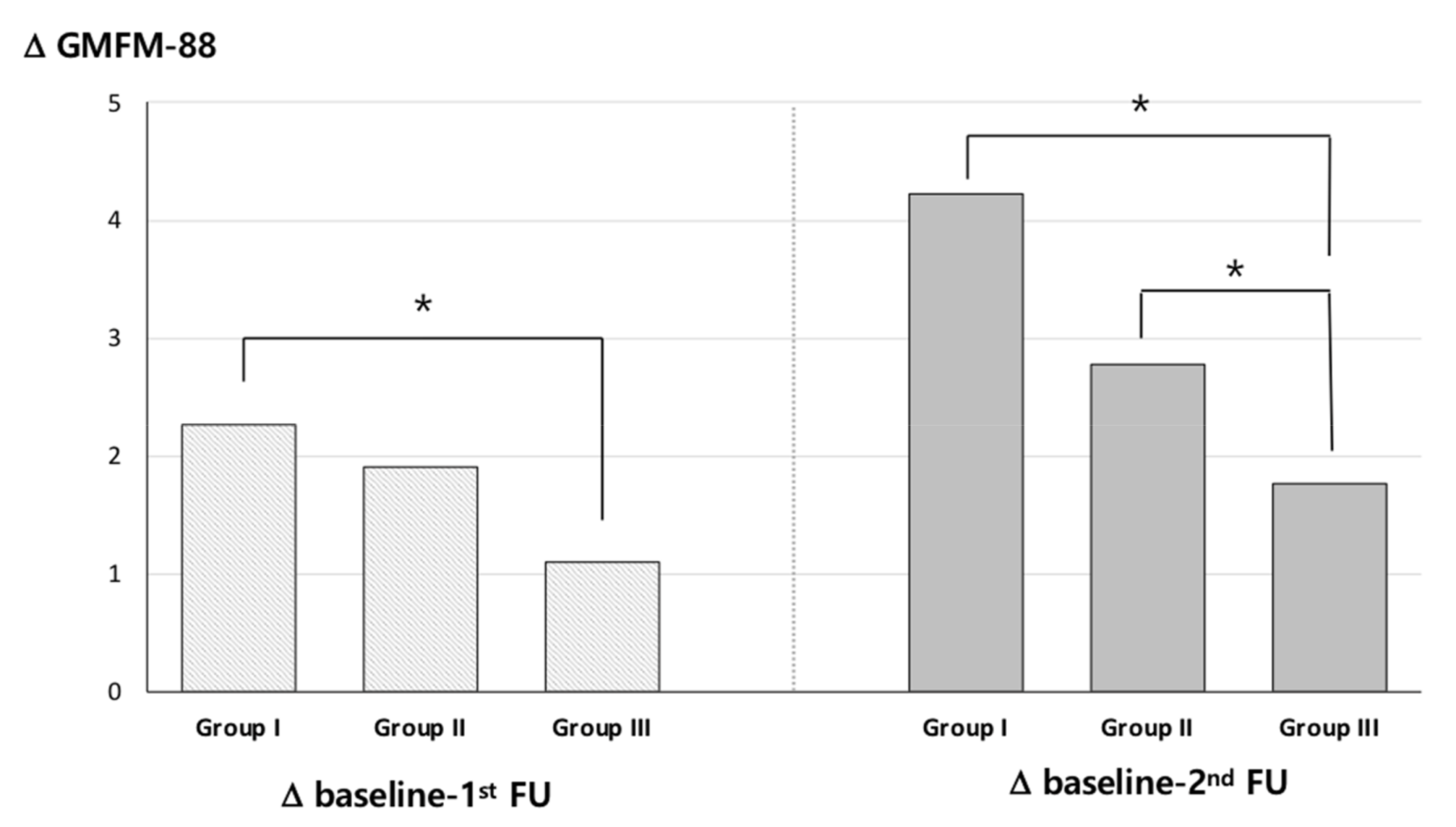
| Characteristic | Total 591 Patients |
|---|---|
| Gender, male | 340 (57.5%) |
| Topography classification | |
| Unilateral | 137 (23.2%) |
| Bilateral | 454 (76.8%) |
| GMFCS level (initial) | |
| I | 210 (35.5%) |
| II | 111 (18.8%) |
| III | 114 (19.3%) |
| IV | 91 (15.4%) |
| V | 65 (11.0%) |
| Age at each injection (years) | 4.68 ± 2.22 (2–13) |
| Characteristic | 919 Injections/591 Patients |
|---|---|
| Types of injection | |
| Distal injection only | 502 (54.6%) |
| Proximal injection only | 80 (8.7%) |
| Multi-level injection | 337 (36.7%) |
| Injection dose (unit) | |
| Distal injection only | 7.02 ± 3.19 |
| Proximal injection only | 9.84 ± 5.11 |
| Multi-level injection | 12.88 ± 4.65 |
| Injection site | |
| Unilateral injection | 284 (30.9%) |
| Bilateral injection | 635 (69.1%) |
| GMFM-88 assessment at each time interval | |
| Baseline | 919 |
| 1st follow-up (within 1–2 months after injection) | 825 |
| 2nd follow-up (within 3–6 months after injection) | 523 |
| GMFCS | Injection Site | p-Value | |||
|---|---|---|---|---|---|
| Distal | Proximal | Multilevel | Overall | Post-Hoc | |
| I | 299 (59.6%) | 2 (2.5%) | 49 (14.5%) | <0.001 * | Distal vs. proximal <0.001 * Distal vs. multilevel <0.001 * Proximal vs. multilevel = 0.329 |
| II | 102 (20.3%) | 5 (6.3%) | 73 (21.7%) | ||
| III | 46 (9.2%) | 18 (22.5%) | 100 (29.7%) | ||
| IV | 33 (6.6%) | 20 (25.0%) | 79 (23.4%) | ||
| V | 22 (4.4%) | 35 (43.8%) | 36 (10.7%) | ||
| Group | Injection Occasion | Injection Frequency | Total |
|---|---|---|---|
| I: 1st injection | 1st | 388 | 388 (42.2%) |
| II: 2nd–3rd injection | 2nd | 248 | 381 (41.5%) |
| 3rd | 133 | ||
| III: Multiple injections (≥4 times) | 4th | 63 | 150 (16.3%) |
| 5th | 40 | ||
| 6th | 23 | ||
| 7th | 12 | ||
| 8th | 6 | ||
| 9th | 4 | ||
| 10th | 1 | ||
| 11th | 1 |
| Injection Site | Assessed Muscle and Posture | Assessment Time | p-Value | ||
|---|---|---|---|---|---|
| Baseline | 1st Follow-Up | 2nd Follow-Up | |||
| Modified Ashworth Scale, median (IQR) | |||||
| Calf | Ankle with knee flexion | 2 (1+, 2) | 1+ * (1, 1+) | 1+ * (1, 2) | <0.001 § |
| Calf | Ankle with knee extension | 2 (2, 3) | 1+ * (1+, 2) | 1+ *,† (1+, 2) | <0.001 § |
| Hamstring | Knee flexor | 2 (1+, 2) | 1+ * (1, 1+) | 1+ * (1+, 2) | <0.001 § |
| Hip adductor | Hip adductor with knee flexion | 1+ (1+, 2) | 1 * (1, 1+) | 1+ *,† (1, 2) | <0.001 § |
| Hip adductor | Hip adductor with knee extension | 2 (1+, 2) | 1+ * (1, 1+) | 1+ *,† (1, 2) | <0.001 § |
| Modified Tardieu Scale, R1 (degree) | |||||
| Calf | Ankle with knee flexion | −7.79 (15.09) | 2.56 * (10.60) | −0.04 *,† (13.49) | <0.001 §§ |
| Calf | Ankle with knee extension | −17.88 (14.28) | −4.90 * (13.38) | −10.80 *,† (13.05) | <0.001 §§ |
| Hamstring | Knee flexor | 67.24 (17.11) | 49.22 * (16.18) | 59.69 *,† (21.25) | <0.001 §§ |
| Hip adductor | Hip adductor with knee flexion | 35.71 (13.84) | 48.80 * (12.90) | 43.08 * (14.98) | <0.001 §§ |
| Hip adductor | Hip adductor with knee extension | 16.93 (7.85) | 28.75 * (8.33) | 23.72 *,† (10.62) | <0.001 §§ |
| Modified Tardieu Scale, R2 (degree) | |||||
| Calf | Ankle with knee flexion | 17.14 (12.73) | 23.36 * (11.33) | 21.93 * (11.50) | <0.001 §§ |
| Calf | Ankle with knee extension | 7.05 (11.72) | 13.32 * (10.73) | 11.34 * (10.77) | <0.001 §§ |
| Hamstring | Knee flexor | 38.49 (15.65) | 29.79 * (12.92) | 36.35 * (16.04) | <0.001 §§ |
| Hip adductor | Hip adductor with knee flexion | 57.95 (12.13) | 63.21 * (10.48) | 61.63 (13.93) | 0.001 §§ |
| Hip adductor | Hip adductor with knee extension | 30.96 (10.19) | 38.56 * (7.26) | 34.63 * (9.63) | <0.001 §§ |
| Serial Cast | Age Group at Each Injection | p-Value | ||||
|---|---|---|---|---|---|---|
| I: 2–3 Years | II: 4–6 Years | III: 7–9 Years | IV: >10 Years | Overall | Post-Hoc | |
| Cast/Total | 50/403 (12.4%) | 83/393 (21.1%) | 31/92 (33.7%) | 12/31 (38.7%) | <0.001 * | I vs. II = 0.006 * I vs. III < 0.001 * I vs. IV < 0.001 * II vs. III = 0.084 II vs. IV = 0.246 III vs. IV > 0.999 |
| GMFM-88 | p-Value | |||
|---|---|---|---|---|
| Baseline | 1st Follow-Up | 2nd Follow-Up | ||
| Functional level | ||||
| Ambulatory (GMFCS I-III) | 76.57 (20.83) | 78.77 (19.47) * | 80.96 (18.70) *,† | <0.001§ |
| Non-ambulatory (GMFCS IV-V) | 30.27 (20.98) | 32.00 (21.71) * | 34.32 (19.78) * | <0.001§ |
| Injection occasion | ||||
| 1st injection | 59.55 (30.30) | 61.38 (29.97) * | 64.95 (28.37) *,† | <0.001§ |
| 2nd-3rd injection | 67.52 (27.54) | 70.84 (27.96) * | 70.37 (27.96) *,† | <0.001§ |
| 4th or more injection | 74.17 (25.30) | 76.20 (23.78) * | 75.48 (25.27) * | 0.005 § |
| At Primary End-Point (1–2 Months after Injection) | At Follow-Up (3–6 Months after Injection) | |||
|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |
| Variable | B (SE) | B (SE) | B (SE) | B (SE) |
| Age at injection | −0.30 * (0.07) | −0.23 * (0.07) | −0.63 * (0.13) | −0.51 * (0.15) |
| GMFCS | - | - | ||
| Level I-III | ref | ref | ||
| Level IV-V | −0.42 (0.34) | −0.86 (0.69) | ||
| Body involvement | - | - | ||
| Unilateral | ref | ref | ||
| Bilateral | 0.61 (0.36) | 0.97 (0.18) | ||
| Injection type | ||||
| Distal only | ref | ref | ref | ref |
| Proximal only | −1.46 * (0.54) | −1.31 * (0.54) | −3.08 * (1.10) | −2.75 * (1.07) |
| Multilevel | 0.73 * (0.31) | 0.60 (0.31) | 0.41 (0.64) | 0.11 (0.63) |
| Injection Occasion | ||||
| 1st injection | ref | ref | ref | ref |
| 2nd, 3rd injection | −0.35 (0.32) | −0.23 (0.32) | −1.46 * (0.66) | −0.99 (0.66) |
| ≥4th injection | −1.16 * (0.45) | −6.51 (0.47) | −2.48 * (0.88) | −1.22 (0.93) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.Y.; Kim, S.K.; Park, E.S. The Effect of Botulinum Toxin Injections on Gross Motor Function for Lower Limb Spasticity in Children with Cerebral Palsy. Toxins 2019, 11, 651. https://doi.org/10.3390/toxins11110651
Choi JY, Kim SK, Park ES. The Effect of Botulinum Toxin Injections on Gross Motor Function for Lower Limb Spasticity in Children with Cerebral Palsy. Toxins. 2019; 11(11):651. https://doi.org/10.3390/toxins11110651
Chicago/Turabian StyleChoi, Ja Young, Seung Ki Kim, and Eun Sook Park. 2019. "The Effect of Botulinum Toxin Injections on Gross Motor Function for Lower Limb Spasticity in Children with Cerebral Palsy" Toxins 11, no. 11: 651. https://doi.org/10.3390/toxins11110651
APA StyleChoi, J. Y., Kim, S. K., & Park, E. S. (2019). The Effect of Botulinum Toxin Injections on Gross Motor Function for Lower Limb Spasticity in Children with Cerebral Palsy. Toxins, 11(11), 651. https://doi.org/10.3390/toxins11110651





