The Streptococcus pneumoniae yefM-yoeB and relBE Toxin-Antitoxin Operons Participate in Oxidative Stress and Biofilm Formation
Abstract
1. Introduction
2. Results
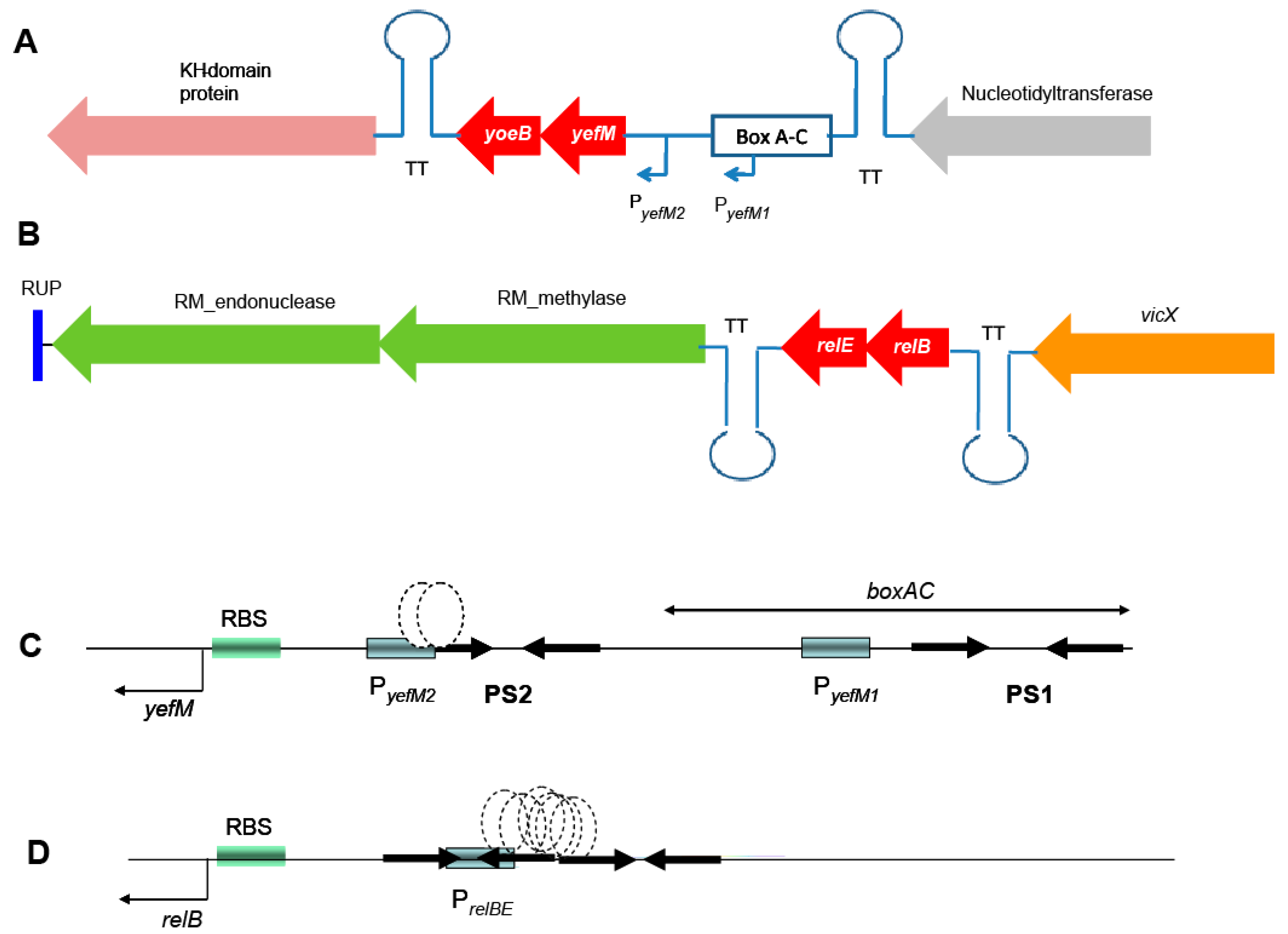
2.1. Genetic Organization of the Pneumococcal Operons yefM-yoeB and relBE
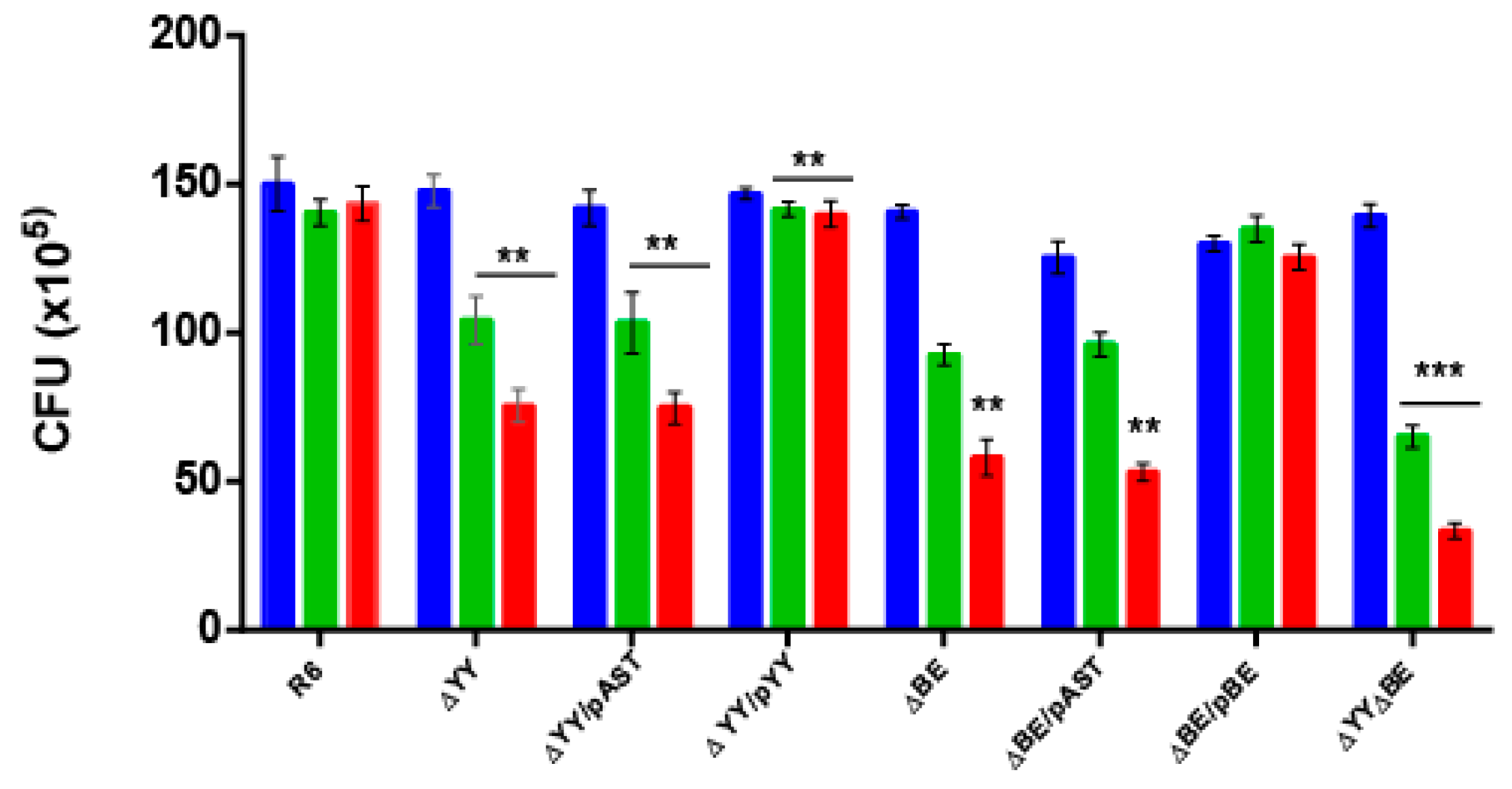
2.2. Deletion of the Pneumococcal Operons Reduce Response to Oxidative Stress
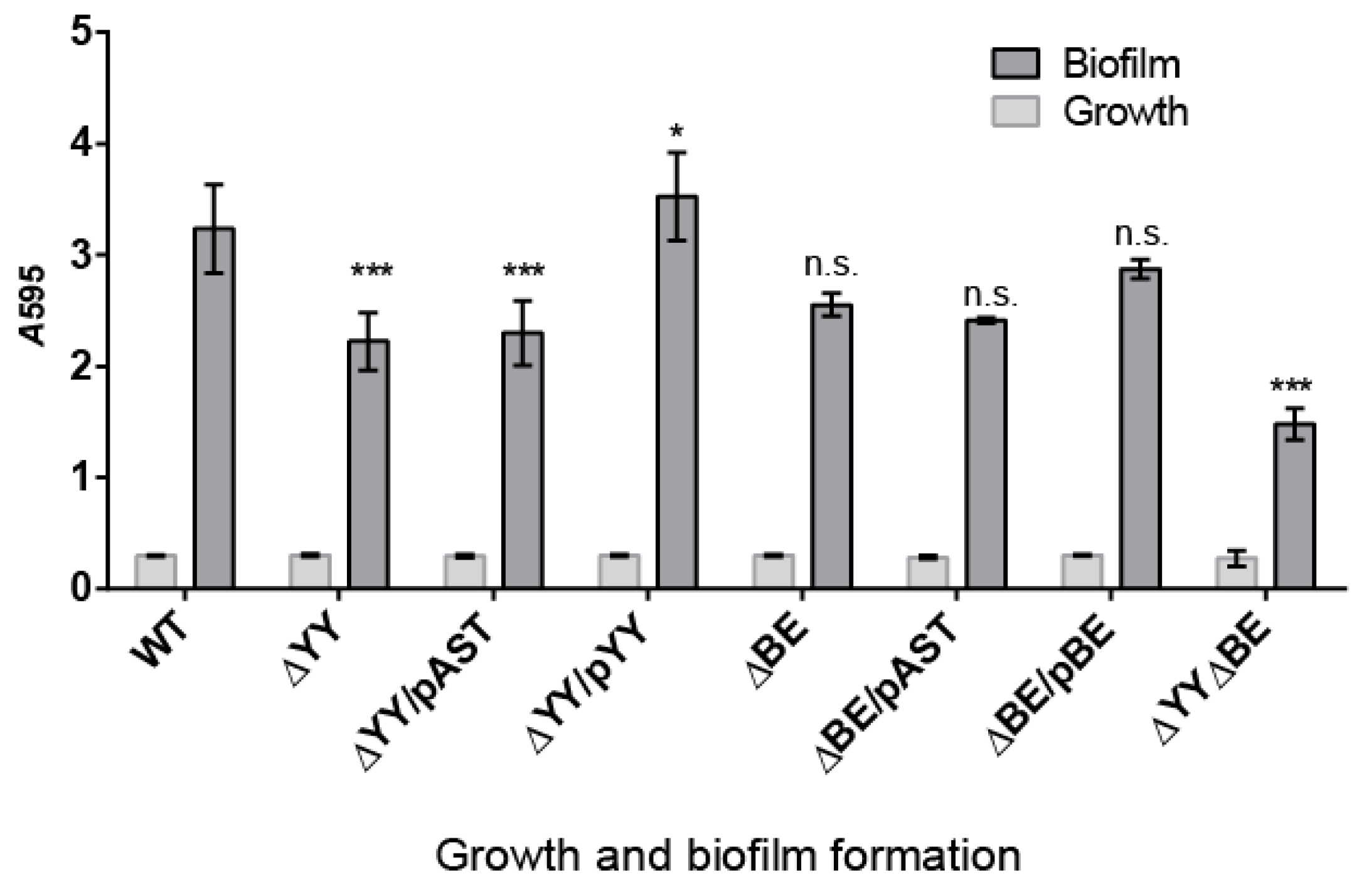
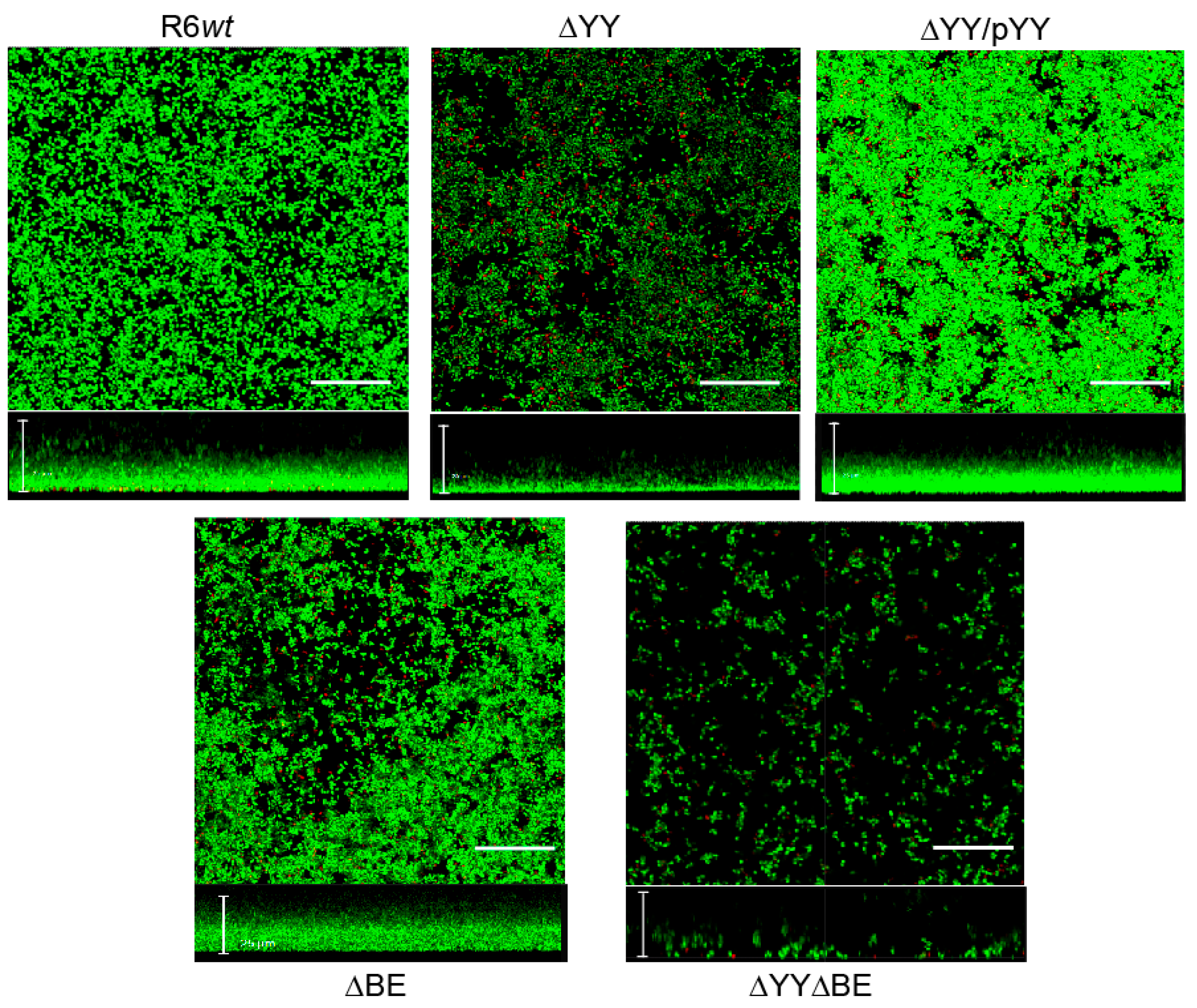
2.3. Pneumococcal Cells Lacking the RNA-Interferase Operons Have Reduced Biofilm Formation
3. Discussion
4. Materials and Methods
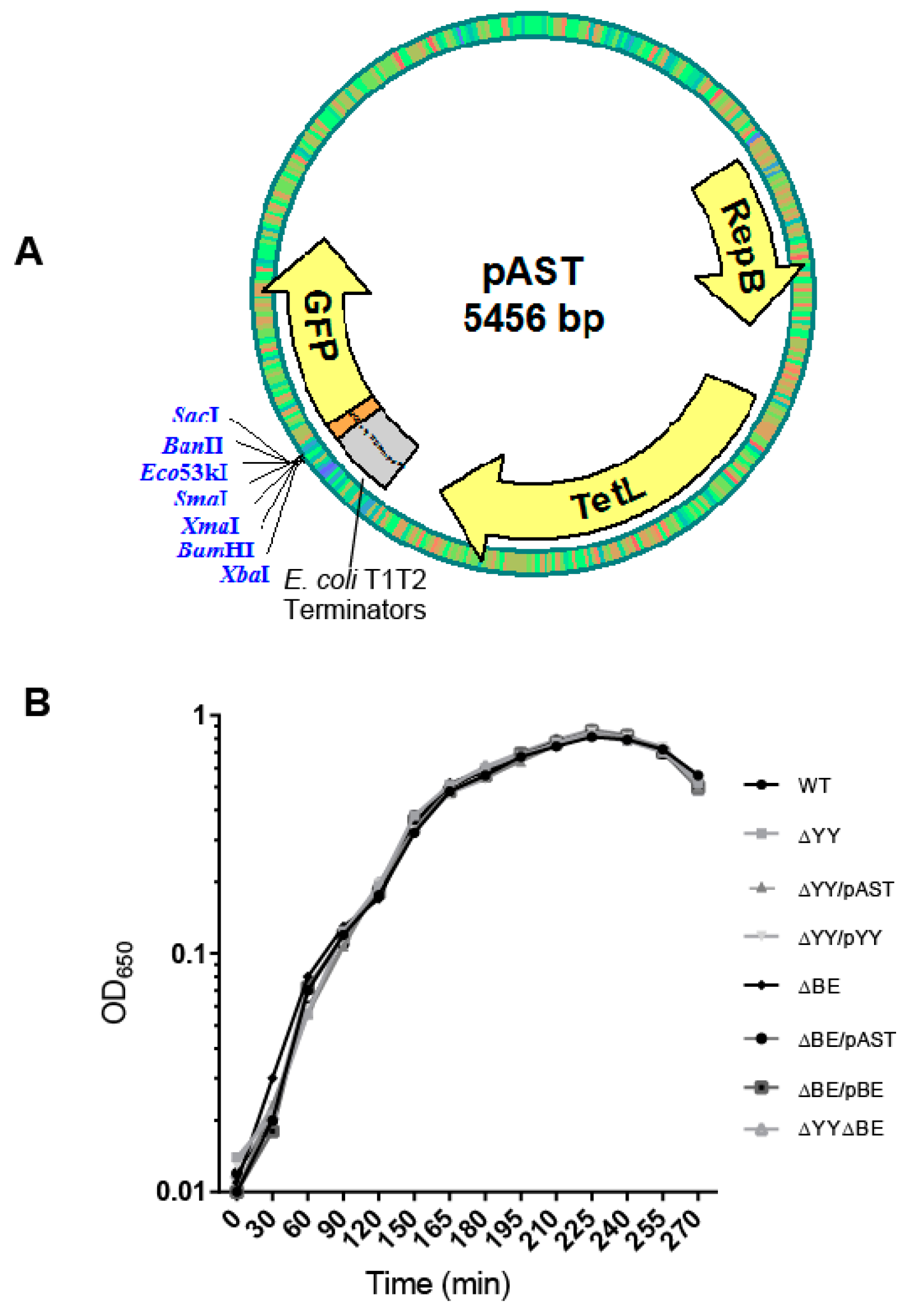
4.1. Culture Conditions, Plasmids, and Construction of Bacterial Strains
4.2. Oxidative Stress Assays
4.3. Biofilm Formation and Quantification
4.4. Confocal Laser Scanning Microscopy (CLSM)
4.5. Statistical and Computer Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| bp | base pair(s) |
| CFU | colony-forming units |
| CLSM | Confocal Laser Scanning Microscopy |
| nt | nucleotide(s) |
| OD | optical density |
| TA | Toxin-Antitoxin |
| wt | wild type |
References
- Gamez, G.; Hammerschmidt, S. Combat pneumococcal infections: Adhesins as candidates for protein-based vaccine development. Curr. Drug Targets 2012, 13, 323–337. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.L.; Wolfson, L.J.; Watt, J.P.; Henkle, E.; Deloria-Knoll, M.; McCall, N.; Lee, E.; Mulholland, K.; Levine, O.S.; Cherian, T. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: Global estimates. Lancet 2009, 374, 893–902. [Google Scholar] [CrossRef]
- Hanage, W.P.; Fraser, C.; Tang, J.; Connor, T.R.; Corander, J. Hyper-recombination, diversity, and antibiotic resistance in pneumococcus. Science 2009, 324, 1454–1457. [Google Scholar] [CrossRef] [PubMed]
- Darrieux, M.; Goulart, C.; Briles, D.; Leite, L.C.D.C. Current status and perspectives on protein-based pneumococcal vaccines. Crit. Rev. Microbiol. 2015, 41, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Farrell, D.J.; Klugman, K.P.; Pichichero, M. Increased antimicrobial resistance among nonvaccine serotypes of Streptococcus pneumoniae in the pediatric population after the introduction of 7-valent pneumococcal vaccine in the United States. Pediatr. Infect. Dis. J. 2007, 26, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.T.; Balsa, D.; Espinosa, M. One cannot rule them all: Are bacterial toxins-antitoxins druggable? FEMS Microbiol. Rev. 2015, 39, 522–540. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.C.; Balsa, D.; Cherny, I.; Christensen, S.K.; Espinosa, M.; Francuski, D.; Gazit, E.; Gerdes, K.; Hitchin, E.; Martín, M.T.; et al. Bacterial toxin-antitoxin systems as targets for the development of novel antibiotics. In Enzyme-Mediated Resistance to Antibiotics: Mechanisms, Dissemination, and Prospects for Inhibition; Bonomo, R.A., Tolmasky, M.E., Eds.; ASM Press: Washington, DC, USA, 2007; pp. 313–329. [Google Scholar]
- Gerdes, K. Prokaryotic toxin-antitoxins. In Prokaryotic Toxin-Antitoxins; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Mutschler, H.; Meinhart, A. Ε/ζ systems: Their role in resistance, virulence, and their potential for antibiotic development. J. Mol. Med. 2011, 89, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.T.; Espinosa, M. The Antibacterials That Have Yet to Be Found. Atlas of Science 2016. Available online: https://atlasofscience.org/the-antibacterials-that-have-yet-to-be-found/ (accessed on 24 February 2016).
- Fernández-Bachiller, M.; Brzozowska, I.; Odolczyk, N.; Zielenkiewicz, U.; Zielenkiewicz, P.; Rademann, J. Mapping protein–protein interactions of the resistance-related bacterial zeta toxin–epsilon antitoxin complex (ε2ζ2) with high affinity peptide ligands using fluorescence polarization. Toxins 2016, 8, 222. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.T.; Espinosa, M.; Yeo, C.C. Keeping the wolves at bay: Antitoxins of prokaryotic type II toxin-antitoxin systems. Front. Mol. Biosci. 2016, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.T.; Moreno-Córdoba, I.; Yeo, C.C.; Espinosa, M. Toxin-antitoxin genes of the Gram-positive pathogen Streptococcus pneumoniae: so few and yet so many. Microbiol. Mol. Biol. Rev. 2012, 76, 773–791. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pino, A.; Christensen-Dalsgaard, M.; Wyns, L.; Yarmolinsky, M.B.; Magnuson, R.D.; Gerdes, K.; Loris, R. Doc of prophage P1 is inhibited by its antitoxin partner Phd through fold complementation. J. Biol. Chem. 2008, 283, 30821–30827. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.T.; Yeo, C.C.; Sadowy, E.; Espinosa, M. Functional validation of putative toxin-antitoxin genes from the Gram-positive pathogen Streptococcus pneumoniae: phd-doc is the fourth bona-fide operon. Front. Microbiol. 2014, 5, 677. [Google Scholar] [CrossRef] [PubMed]
- Khoo, S.K.; Loll, B.; Chan, W.T.; Shoeman, R.L.; Ngoo, L.; Yeo, C.C.; Meinhart, A. Molecular and structural characterization of the PezAT chromosomal toxin-antitoxin system of the human pathogen Streptococcus pneumoniae. J. Biol. Chem. 2007, 282, 19606–19618. [Google Scholar] [CrossRef] [PubMed]
- Mutschler, H.; Gebhardt, M.; Shoeman, R.L.; Meinhart, A. A novel mechanism of programmed cell death in bacteria by toxin-antitoxin systems corrupts peptidoglycan synthesis. PLoS Biol. 2011, 9, e1001033. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.T.; Espinosa, M. The Streptococcus pneumoniae pezAT toxin antitoxin system reduces β-lactam resistance and genetic competence. Front. Microbiol. 2016, 7, 1322. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Córdoba, I.; Diago-Navarro, E.; Barendregt, A.; Heck, A.J.R.; Alfonso, C.; Díaz-Orejas, R.; Nieto, C.; Espinosa, M. The toxin antitoxin proteins RelBE2Spn of Streptococcus pneumoniae: Characterization and association to their DNA target. Proteins 2012, 80, 1834–1846. [Google Scholar] [CrossRef] [PubMed]
- Nieto, C.; Pellicer, T.; Balsa, D.; Christensen, S.K.; Gerdes, K.; Espinosa, M. The chromosomal relBE2 toxin–antitoxin locus of Streptococcus pneumoniae: Characterization and use of a bioluminescence resonance energy transfer assay to detect toxin–antitoxin interaction. Mol. Microbiol. 2006, 59, 1280–1296. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.T.; Nieto, C.; Harikrishna, J.A.; Khoo, S.K.; Yasmin Othman, R.; Espinosa, M.; Yeo, C.C. Genetic regulation of the yefM-yoeB toxin-antitoxin locus of Streptococcus pneumoniae. J. Bacteriol. 2011, 193, 4612–4625. [Google Scholar] [CrossRef] [PubMed]
- Nieto, C.; Cherny, I.; Khoo, S.K.; García de Lacoba, M.; Chan, W.T.; Yeo, C.C.; Gazit, E.; Espinosa, M. The yefM-yoeB toxin-antitoxin systems of Escherichia coli and Streptococcus pneumoniae: Functional and structural correlation. J. Bacteriol. 2007, 189, 1266–1278. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.K.; Gerdes, K. RelE toxins from bacteria and archaea cleave mRNAs on translating ribosomes, which are rescued by tmRNA. Mol. Microbiol. 2003, 48, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, K.; Christensen, S.K.; Løbner-Olensen, A. Prokaryotic toxin–antitoxin stress response loci. Nat. Rev. Microbiol. 2005, 3, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Nieto, C.; Sadowy, E.; de la Campa, A.G.; Hryniewicz, W.; Espinosa, M. The relBE2Spn toxin-antitoxin system of Streptococcus pneumoniae: Role in antibiotic tolerance and functional conservation in clinical isolates. PLoS ONE 2010, 5, e11289. [Google Scholar] [CrossRef] [PubMed]
- Lioy, V.S.; Martin, M.T.; Camacho, A.G.; Lurz, R.; Antelmann, H.; Hecker, M.; Hitchin, E.; Ridge, Y.; Wells, J.M.; Alonso, J.C. pSM19035-encoded ζ toxin induces stasis followed by death in a subpopulation of cells. Microbiology 2006, 152, 2365–2379. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.T.; Moreno-Córdoba, I.; Yeo, C.C.; Espinosa, M. Toxin-antitoxin loci in Streptococcus pneumoniae. In Prokaryotic Toxin-Antitoxins; Gerdes, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 315–339. [Google Scholar]
- Hoskins, J.; Alborn, W.E., Jr.; Arnold, J.; Blaszczak, L.C.; Burgett, S.; DeHoff, B.S.; Estrem, S.T.; Fritz, L.; Fu, D.J.; Fuller, W.; et al. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 2001, 183, 5709–5717. [Google Scholar] [CrossRef] [PubMed]
- Valverde, R.; Edwards, L.; Regan, L. Structure and function of KH domains. FEBS J. 2008, 275, 2712–2726. [Google Scholar] [CrossRef] [PubMed]
- Nolle, N.; Schuster, C.F.; Bertram, R. Two paralogous yefM-yoeB loci from Staphylococcus equorum encode functional toxin–antitoxin systems. Microbiology 2013, 159, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- López, P.; Espinosa, M.; Stassi, D.L.; Lacks, S.A. Facilitation of plasmid transfer in Streptococcus pneumoniae by chromosomal homology. J. Bacteriol. 1982, 150, 692–701. [Google Scholar] [PubMed]
- Song, J.-H.; Ko, K.S.; Lee, J.-Y.; Baek, J.Y.; Oh, W.S.; Yoon, H.S.; Jeong, J.-Y.; Chun, J. Identification of essential genes in Streptococcus pneumoniae by allelic replacement mutagenesis. Mol. Cells 2005, 19, 365–374. [Google Scholar] [PubMed]
- Ballester, S.; López, P.; Alonso, J.C.; Espinosa, M.; Lacks, S.A. Selective advantage of deletions enhancing chloramphenicol acetyltransferase gene expression in Streptococcus pneumoniae plasmids. Gene 1986, 41, 153–163. [Google Scholar] [CrossRef]
- Ruiz-Cruz, S.; Solano-Collado, V.; Espinosa, M.; Bravo, A. Novel plasmid-based genetic tools for the study of promoters and terminators in Streptococcus pneumoniae and Enterococcus faecalis. J. Microbiol. Methods 2010, 83, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, M. Plasmids as models to study macromolecular interactions: The pMV158 paradigm. Res. Microbiol. 2013, 164, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Brosius, J.; Dull, T.J.; Sleeter, D.D.; Noller, H.F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J. Mol. Biol. 1981, 148, 107–127. [Google Scholar] [CrossRef]
- Miller, W.G.; Lindow, S.E. An improved GFP cloning cassette designed for prokaryotic transcriptional fusions. Gene 1997, 191, 149–153. [Google Scholar] [CrossRef]
- Saluja, S.K.; Weiser, J.N. The genetic basis of colony opacity in Streptococcus pneumoniae: Evidence for the effect of BOX elements on the frequency of phenotypic variation. Mol. Microbiol. 1995, 16, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Buts, L.; Lah, J.; Dao-Thi, M.H.; Wyns, L.; Loris, R. Toxin-antitoxin modules as bacterial metabolic stress managers. Trends Biochem. Sci. 2005, 30, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.K.; Mikkelsen, M.; Pedersen, K.; Gerdes, K. RelE, a global inhibitor of translation, is activated during nutritional stress. Proc. Natl. Acad. Sci. USA 2001, 98, 14328–14333. [Google Scholar] [CrossRef] [PubMed]
- Lioy, V.S.; Machon, C.; Tabone, M.; Gonzalez-Pastor, J.E.; Daugelavicius, R.; Ayora, S.; Alonso, J.C. The ζ toxin induces a set of protective responses and dormancy. PLoS ONE 2012, 7, e30282. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wood, T.K. Toxin-antitoxin systems influence biofilm and persister cell formation and the general stress response. Appl. Environ. Microbiol. 2011, 77, 5577–5583. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, E.; Gerdes, K. Molecular mechanisms underlying bacterial persisters. Cell 2014, 157, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Gotfredsen, M.; Gerdes, K. Toxin-antitoxin modules may regulate synthesis of macromolecules during nutritional stress. J. Bacteriol. 2002, 182, 561–572. [Google Scholar]
- Forman, H.J.; Torres, M. Reactive oxygen species and cell signaling. Am. J. Resp. Crit. Care Med. 2002, 166, S4–S8. [Google Scholar] [CrossRef] [PubMed]
- Fittipaldi, N.; Segura, M.; Grenier, D.; Gottschalk, M. Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Future Microbiol. 2012, 7, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Pericone, C.D.; Park, S.; Imlay, J.A.; Weiser, J.N. Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the toxic effects of the Fenton reaction. J. Bacteriol. 2003, 185, 6815–6825. [Google Scholar] [CrossRef] [PubMed]
- Bortoni, M.E.; Terra, V.S.; Hinds, J.; Andrew, P.W.; Yesilkaya, H. The pneumococcal response to oxidative stress includes a role for Rgg. Microbiology 2009, 155, 4123–4134. [Google Scholar] [CrossRef] [PubMed]
- Chionh, Y.H.; McBee, M.; Babu, I.R.; Hia, F.; Lin, W.; Zhao, W.; Cao, J.; Dziergowska, A.; Malkiewicz, A.; Begley, T.J.; et al. tRNA-mediated codon-biased translation in mycobacterial hypoxic persistence. Nat. Commun. 2016, 7, 13302. [Google Scholar] [CrossRef] [PubMed]
- Piñas, G.E.; Cortes, P.R.; Albarracín-Orio, A.G.; Echenique, J. Acidic stress induces autolysis by a CSP-independent ComE pathway in Streptococcus pneumoniae. Microbiology 2008, 154, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Martín-Galiano, A.J.; Overweg, K.; Ferrándiz, M.J.; Reuter, M.; Wells, J.M.; de la Campa, A.G. Transcriptional analysis of the acid tolerance response in Streptococcus pneumoniae. Microbiology 2005, 151, 3935–3946. [Google Scholar] [CrossRef] [PubMed]
- Versiek, J. Trace elements in human body fluids and tissues. Crit. Rev. Clin. Lab. Sci. 1985, 22, 97–184. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, F.E.; Kazmierczak, K.M.; Lisher, J.P.; Winkler, M.E.; Giedroc, D. Interplay between manganese and zinc homeostasis in the human pathogen Streptococcus pneumoniae. Metallomics 2011, 3, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Hu, F.Z.; Gieseke, A.; Nistico, L.; Nguyen, D.; Hayes, J.; Forbes, M.; Greenberg, D.P.; Dice, B.; Burrows, A.; et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 2006, 296, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Wang, X.; Ma, Q.; Zhang, X.S.; Wood, T.K. Toxin-antitoxin systems in Escherichia coli influence biofilm formation through YigK (TabA) and fimbriae. J. Bacteriol. 2009, 191, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Kolodkin-Gal, I.; Verdiger, R.; Shlosberg-Fedida, A.; Engelberg-Kulka, H. A differential effect of Escherichia coli toxin-antitoxin systems on cell death in liquid media and biofilm formation. PLoS ONE 2009, 4, e6785. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Behiels, E.; Devreese, B. Toxin-antitoxin systems: Their role in persistence, biofilm formation and pathogenicity. Pathog. Dis. 2014, 70, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Fleming, B.A.; Mulvey, M.A. Toxin–antitoxin systems as regulators of bacterial fitness and virulence. In Stress and Environmental Regulation of Gene Expression and Adaptation in Bacteria; de Bruijn, F.J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 437–445. [Google Scholar]
- Song, S.; Wood, T.K. Post-segregational killing and phage inhibition are not mediated by cell death through toxin/antitoxin systems. Front. Microbiol. 2018, 9, 814. [Google Scholar] [CrossRef] [PubMed]
- Moscoso, M.; García, E.; López, R. Pneumococcal biofilms. Int. Microbiol. 2009, 12, 77–85. [Google Scholar] [PubMed]
- Moscoso, M.; García, E.; López, R. Biofilm formation by Streptococcus pneumoniae: Role of choline, extracellular DNA, and capsular polysaccharide in microbial accretion. J. Bacteriol. 2006, 188, 7785–7795. [Google Scholar] [CrossRef] [PubMed]
- Domenech, M.; Araújo-Bazán, L.; García, E.; Moscoso, M. In vitro biofilm formation by Streptococcus pneumoniae as a predictor of post-vaccination emerging serotypes colonizing the human nasopharynx. Environ. Microbiol. 2014, 16, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- González Barrios, A.F.; Zuo, R.; Hashimoto, Y.; Yang, L.; Bentley, W.E.; Wood, T.K. Autoinducer 2 controls biofilm formation in Escherichia coli through a novel motility quorum-sensing regulator (MqsR, B3022). J. Bacteriol. 2006, 188, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kim, Y.; Hong, S.H.; Ma, Q.; Brown, B.L.; Pu, M.; Tarone, A.M.; Benedik, M.; Peti, W.; Page, R.; et al. Antitoxin MqsA helps mediate the bacterial general stress response. Nat. Chem. Biol. 2011, 7, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Inouye, M. Characterization of the interactions within the mazEF addiction module of Escherichia coli. J. Biol. Chem. 2003, 278, 32300–32306. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Inouye, M. Type II toxin-antitoxin loci: The mazEF family. In Prokaryotic Toxin-Antitoxin; Gerdes, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 107–136. [Google Scholar]
- Valadbeigi, H.; Sadeghifard, N.; Salehi, M.B. Assessment of biofilm formation in Pseudomonas aeruginosa by antisense mazE-PNA. Microb. Pathog. 2017, 104, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Kędzierska, B.; Hayes, F. Emerging roles of toxin-antitoxin modules in bacterial pathogenesis. Molecules 2016, 21, 790. [Google Scholar] [CrossRef] [PubMed]
- Conlon, B.P.; Nakayasu, E.S.; Fleck, L.E.; LaFleur, M.D.; Isabella, V.M.; Coleman, K.; Leonard, S.N.; Smith, R.D.; Adkins, J.N.; Lewis, K. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature 2013, 503, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Guilhen, C.; Forestier, C.; Balestrino, D. Biofilm dispersal: Multiple elaborate strategies for dissemination of bacteria with unique properties. Mol. Microbiol. 2017, 105, 188–210. [Google Scholar] [CrossRef] [PubMed]
- Lacks, S.A.; Hotchkiss, R. A study of the genetic material determining an enzyme activity in pneumococcus. Biochim. Biohys. Acta 1960, 39, 508–517. [Google Scholar] [CrossRef]
- Lacks, S.A.; Lopez, P.; Greenberg, B.; Espinosa, M. Identification and analysis of genes for tetracycline resistance and replication functions in the broad-host-range plasmid pLS1. J. Mol. Biol. 1986, 192, 753–765. [Google Scholar] [CrossRef]
- Moscoso, M.; Domenech, M.; García, E. Vancomycin tolerance in clinical and laboratory Streptococcus pneumoniae isolates depends on reduced enzyme activity of the major LytA autolysin or cooperation between CiaH histidine kinase and capsular polysaccharide. Mol. Microbiol. 2010, 77, 1052–1064. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.K.; Li, H.; Claverys, J.P.; Morrison, D.A. An rpsl cassette, Janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl. Environ. Microbiol. 2001, 67, 5190–5196. [Google Scholar] [CrossRef] [PubMed]
| Primers | Sequences 5′ → 3′ | Restriction Sites * |
|---|---|---|
| kan-F | AGCAGAGCTCCTTATCGATACCGTCGACCTC | SacI |
| kan-R | AGCAACTAGTCCCCTATCTAGCGAACTTTTA | SpeI |
| CmF | CGGATTTTATGACCGATGATG | - |
| CmR | TAACGCGGCAGGTTAGTGAC | - |
| KmF | CTTATCGATACCGTCGACCTC | - |
| KmR | CCCCTATCTAGCGAACTTTTAG | - |
| L-F yefMyoeB | TTTCTTAGAACGTTTTATGCCTTC | - |
| L-R yefMyoeB | GAGGTCGACGGTATCGATAAGCGCGATTTGAATTTGATTTTCG | - |
| R-F yefMyoeB | CTAAAAGTTCGCTAGATAGGGGGTCTACTGTAAAGTAGGCTTTTTC | - |
| R-R yefMyoeB | CTCGTCAAATTGTCGTCCTT | - |
| L-F relBE | ATGAAAAGACCTGGCAAGCTATG | - |
| L-R relBE | CATCATCGGTCATAAAATCCGTATAAAAAGAACACCTTCTCAGCG | - |
| R-F relBE | GTCACTAACCTGCCCCGTTAGGTCATCGGAGAGATATTTATTGA | - |
| R-R relBE | GACTTCATCTGAAACCTCACG | - |
| Sec 1.1 | CAAACTAAGTCTACTGAAAGGTAGG | - |
| Sec 2.1 | TGTTTTTACCTCATTTTATTGTTATTCC | - |
| yefMyoeB-F | CAGAGGATCCTCTACTGAAAGGTAGGCTTT | BamHI |
| yefMyoeB-R | CAGCGAGCTCAAATAGTAGTTTAGTAGAGA | SacI |
| relBE-F | CAGAGGATCCAAAGAACGCTGAGAAGGTGT | BamHI |
| relBE-R | CAGCGAGCTCGTAAGCCCTATTATATCATA | SacI |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, W.T.; Domenech, M.; Moreno-Córdoba, I.; Navarro-Martínez, V.; Nieto, C.; Moscoso, M.; García, E.; Espinosa, M. The Streptococcus pneumoniae yefM-yoeB and relBE Toxin-Antitoxin Operons Participate in Oxidative Stress and Biofilm Formation. Toxins 2018, 10, 378. https://doi.org/10.3390/toxins10090378
Chan WT, Domenech M, Moreno-Córdoba I, Navarro-Martínez V, Nieto C, Moscoso M, García E, Espinosa M. The Streptococcus pneumoniae yefM-yoeB and relBE Toxin-Antitoxin Operons Participate in Oxidative Stress and Biofilm Formation. Toxins. 2018; 10(9):378. https://doi.org/10.3390/toxins10090378
Chicago/Turabian StyleChan, Wai Ting, Mirian Domenech, Inmaculada Moreno-Córdoba, Verónica Navarro-Martínez, Concha Nieto, Miriam Moscoso, Ernesto García, and Manuel Espinosa. 2018. "The Streptococcus pneumoniae yefM-yoeB and relBE Toxin-Antitoxin Operons Participate in Oxidative Stress and Biofilm Formation" Toxins 10, no. 9: 378. https://doi.org/10.3390/toxins10090378
APA StyleChan, W. T., Domenech, M., Moreno-Córdoba, I., Navarro-Martínez, V., Nieto, C., Moscoso, M., García, E., & Espinosa, M. (2018). The Streptococcus pneumoniae yefM-yoeB and relBE Toxin-Antitoxin Operons Participate in Oxidative Stress and Biofilm Formation. Toxins, 10(9), 378. https://doi.org/10.3390/toxins10090378





