Vampire Venom: Vasodilatory Mechanisms of Vampire Bat (Desmodus rotundus) Blood Feeding
Abstract
1. Introduction
2. Results
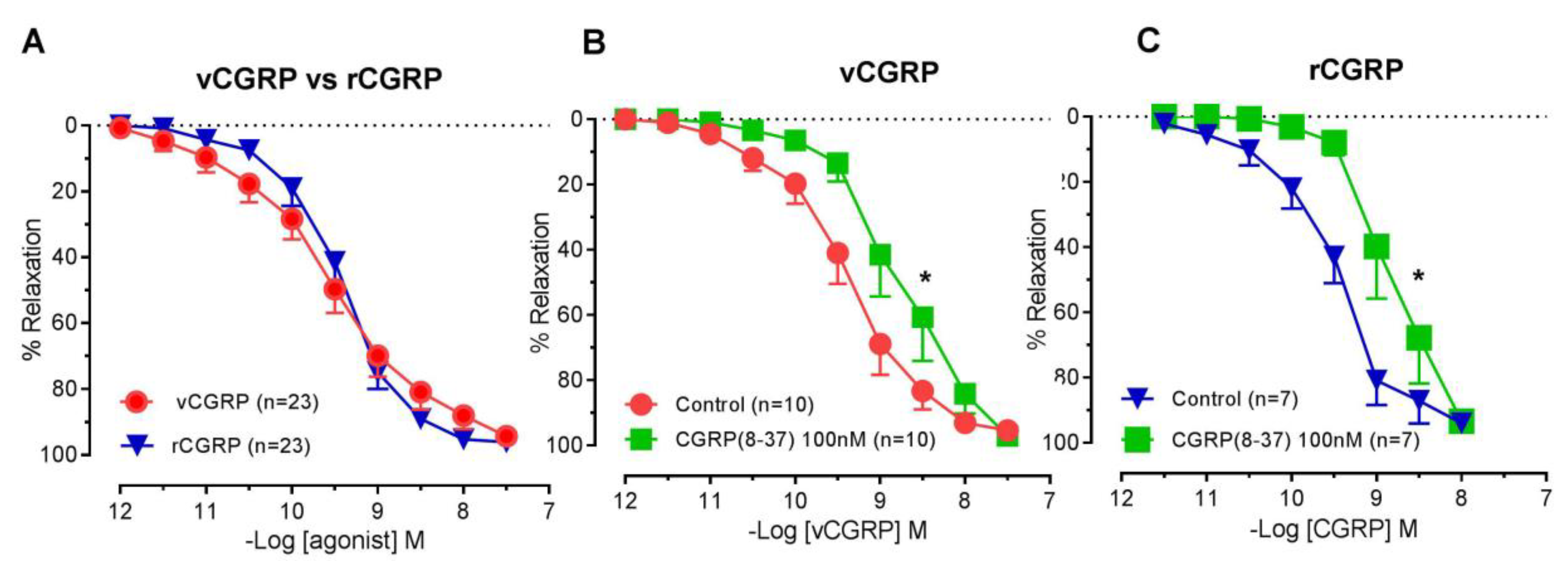
2.1. Vasorelaxant Responses to D. rotundus vCGRP and rCGRP
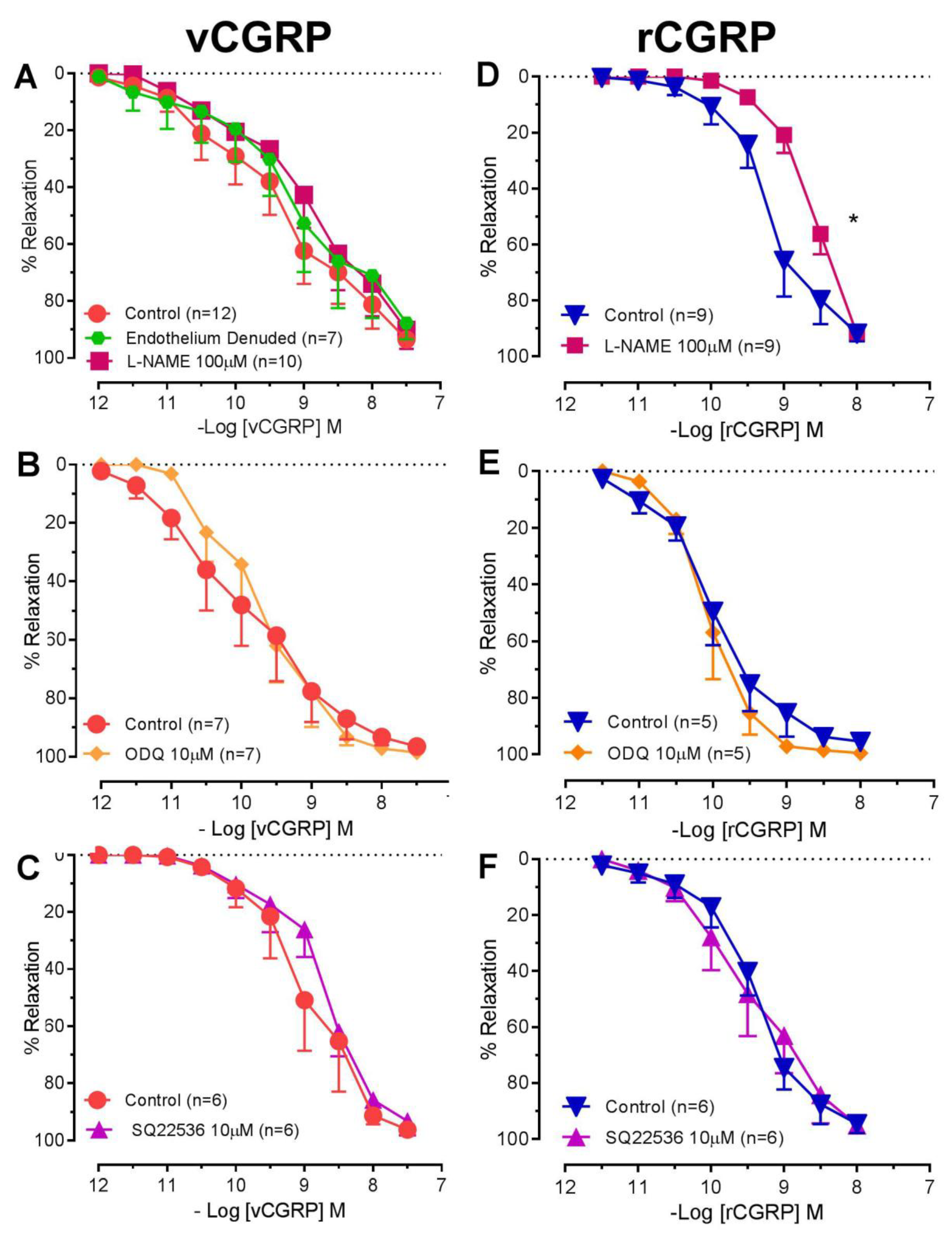
2.2. Contribution of NO-SGC and Adenylate Cyclase to D. rotundus vCGRP and rCGRP Mediated Relaxation
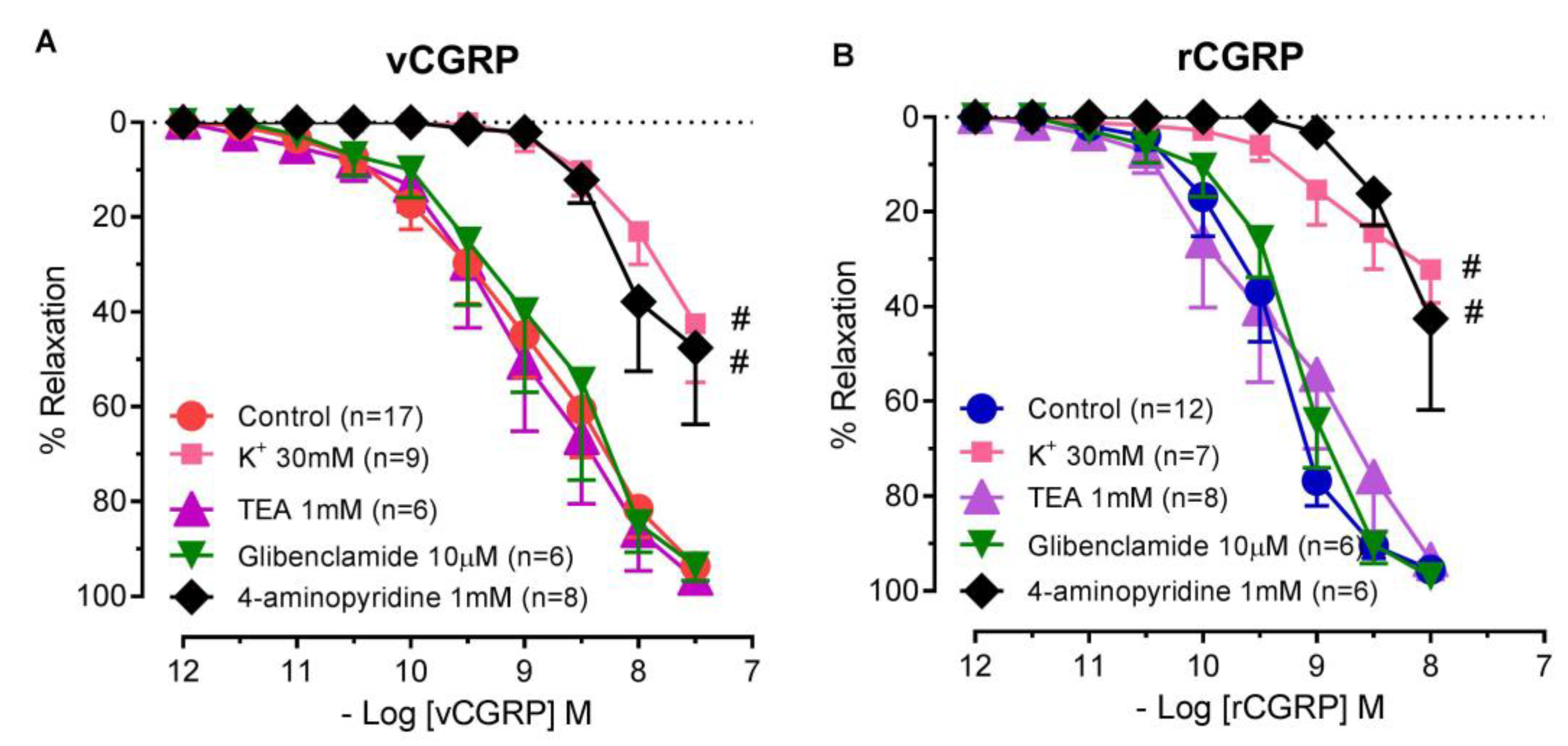
2.3. Contribution of Potassium Channels to D. rotundus vCGRP and rCGRP Mediated Relaxation
3. Discussion
4. Materials and Methods
4.1. Isolation of Rat Small Mesenteric Arteries
4.2. Vasorelaxation Experiments
4.3. Data Analysis and Statistical Procedures
4.4. Reagents
Author Contributions
Funding
Conflicts of Interest
References
- Greenhall, A.M. Natural History of Vampire Bats; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Delpietro, H.; Marchevsky, N.; Simonetti, E. Relative population densities and predation of the common vampire bat (Desmodus rotundus) in natural and cattle-raising areas in north-east argentina. Prev. Vet. Med. 1992, 14, 13–20. [Google Scholar] [CrossRef]
- Disanto, P.E. Anatomy and histochemistry of the salivary glands of the vampire bat, Desmodus rotundus murinus. J. Morphol. 1960, 106, 301–335. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.Z.; Tablante, A.; Bartoli, F.; Beguin, S.; Hemker, H.; Apitz-Castro, R. Expression of biological activity of draculin, the anticoagulant factor from vampire bat saliva, is strictly dependent on the appropriate glycosylation of the native molecule. Biochim. Biophys. Acta Gen. Subj. 1998, 1425, 291–299. [Google Scholar] [CrossRef]
- Fernandez, A.Z.; Tablante, A.; Beguín, S.; Hemker, H.C.; Apitz-Castro, R. Draculin, the anticoagulant factor in vampire bat saliva, is a tight-binding, noncompetitive inhibitor of activated factor X. Biochim. Biophys. Acta Protein Struct. Mol. Enzym. 1999, 1434, 135–142. [Google Scholar] [CrossRef]
- Apitz-Castro, R.; Beguin, S.; Tablante, A.; Bartoli, F.; Holt, J.; Hemker, H. Purification and partial characterization of draculin, the anticoagulant factor present in the saliva of vampire bats (Desmodus rotundus). Thromb. Haemost. 1995, 73, 94–100. [Google Scholar] [CrossRef]
- Batista-da-Costa, M.; Bonito, R.F.; Nishioka, S. An outbreak of vampire bat bite in a Brazilian village. Trop. Med. Parasitol. 1993, 44, 219–220. [Google Scholar]
- Schneider, M.C.; Aron, J.; Santos-Burgoa, C.; Uieda, W.; Ruiz-Velazco, S. Common vampire bat attacks on humans in a village of the amazon region of Brazil. Cadernos Saúde Pública 2001, 17, 1531–1536. [Google Scholar] [CrossRef]
- Schneider, M.C.; Romijn, P.C.; Uieda, W.; Tamayo, H.; Silva, D.F.D.; Belotto, A.; Silva, J.B.d.; Leanes, L.F. Rabies transmitted by vampire bats to humans: An emerging zoonotic disease in Latin America? Revista Panamericana Salud Pública 2009, 25, 260–269. [Google Scholar] [CrossRef]
- McCarthy, T.J. Human depredation by vampire bats (Desmodus rotundus) following a hog cholera campaign. Am. J. Trop. Med. Hyg. 1989, 40, 320–322. [Google Scholar] [CrossRef]
- Constantine, D.G. Bats in Relation to the Health Welfare and Economy of Man; Academic Press Incorporated: Cambridge, MA, USA, 1970. [Google Scholar]
- Basanova, A.; Baskova, I.; Zavalova, L. Vascular–platelet and plasma hemostasis regulators from bloodsucking animals. Biochemistry 2002, 67, 143–150. [Google Scholar] [PubMed]
- Tellgren-Roth, Å.; Dittmar, K.; Massey, S.E.; Kemi, C.; Tellgren-Roth, C.; Savolainen, P.; Lyons, L.A.; Liberles, D.A. Keeping the blood flowing—Plasminogen activator genes and feeding behavior in vampire bats. Naturwissenschaften 2009, 96, 39–47. [Google Scholar] [CrossRef]
- Hawkey, C. Plasminogen activator in saliva of the vampire bat Desmodus rotundus. Nature 1966, 211, 434–435. [Google Scholar] [CrossRef] [PubMed]
- Hawkey, C. Inhibitor of platelet aggregation present in saliva of the vampire bat Desmodus rotundus. Br. J. Haematol. 1967, 13, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Champagne, D.E.; Ribeiro, J. Sialokinin I and II: Vasodilatory tachykinins from the yellow fever mosquito Aedes aegypti. Proc. Nat. Acad. Sci. USA 1994, 91, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.; Nussenzveig, R.H.; Tortorella, G. Salivary vasodilators of Aedes triseriatus and Anopheles gambiae (Diptera: Culicidae). J. Med. Èntomol. 1994, 31, 747–753. [Google Scholar] [CrossRef]
- Nussenzveig, R.H.; Bentley, D.L.; Ribeiro, J. Nitric oxide loading of the salivary nitric-oxide-carrying hemoproteins (nitrophorins) in the blood-sucking bug Rhodnius prolixus. J. Exp. Boil. 1995, 198, 1093–1098. [Google Scholar]
- Valenzuela, J.G.; Walker, F.; Ribeiro, J. A salivary nitrophorin (nitric-oxide-carrying hemoprotein) in the bedbug Cimex lectularius. J. Exp. Boil. 1995, 198, 1519–1526. [Google Scholar]
- Lerner, E.A.; Iuga, A.O.; Reddy, V.B. Maxadilan, a PAC1 receptor agonist from sand flies. Peptides 2007, 28, 1651–1654. [Google Scholar] [CrossRef]
- Lerner, E.; Ribeiro, J.; Nelson, R.J.; Lerner, M. Isolation of maxadilan, a potent vasodilatory peptide from the salivary glands of the sand fly Lutzomyia longipalpis. J. Boil. Chem. 1991, 266, 11234–11236. [Google Scholar]
- Ma, D.; Xu, X.; An, S.; Liu, H.; Yang, X.; Andersen, J.F.; Wang, Y.; Tokumasu, F.; Ribeiro, J.M.; Francischetti, I.M. A novel family of RGD-containing disintegrin (Tablysin-15) from the salivary gland of the horsefly Tabanus yao targets integrins αIIbβ3 and αvβ3 and inhibits platelet aggregation and angiogenesis. Thromb. Haemost. 2011, 105, 1032–1045. [Google Scholar] [PubMed]
- Tu, A.T.; Motoyashiki, T.; Azimov, D.A. Bioactive compounds in tick and mite venoms (saliva). Toxin Rev. 2005, 24, 143–174. [Google Scholar] [CrossRef]
- Greenhall, A.M. Feeding behavior. Nat. Hist. Vampire Bats 1988, 111–131. [Google Scholar]
- Low, D.H.W.; Sunagar, K.; Undheim, E.A.B.; Ali, S.A.; Alagon, A.C.; Ruder, T.; Jackson, T.N.W.; Pineda Gonzalez, S.; King, G.F.; Jones, A.; et al. Dracula’s children: Molecular evolution of vampire bat venom. J. Proteom. 2013, 89, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Takasaki, K.; Saito, A.; Goto, K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature 1988, 335, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Thom, S.M.; Hughes, A.D.; Goldberg, P.; Martin, G.; Schachter, M.; Sever, P.S. The actions of calcitonin gene related peptide and vasoactive intestinal peptide as vasodilators in man in vivo and in vitro. Br. J. Clin. Pharmacol. 1987, 24, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Steenbergh, P.; Höppener, J.; Zandberg, J.; Visser, A.; Lips, C.; Jansz, H. Structure and expression of the human calcitonin/CGRP genes. FEBS Lett. 1986, 209, 97–103. [Google Scholar] [CrossRef]
- Rosenfeld, M.G.; Mermod, J.-J.; Amara, S.G.; Swanson, L.W.; Sawchenko, P.E.; Rivier, J.; Vale, W.W.; Evans, R.M. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature 1983, 304, 129–135. [Google Scholar] [CrossRef]
- Russell, F.A.; King, R.; Smillie, S.-J.; Kodji, X.; Brain, S.D. Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef]
- Lei, S.; Mulvany, M.J.; Nyborg, N.C.B. Characterization of the CGRP receptor and mechanisms of action in rat mesenteric small arteries. Pharmacol. Toxicol. 1994, 74, 130–135. [Google Scholar] [CrossRef]
- Boussery, K.; Delaey, C.; Van de Voorde, J. The vasorelaxing effect of CGRP and natriuretic peptides in isolated bovine retinal arteries. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Ishihara, I.; Ishikawa, T.; Saito, A.; Goto, K. Vasodilating effects of human and rat calcitonin gene-related peptides in isolated porcine coronary arteries. Naunyn-Schmiedebergs Arch. Pharmacol. 1987, 336, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Brain, S.D.; Grant, A.D. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol. Rev. 2004, 84, 903–934. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.W.; Marshall, I. Human α-calcitonin gene-related peptide stimulates adenylate cyclase and guanylate cyclase and relaxes rat thoracic aorta by releasing nitric oxide. Br. J. Pharmacol. 1992, 107, 691–696. [Google Scholar] [CrossRef] [PubMed]
- McNeish, A.J.; Roux, B.T.; Aylett, S.B.; Van Den Brink, A.M.; Cottrell, G.S. Endosomal proteolysis regulates calcitonin gene-related peptide responses in mesenteric arteries. Br. J. Pharmacol. 2012, 167, 1679–1690. [Google Scholar] [CrossRef]
- Zygmunt, P.M.; Ryman, T.; HÖGestÄTt, E.D. Regional differences in endothelium-dependent relaxation in the rat: Contribution of nitric oxide and nitric oxide-independent mechanisms. Acta Physiol. Scand. 1995, 155, 257–266. [Google Scholar] [CrossRef]
- Nelson, M.T.; Huang, Y.; Brayden, J.E.; Hescheler, J.; Standen, N.B. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature 1990, 344, 770–773. [Google Scholar] [CrossRef]
- Quayle, J.M.; Bonev, A.D.; Brayden, J.E.; Nelson, M.T. Calcitonin gene-related peptide activated ATP-sensitive K+ currents in rabbit arterial smooth muscle via protein kinase A. J. Physiol. 1994, 475, 9–13. [Google Scholar] [CrossRef]
- Bol, M.; Leybaert, L.; Vanheel, B. Influence of methanandamide and CGRP on potassium currents in smooth muscle cells of small mesenteric arteries. Pflügers Arch. Eur. J. Physiol. 2012, 463, 669–677. [Google Scholar] [CrossRef]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S. The toxicogenomic multiverse: Convergent recruitment of proteins into animal venoms. Annu. Rev. Genom. Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef]
- Fry, B.G. From genome to “venome”: Molecular origin and evolution of the snake venom proteome inferred from phylogenetic analysis of toxin sequences and related body proteins. Genome Res. 2005, 15, 403–420. [Google Scholar] [CrossRef]
- Aubdool, A.A.; Thakore, P.; Argunhan, F.; Smillie, S.-J.; Schnelle, M.; Srivastava, S.; Alawi, K.M.; Wilde, E.; Mitchell, J.; Farrell-Dillon, K. A novel α-calcitonin gene-related peptide analogue protects against end-organ damage in experimental hypertension, cardiac hypertrophy, and heart failureclinical perspective. Circulation 2017, 136, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Ruder, T.; Ali, S.A.; Ormerod, K.; Brust, A.; Roymanchadi, M.-L.; Ventura, S.; Undheim, E.A.; Jackson, T.N.; Mercier, A.J.; King, G.F. Functional characterization on invertebrate and vertebrate tissues of tachykinin peptides from octopus venoms. Peptides 2013, 47, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Andrews, K.L.; Irvine, J.C.; Tare, M.; Apostolopoulos, J.; Favaloro, J.L.; Triggle, C.R.; Kemp-Harper, B.K. A role for nitroxyl (HNO) as an endothelium-derived relaxing and hyperpolarizing factor in resistance arteries. Br. J. Pharmacol. 2009, 157, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Yuill, K.H.; Yarova, P.; Kemp-Harper, B.K.; Garland, C.J.; Dora, K.A. A novel role for HNO in local and spreading vasodilatation in rat mesenteric resistance arteries. Antioxid. Redox Signal. 2011, 14, 1625–1635. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.R.; Yallampalli, C. Endothelium-independent relaxation by adrenomedullin in pregnant rat mesenteric artery: Role of camp-dependent protein kinase a and calcium-activated potassium channels. J. Pharmacol. Exp. Ther. 2006, 317, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Oliver, K.; Kane, S.; Salvatore, C.; Mallee, J.; Kinsey, A.; Koblan, K.; Keyvan-Fouladi, N.; Heavens, R.; Wainwright, A.; Jacobson, M. Cloning, characterization and central nervous system distribution of receptor activity modifying proteins in the rat. Eur. J. Neurosci. 2001, 14, 618–628. [Google Scholar] [CrossRef]
- Rorabaugh, B.R.; Scofield, M.A.; Smith, D.D.; Jeffries, W.B.; Abel, P.W. Functional calcitonin gene-related peptide subtype 2 receptors in porcine coronary arteries are identified as calcitonin gene-related peptide subtype 1 receptors by radioligand binding and reverse transcription-polymerase chain reaction. J. Pharmacol. Exp. Ther. 2001, 299, 1086–1094. [Google Scholar]
- Isomoto, S.; Kondo, C.; Yamada, M.; Matsumoto, S.; Higashiguchi, O.; Horio, Y.; Matsuzawa, Y.; Kurachi, Y. A novel sulfonylurea receptor forms with BIR (Kir6. 2) a smooth muscle type ATP-sensitive K+ channel. J. Boil. Chem. 1996, 271, 24321–24324. [Google Scholar] [CrossRef]
- Favaloro, J.L.; Kemp-Harper, B.K. Redox variants of NO (NO· and HNO) elicit vasorelaxation of resistance arteries via distinct mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1274–H1280. [Google Scholar] [CrossRef]
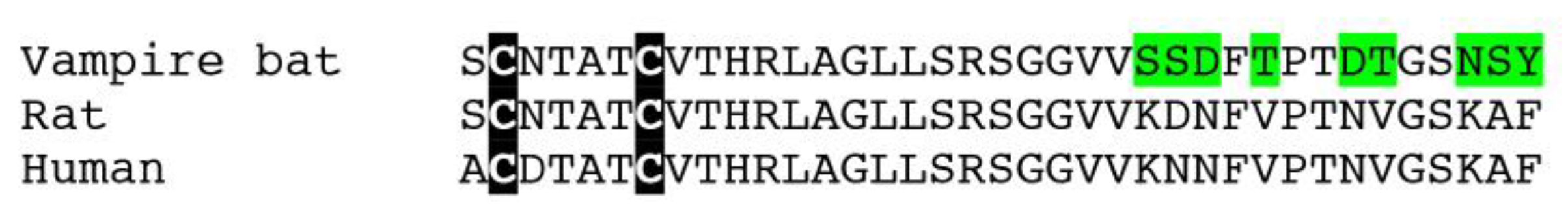
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakumanu, R.; Hodgson, W.C.; Ravi, R.; Alagon, A.; Harris, R.J.; Brust, A.; Alewood, P.F.; Kemp-Harper, B.K.; Fry, B.G. Vampire Venom: Vasodilatory Mechanisms of Vampire Bat (Desmodus rotundus) Blood Feeding. Toxins 2019, 11, 26. https://doi.org/10.3390/toxins11010026
Kakumanu R, Hodgson WC, Ravi R, Alagon A, Harris RJ, Brust A, Alewood PF, Kemp-Harper BK, Fry BG. Vampire Venom: Vasodilatory Mechanisms of Vampire Bat (Desmodus rotundus) Blood Feeding. Toxins. 2019; 11(1):26. https://doi.org/10.3390/toxins11010026
Chicago/Turabian StyleKakumanu, Rahini, Wayne C. Hodgson, Ravina Ravi, Alejandro Alagon, Richard J. Harris, Andreas Brust, Paul F. Alewood, Barbara K. Kemp-Harper, and Bryan G. Fry. 2019. "Vampire Venom: Vasodilatory Mechanisms of Vampire Bat (Desmodus rotundus) Blood Feeding" Toxins 11, no. 1: 26. https://doi.org/10.3390/toxins11010026
APA StyleKakumanu, R., Hodgson, W. C., Ravi, R., Alagon, A., Harris, R. J., Brust, A., Alewood, P. F., Kemp-Harper, B. K., & Fry, B. G. (2019). Vampire Venom: Vasodilatory Mechanisms of Vampire Bat (Desmodus rotundus) Blood Feeding. Toxins, 11(1), 26. https://doi.org/10.3390/toxins11010026






