Multi-Mycotoxin Occurrence in Dairy Cattle Feeds from the Gauteng Province of South Africa: A Pilot Study Using UHPLC-QTOF-MS/MS
Abstract
1. Introduction
2. Results
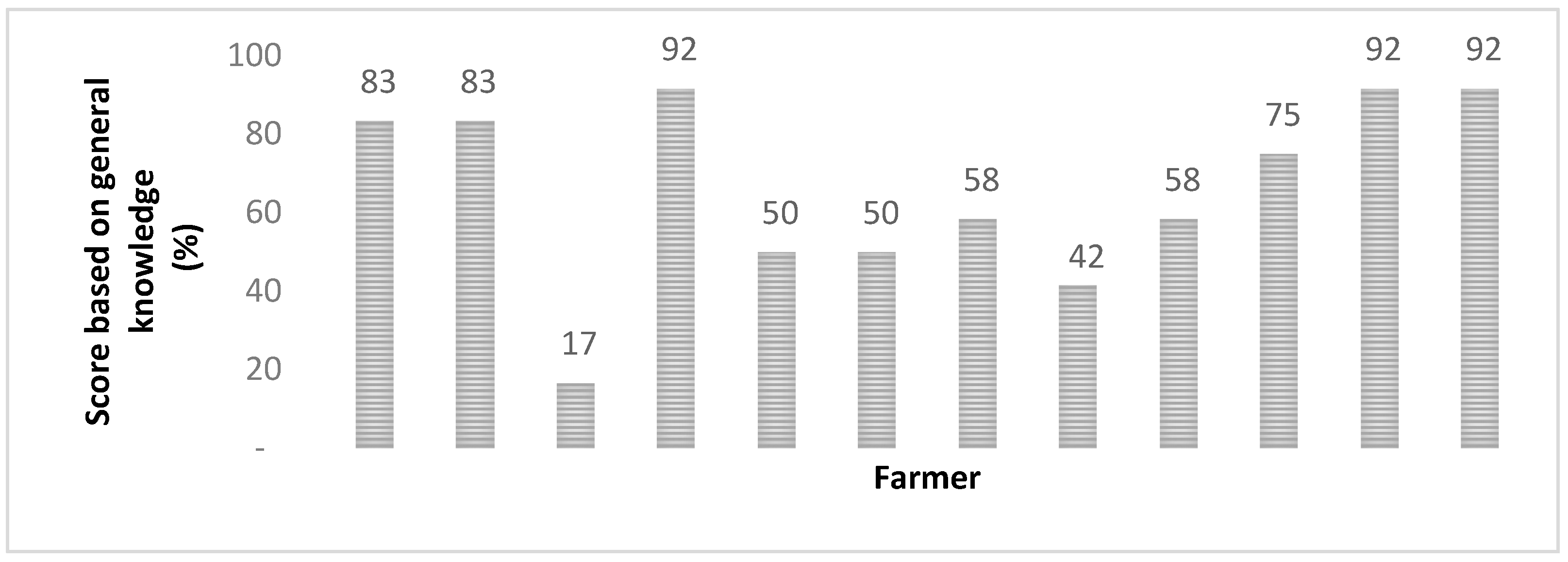
2.1. Farmer Perception Survey
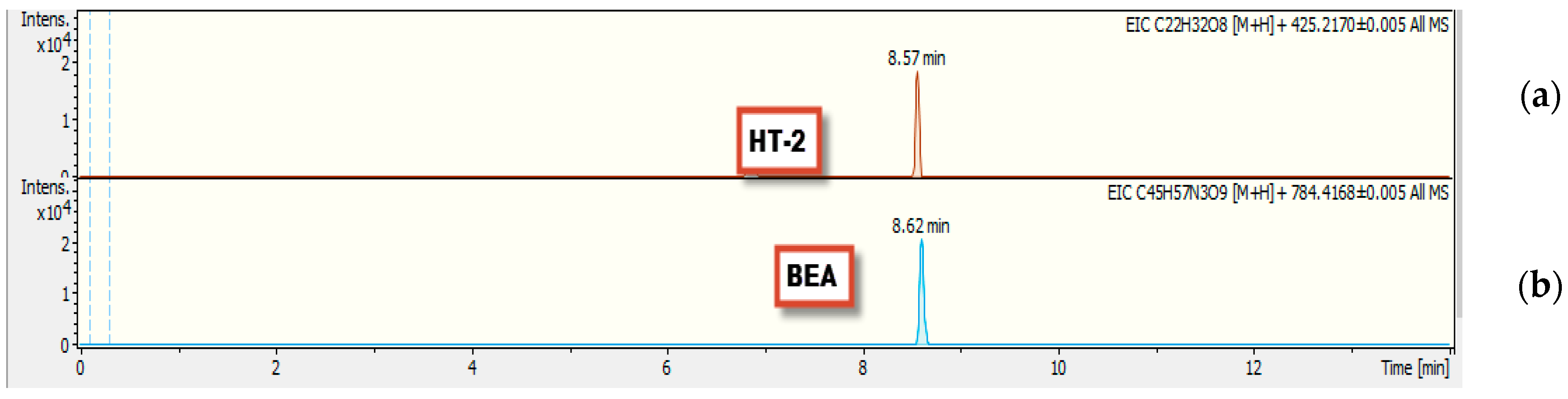
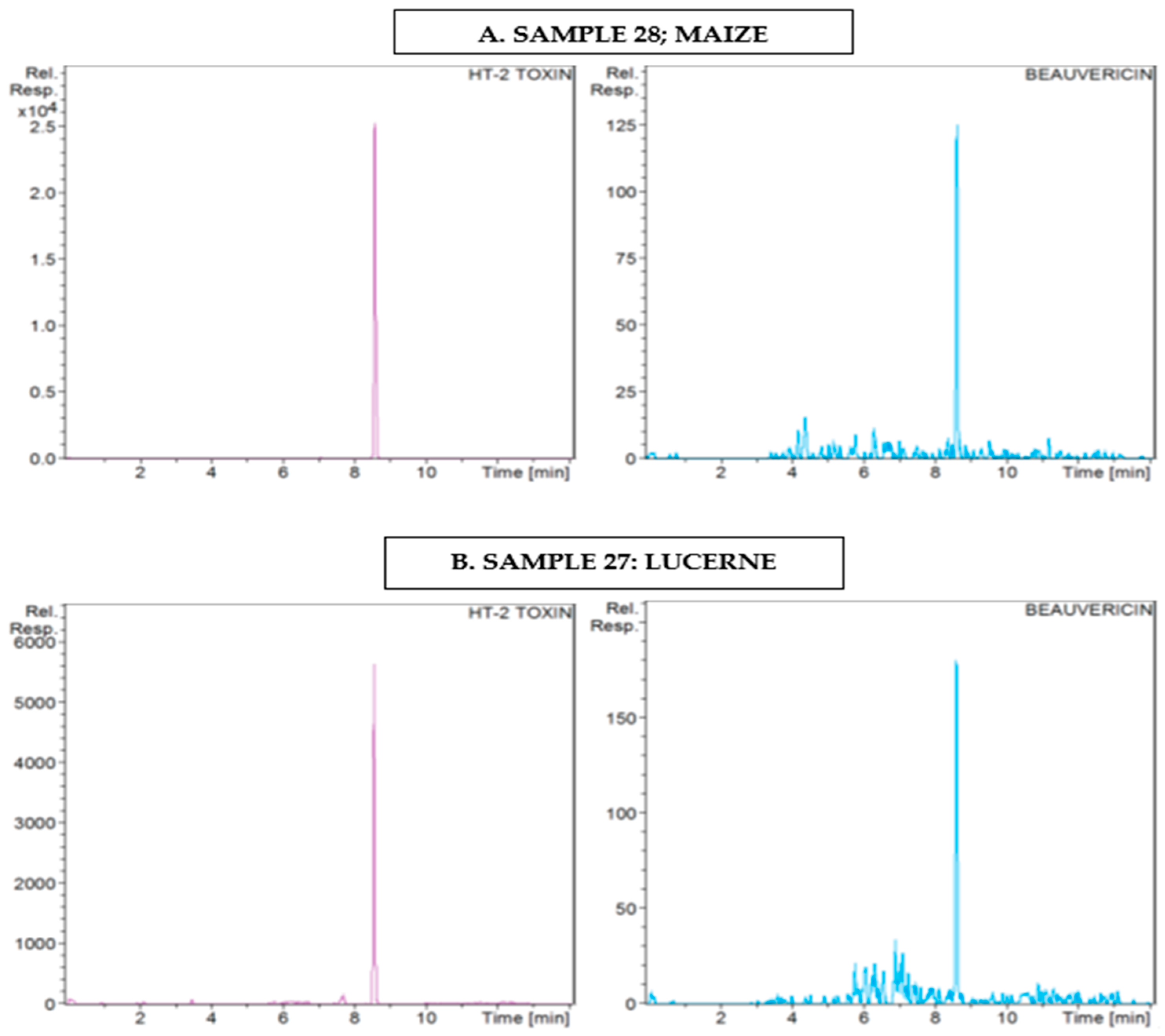
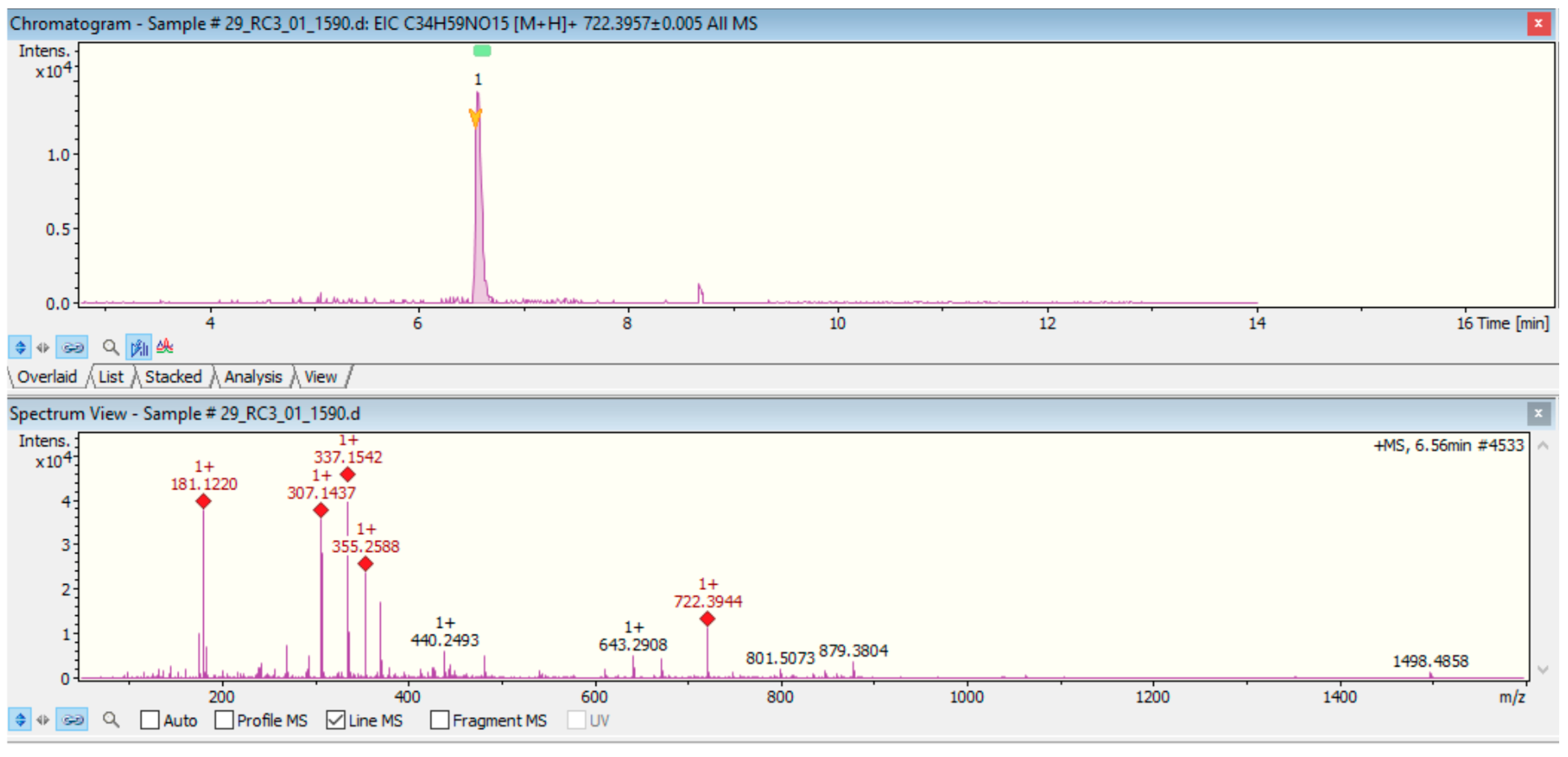
2.2. Qualitative Screening Identification
2.3. Quantitative Screening
2.3.1. Method Performance Characteristics
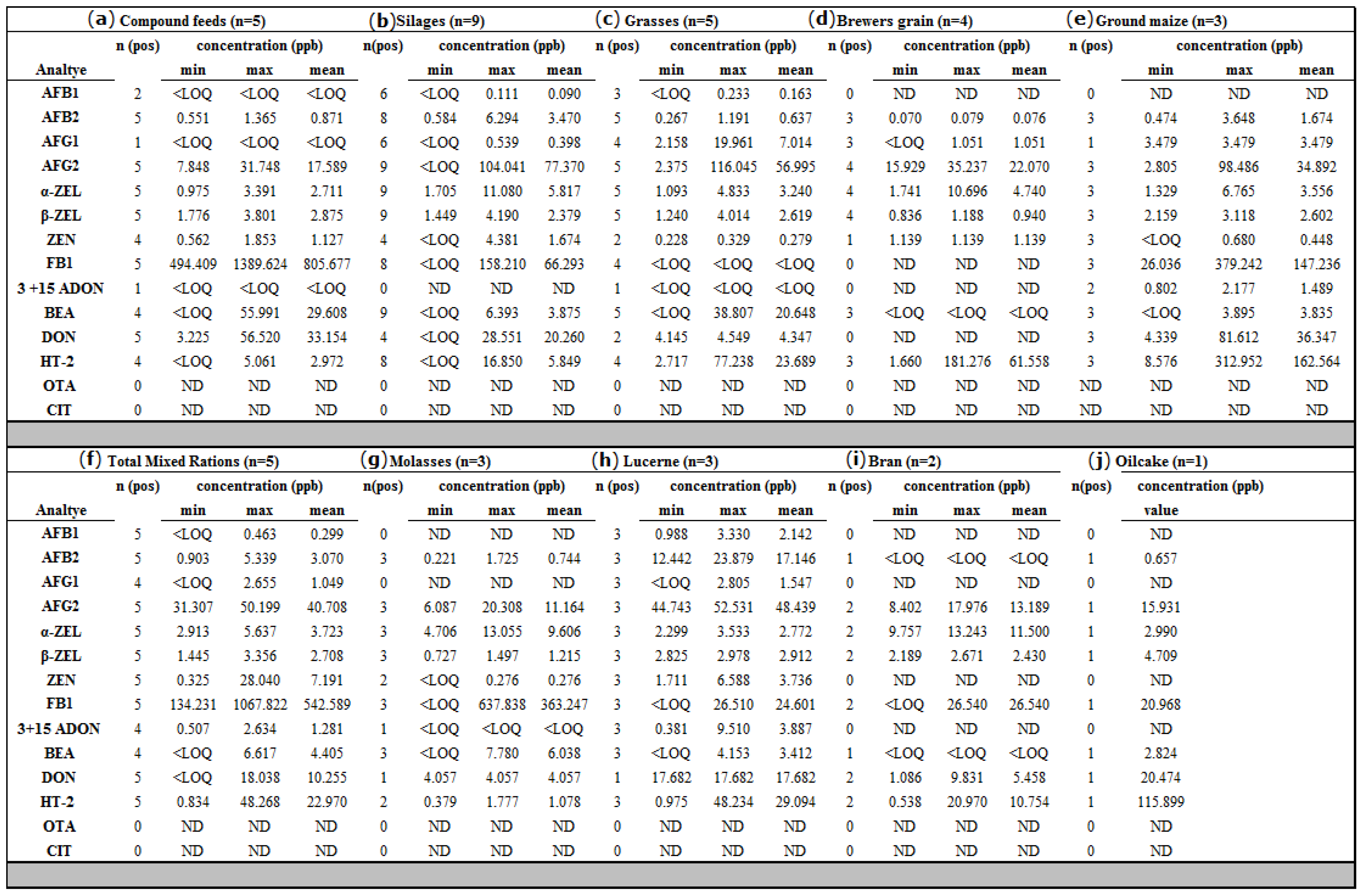
2.3.2. Natural Occurrence of Mycotoxins in Dairy Feed Samples
3. Discussion
3.1. Natural Occurrence in Feeds
3.1.1. Aflatoxins
3.1.2. Fumonisins
3.1.3. Trichothecenes
3.1.4. Zearalenones
3.1.5. Beauvericin
3.1.6. Ochratoxin A
3.2. Mycotoxin Co-Occurrence
4. Conclusions
5. Materials and Methods
5.1. Study Sites and Socio-Demographic Survey
5.2. Samples
5.3. Standard Solutions and Reagents
- Aflatoxin mix: AFB1, AFB2, AFG1 and AFG2 in acetonitrile at concentrations of 50 ppb each.
- Multi mix: DON, FB1, BEA, and CIT at individual concentrations of 2000 ppb each; HT-2, ZEN, α-ZEL, β-ZEL, 15-ADON and 3-ADON at 500 ppb each; and OTA at 135 ppb.
5.4. Sample Extraction
5.5. LC-QTOF-MS/MS Conditions
- 2.5, 5, 15, 25 and 50 ppb for AFB1, AFB2, AFG1 and AFG2
- 50, 100, 200, 500, 1000 and 2000 ppb for DON, FB1, BEA, and CIT
- 12.5, 25, 50, 125, 250 and 500 ppb for HT-2, ZEN, α-ZEL, β-ZEL, 15-ADON and 3-ADON
- 3.375, 6.75, 13.5, 33.75, 67.5 and 135 ppb for OTA.
5.6. Ethical Clearance of the Study
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Qualitative Results
| Sample | Sample Type | REGULATED MYCOTOXINS | OTHER MYCOTOXINS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AFB1 | AFB2 | AFG1 | AFG2 | ZEN | FB1 | DON | HT-2 | BEA | ADONs | α-ZEL | β-ZEL | ||
| # 12 | Pellets | ||||||||||||
| # 29 | Dairy maize meal | ||||||||||||
| # 33 | Dairy maize meal | ||||||||||||
| # 34 | Pellets | ||||||||||||
| # 39 | Dairy Pellets | ||||||||||||
| # 01 | Mixed TMR | ||||||||||||
| # 10 | 17% TMR | ||||||||||||
| # 11 | 19% TMR | ||||||||||||
| # 23 | TMR Semi-complete | ||||||||||||
| # 40 | TMR | ||||||||||||
| # 26 | Oilcake | ||||||||||||
| # 09 | Ground maize | ||||||||||||
| # 16 | Maize grain | ||||||||||||
| # 28 | Maize | ||||||||||||
| # 02 | Teff | ||||||||||||
| # 06 | Dried grass | ||||||||||||
| # 13 | Dried grass | ||||||||||||
| #14 | Dried grass | ||||||||||||
| # 22 | Dried grass | ||||||||||||
| # 27 | Lucerne | ||||||||||||
| # 37 | Lucerne | ||||||||||||
| # 38 | Lucerne Pellets | ||||||||||||
| # 25 | Bran | ||||||||||||
| # 31 | Bran | ||||||||||||
| #03 | Maize silage | ||||||||||||
| #04 | Maize silage | ||||||||||||
| #07 | Maize silage | ||||||||||||
| #18 | Maize silage | ||||||||||||
| #19 | Grass silage | ||||||||||||
| #21 | Silage | ||||||||||||
| #24 | Maize silage | ||||||||||||
| #35 | Silage | ||||||||||||
| #17 | M. silage + BG | ||||||||||||
| # 08 | Brewers grain | ||||||||||||
| # 20 | Brewers grain | ||||||||||||
| # 32 | Brewers grain | ||||||||||||
| # 36 | Brewers grain | ||||||||||||
| #05 | Molasses meal | ||||||||||||
| #15 | Molasses meal | ||||||||||||
| #30 | Molasses | ||||||||||||
| POSITIVE n pos(N) | 19/40 | 37/40 | 22/40 | 40/40 | 24/40 | 34/40 | 24/40 | 35/40 | 36/40 | 12/40 | 40/40 | 40/40 | |
Appendix B. Spectral Interpretation Output (FB1)
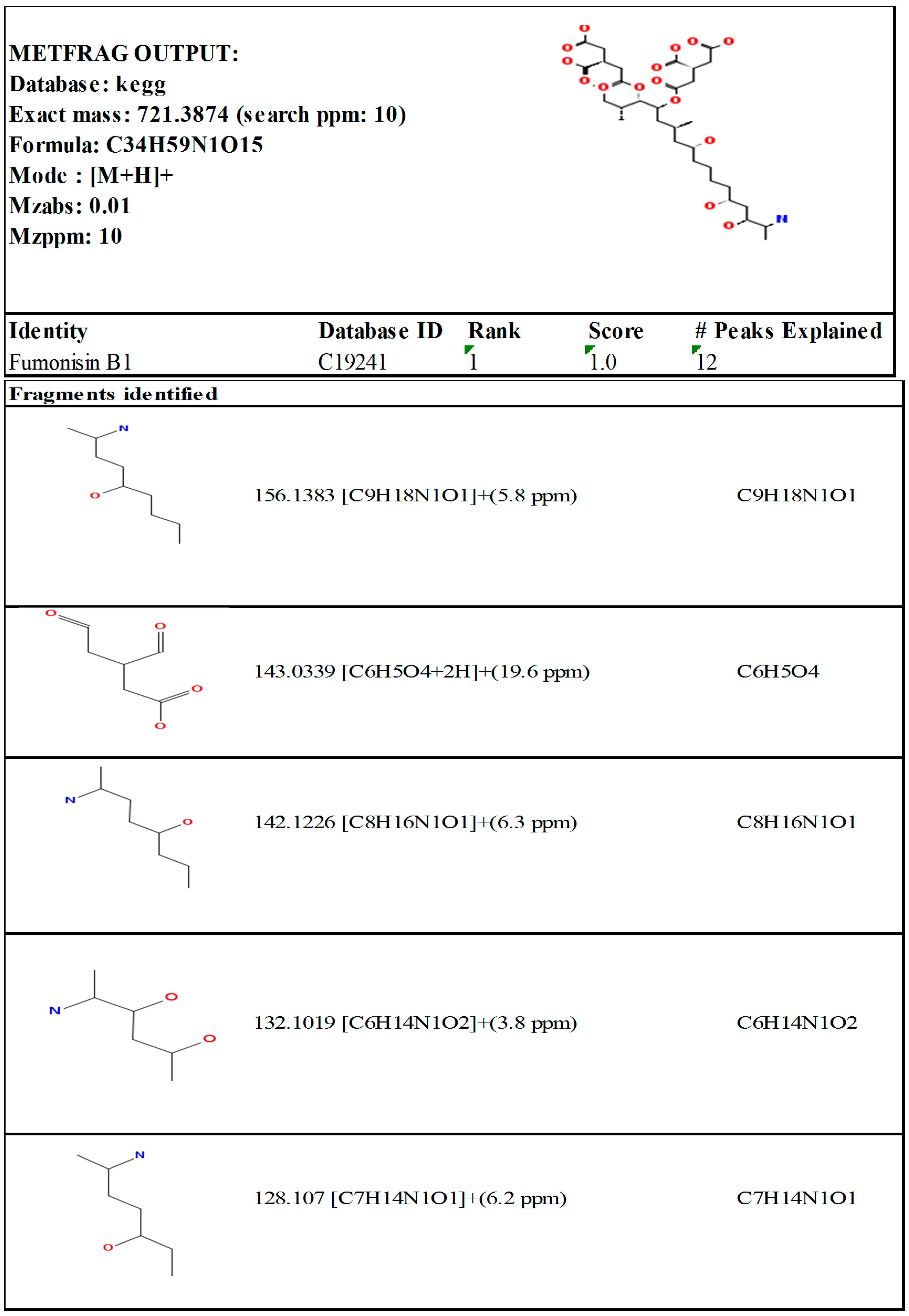

References
- DAFF (Department of Agriculture, Forestry and Fisheries). A Profile of the South African Dairy Market Value Chain; DAFF: Cairns, Queensland, 2011. Available online: http://www.daff.gov.za (accessed on 22 October 2013).
- DAFF (Department of Agriculture, Forestry and Fisheries). A Profile of the South African Dairy Market Value Chain; DAFF: Cairns, Queensland, 2014. Available online: http://www.daff.gov.za (accessed on 29 May 2015).
- Mngadi, P.T.; Govinden, R.; Odhav, B. Co-occurring mycotoxins in animal feeds. Afr. J. Biotechnol. 2008, 13, 2239–2243. [Google Scholar]
- Piva, A.; Fabio, G. Nutritional approaches to reduce the impact of mycotoxins. In Biotechnology in the Feed Industry: Proceedings of the Alltech’s 15th Annual Symposium; Lyons, T.P., Jacques, K.A., Eds.; Nottingham University Press: Nottingham, UK, 1999; pp. 381–399. [Google Scholar]
- Fink-Gremmels, J. Mycotoxins in cattle feeds and carry-over to dairy milk: A review. Food Addit. Contam. Part A 2008, 25, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Hanif, N.Q. Mycotoxin contamination in cattle feed and feed ingredients. Pak. Vet. J. 2009, 29, 211–213. [Google Scholar]
- Whitlow, L.W.; Diaz, D.E.; Hopkins, B.A.; Hagler, W.M. Mycotoxins and milk safety: The potential of block transfer to milk. In Biotechnology in the Feed Industry: Proceedings of Alltech’s 16th Annual Symposium; Lyons, T.P., Jacques, K.A., Eds.; Nottingham University Press: Nottingham, UK, 2000; pp. 391–408. [Google Scholar]
- Njobeh, P.B.; Dutton, M.F.; Åberg, A.; Haggblom, P. Estimation of multi-mycotoxin contamination in South African compound feeds. Toxins 2012, 4, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Dutton, M.F.; Mwanza, M.; de Kock, S.; Khilosia, L. Mycotoxins in South African foods: A case study on AFM1 in milk. Mycotoxin Res. 2012, 28, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Mwanza, M.; Abdel-Hadi, A.; Ali, A.; Egbuta, M. Evaluation of analytical assays efficiency to detect AFM1 in milk from selected areas in Egypt and South Africa. J. Dairy Sci. 2015, 98, 6660–6667. [Google Scholar] [CrossRef] [PubMed]
- Naicker, D.; Marais, G.J.; Van den Berg, H.; Masango, M.G. Some fungi, zearalenone and other mycotoxins in chicken rations, stock feedstuffs, lucerne and pasture grasses in the communal farming area of Rhenosterkop in South Africa. J. S. Afr. Vet. Assoc. 2007, 78, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Sirhan, A.; Tan, G.; Wong, R. Determination of aflatoxins in food using liquid chromatography coupled with electrospray ionization quadrupole time of flight mass spectrometry (LC-ESI-QTOF-MS/MS). Food Control 2013, 31, 35–44. [Google Scholar] [CrossRef]
- Nonaka, Y.; Saito, K.; Hanioka, N.; Narimatsu, S.; Kataoka, H. Determination of aflatoxins in food samples by automated on-line in-tube solid-phase microextraction coupled with liquid chromatography–mass spectrometry. J. Chromatogr. A 2009, 1216, 4416–4422. [Google Scholar] [CrossRef] [PubMed]
- Lopez Grio, S.; Garrido Frenich, A.; Martínez Vidal, J.; Romero-González, R. Determination of aflatoxins B1, B2, G1, G2 and ochratoxin A in animal feed by ultra-high-performance liquid chromatography-tandem mass spectrometry. J. Sep. Sci. 2010, 33, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Oueslati, S.; Romero-González, R.; Lasram, S.; Frenich, A.; Vidal, J. Multi-mycotoxin determination in cereals and derived products marketed in Tunisia using ultra-high-performance liquid chromatography coupled to triple quadrupole mass spectrometry. Food Chem. Toxicol. 2012, 50, 2376–2381. [Google Scholar] [CrossRef] [PubMed]
- Matumba, L.; Sulyok, M.; Njoroge, S.; Njumbe Ediage, E.; Van Poucke, C.; De Saeger, S.; Krska, R. Uncommon occurrence ratios of aflatoxin B1, B2, G1, and G2 in maize and groundnuts from Malawi. Mycotoxin Res. 2014, 31, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Yibadatihan, S.; Jinap, S.; Mahyudin, N. Simultaneous determination of multi-mycotoxins in palm kernel cake (PKC) using liquid chromatography-tandem mass spectrometry (LC-MS/MS). Food Addit. Contam. Part A 2014, 31, 2071–2079. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, J.P.F.; Macdonald, A.M.C. Mycotoxins. Anim. Feed Sci. Technol. 1997, 69, 155–166. [Google Scholar] [CrossRef]
- Bakırdere, S.; Bora, S.; Bakırdere, E.; Aydın, F.; Arslan, Y.; Komesli, O.; Aydın, I.; Yıldırım, E. Aflatoxin species: Their health effects and determination methods in different foodstuffs. Cent. Eur. J. Chem. 2012, 10, 675–685. [Google Scholar] [CrossRef]
- Fallah, A.; Pirali-Kheirabadi, E.; Rahnama, M.; Saei-Dehkordi, S.; Pirali-Kheirabadi, K. Mycoflora, aflatoxigenic strains of Aspergillus section Flavi and aflatoxins in fish feed. Qual. Assur. Saf. Crops 2014, 6, 419–424. [Google Scholar] [CrossRef]
- Sharma, R.K.; Parisi, S. Aflatoxins in Indian food products. In Toxins and Contaminants in Indian Food Products, 1st ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 13–24. ISBN 978-3-319-48049-7. [Google Scholar]
- Schmidt-Heydt, M.; Rufer, C.E.; Abdel-Hadi, A.; Magan, N.; Geisen, R. The production of aflatoxin B1 or G1 by Aspergillus parasiticus at various combinations of temperature and water activity is related to the ratio of aflS to aflR expression. Mycotoxin Res. 2010, 26, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Ayres, J.C.; Koehler, P.E. Influence of temperature cycling on the production of aflatoxins B1 and G1 by Aspergillus parasiticus. Appl. Environ. Microbiol. 1980, 40, 333–336. [Google Scholar] [PubMed]
- Holmes, R.A.; Boston, R.S.; Payne, G.A. Diverse inhibitors of aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 2008, 78, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Carbone, I.; Jakobek, J.; Ramirez-Prado, J.; Horn, B. Recombination, balancing selection and adaptive evolution in the aflatoxin gene cluster of Aspergillus parasiticus. Mol. Ecol. 2007, 16, 4401–4417. [Google Scholar] [CrossRef] [PubMed]
- WHO-IPCS (World Health Organization/International Programme on Chemical Safety). Fumonisin B1—Environmental Health Criteria 219; World Health Organization: Geneva, Switzerland, 2000. Available online: http://www.inchem.org/documents/ehc/ehc/ehc219.htm (accessed on 27 May 2015).
- Rodrigues, I.; Handl, J.; Binder, E. Mycotoxin occurrence in commodities, feeds and feed ingredients sourced in the Middle East and Africa. Food Addit. Contam. 2011, 4, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Rheeder, J.P.; Marasas, W.F.O.; Thiel, P.G.; Sydenham, E.W.; Shephard, G.S.; van Schalkwyk, D.J. Fusarium moniliforme and fumonisins in corn in relation to human oesophageal cancer in Transkei. Phytopathology 1992, 82, 353–357. [Google Scholar] [CrossRef]
- Shephard, G.S.; Marasas, W.F.O.; Leggott, N.L.; Yazdanpanah, H.; Rahimian, H.; Safavi, N. Natural occurrence of fumonisins in corn from Iran. J. Agric. Food Chem. 2000, 48, 1860–1864. [Google Scholar] [CrossRef] [PubMed]
- Krska, R.; Welziga, E.; Boudra, H. Analysis of Fusarium toxins in feed. Anim. Feed Sci. Technol. 2007, 137, 241–264. [Google Scholar] [CrossRef]
- EC (European Commission). Commission recommendation 2006/576/EC on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 toxins and fumonisins in products for animal feeding. Off. J. Eur. Union L 2006, 229, 7–9. [Google Scholar]
- Hickert, S.; Gerding, J.; Ncube, E.; Hübner, F.; Flett, B.; Cramer, B.; Humpf, H. A new approach using micro HPLC-MS/MS for multi-mycotoxin analysis in maize samples. Mycotoxin Res. 2015, 31, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Chilaka, C.A.; De Kock, S.; Phoku, J.Z.; Mwanza, M.; Egbuta, M.A.; Dutton, M.F. Fungal and mycotoxin contamination of South African commercial maize. J. Food Agric. Environ. 2012, 10, 296–303. [Google Scholar]
- Keller, L.; Pereyra, C.; Cavaglieri, L.; Dalcero, A.; Rosa, C. Fungi and mycotoxins from pre- and poststorage brewer’s grain intended for bovine intensive rearing. ISRN Vet. Sci. 2012, 2012, 396590. [Google Scholar] [CrossRef] [PubMed]
- Botha, C.; Legg, M.; Truter, M.; Sulyok, M. Multitoxin analysis of Aspergillus clavatus infected feed samples implicated in two outbreaks of neuromycotoxicosis in cattle in South Africa. Onderstepoort J. Vet. Res. 2014, 81, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kovalsky, P.; Kos, G.; Nährer, K.; Schwab, C.; Jenkins, T.; Schatzmayr, G.; Sulyok, M.; Krska, R. Co-occurrence of regulated, masked and emerging mycotoxins and secondary metabolites in finished feed and maize: An extensive survey. Toxins 2016, 8, 363. [Google Scholar] [CrossRef] [PubMed]
- Chilaka, C.; De Boevre, M.; Atanda, O.; De Saeger, S. Occurrence of Fusarium mycotoxins in cereal crops and processed products (Ogi) from Nigeria. Toxins 2016, 8, 342. [Google Scholar] [CrossRef] [PubMed]
- Kamala, A.; Ortiz, J.; Kimanya, M.; Haesaert, G.; Donoso, S.; Tiisekwa, B.; De Meulenaer, B. Multiple mycotoxin co-occurrence in maize grown in three agro-ecological zones of Tanzania. Food Control 2015, 54, 208–215. [Google Scholar] [CrossRef]
- Rodrigues, I.; Naehrer, K. A three-year survey on the worldwide occurrence of mycotoxins in feedstuffs and feed. Toxins 2012, 4, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Schatzmayr, G.; Streit, E. Global occurrence of mycotoxins in the food and feed chain: Facts and figures. World Mycotoxin J. 2013, 6, 213–222. [Google Scholar] [CrossRef]
- Guerre, P. Worldwide mycotoxins exposure in pig and poultry feed formulations. Toxins 2016, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin contamination in the EU feed supply chain: A focus on cereal by-products. Toxins 2016, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Shephard, G.; Burger, H.; Gambacorta, L.; Gong, Y.; Krska, R.; Rheeder, J.; Solfrizzo, M.; Srey, C.; Sulyok, M.; Visconti, A.; et al. Multiple mycotoxin exposure determined by urinary biomarkers in rural subsistence farmers in the former Transkei, South Africa. Food Chem. Toxicol. 2012, 62, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; De Saeger, S.; Haesaert, G.; Karlovsky, P.; Oswald, I.; Seefelder, W.; Speijers, G.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2012, 57, 165–186. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, J.P.F.; Placinta, C.M.; Macdonald, A.M.C. Fusarium mycotoxins: A review of global implications for animal health, welfare and productivity. Anim. Feed Sci. Technol. 1999, 80, 183–205. [Google Scholar] [CrossRef]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current situation of mycotoxin contamination and co-occurrence in animal feed: Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef] [PubMed]
- Haggblom, P.; Nordkvist, E. Deoxynivalenol, zearalenone, and Fusarium graminearum contamination of cereal straw; field distribution; and sampling of big bales. Mycotoxin Res. 2015, 31, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Zinedine, A.; Soriano, J.; Moltó, J.; Mañes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Boutigny, A.; Beukes, I.; Small, I.; Zühlke, S.; Spiteller, M.; Van Rensburg, B.; Flett, B.; Viljoen, A. Quantitative detection of Fusarium pathogens and their mycotoxins in South African maize. Plant Path 2012, 61, 522–531. [Google Scholar] [CrossRef]
- Schollenberger, M.; Muller, H.M.; Rufle, M.; Suchy, S.; Planck, S.; Drochner, W. Natural occurrence of 16 Fusarium toxins in grains and feedstuffs of plant origin from Germany. Mycopathologia 2006, 161, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Ezekiel, C.; Bandyopadhyay, R.; Sulyok, M.; Warth, B.; Krska, R. Fungal and bacterial metabolites in commercial poultry feed from Nigeria. Food Addit. Contam. Part A 2012, 29, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Nichea, M.; Palacios, S.; Chiacchiera, S.; Sulyok, M.; Krska, R.; Chulze, S.; Torres, A.; Ramirez, M. Presence of multiple mycotoxins and other fungal metabolites in native grasses from a wetland ecosystem in Argentina intended for grazing cattle. Toxins 2015, 7, 3309–3329. [Google Scholar] [CrossRef] [PubMed]
- Klaric, M.; Rašić, D.; Peraica, M. Deleterious effects of mycotoxin combinations involving Ochratoxin A. Toxins 2013, 5, 1965–1987. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, K.H.; Pettersson, H.; Sandholm, K.; Oslen, M. Metabolism of aflatoxin, ochratoxin, zearalenone, and three trichothecenes by intact rumen fluid, rumen protozoa, and rumen bacteria. Appl. Environ. Microbiol. 1984, 47, 1070–1073. [Google Scholar] [PubMed]
- Cheli, F.; Campagnoli, A.; Dell’Orto, V. Fungal populations and mycotoxins in silages: From occurrence to analysis. Anim. Feed Sci. Technol. 2013, 183, 1–16. [Google Scholar] [CrossRef]
- Gelderblom, W.C.A.; Marasas, W.F.O.; Lebepe-Mazur, S.; Swanevelder, S.; Vessey, C.J.; Hall, P.M. Interaction of FB1 and AFB1 in a short-term carcinogenesis model in rat liver. Toxicol 2002, 171, 161–173. [Google Scholar] [CrossRef]
- Driehuis, F.; Spanjer, M.C.; Scholten, J.M.; Giffel, M.C.T. Occurrence of mycotoxins in maize, grass and wheat silage for dairy cattle in the Netherlands. Food Addit. Contam. Part B 2008, 1, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Spanjer, M.; Rensen, P.; Scholten, J. LC–MS/MS multi-method for mycotoxins after single extraction, with validation data for peanut, pistachio, wheat, maize, cornflakes, raisins and figs. Food Addit. Contam. Part A 2008, 25, 472–489. [Google Scholar] [CrossRef] [PubMed]
- Sulyok, M.; Krska, R.; Schuhmacher, R. Application of a liquid chromatography-tandem mass spectrometric method to multi-mycotoxin determination in raw cereals and evaluation of matrix effects. Food Addit. Contam. 2007, 24, 1184–1195. [Google Scholar] [CrossRef] [PubMed]
- Stroka, J.; Petz, M.; Joerissen, U.; Anklam, E. Investigation of various extractants for the analysis of aflatoxin B1 in different food and feed matrices. Food Addit. Contam. 1999, 16, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Kokkonen, M.; Jestoi, M. A multi-compound LC-MS/MS method for the screening of mycotoxins in grains. Food Anal. Methods 2008, 2, 128–140. [Google Scholar] [CrossRef]

| Concentration of Positive Feedstuff Samples (ppb) N = 40 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Detected Analyte | n (pos) | Incidence (%) | >LOQ (%) | Min (ppb) | Max (ppb) | Mean (ppb) | LOD (ppb) | LOQ (ppb) |
| AFB1 | 19 | 47.5 | 57.9 | <LOQ | 3.33 | 0.74 | 0.02 | 0.06 |
| AFB2 | 37 | 92.5 | 97.3 | <LOQ | 23.88 | 3.06 | 0.05 | 0.16 |
| AFG1 | 22 | 55.0 | 72.7 | <LOQ | 19.96 | 2.55 | 0.05 | 0.15 |
| AFG2 | 40 | 100.0 | 97.5 | <LOQ | 116.04 | 41.27 | 0.06 | 0.19 |
| α-ZEL | 40 | 100.0 | 100.0 | 0.975 | 13.24 | 4.84 | 0.19 | 0.63 |
| β-ZEL | 40 | 100.0 | 100.0 | 0.727 | 4.71 | 2.40 | 0.19 | 0.64 |
| ZEN | 24 | 60.0 | 87.5 | <LOQ | 28.04 | 2.84 | 0.04 | 0.12 |
| FB1 | 34 | 85.0 | 64.7 | <LOQ | 1389.62 | 372.96 | 3.46 | 11.52 |
| ADONs | 12 | 30.0 | 75.0 | <LOQ | 9.51 | 2.20 | 0.08 | 0.27 |
| BEA | 36 | 90.0 | 50.0 | <LOQ | 55.99 | 8.81 | 0.66 | 2.19 |
| DON | 24 | 60.0 | 83.3 | <LOQ | 81.61 | 20.40 | 0.49 | 1.62 |
| HT-2 | 35 | 87.5 | 94.3 | <LOQ | 312.95 | 35.11 | 0.06 | 0.21 |
| OTA | 0 | 0 | ND | ND | ND | ND | 0.08 | 0.26 |
| CIT | 0 | 0 | ND | ND | ND | ND | 0.13 | 0.42 |
| Mycotoxin | Apparent Recoveries in Model Matrices (%) | |||
|---|---|---|---|---|
| Maize | Maize Silage | Brewers Spent Grain | Bran | |
| Aflatoxin B1 | 177 ± 9 | 114 ± 8 | - | - |
| Aflatoxin B2 | 71 ± 8 | 84 ± 12 | - | - |
| Aflatoxin G1 | 73 ± 12 | 54 ± 8 | 61 ± 18 | 76 ± 4 |
| Aflatoxin G2 | 48 ± 13 | 49 ± 11 | 44 ± 12 | 49 ± 9 |
| Acetyldeoxynivalenols | - | - | - | 174 ± 6 |
| α-zearalenol | 100 ± 5 | 99 ± 4 | 99 ± 12 | 100 ± 7 |
| β-zearalenol | 100 ± 9 | 99 ± 9 | 102 ± 5 | 99 ± 5 |
| Citrinin | 26 ± 8 | - | 129 ± 13 | - |
| Beauvericin | 98 ± 12 | 66 ± 5 | 16 ± 4 | 48 ± 16 |
| Deoxynivalenol | 103 ± 12 | 105 ± 12 | - | - |
| HT-2 toxin | 41 ± 7 | - | - | 24 ± 6 |
| Ochratoxin A | 71 ± 21 | 37 ± 13 | 187 ± 9 | 63 ± 12 |
| Zearalenone | 151 ± 12 | 174 ± 8 | - | 153 ± 11 |
| Time (min) | Solvent A (%) | Solvent B (%) |
|---|---|---|
| 0 | 98 | 2 |
| 1.0 | 98 | 2 |
| 8.0 | 0 | 100 |
| 12.0 | 0 | 100 |
| 12.1 | 98 | 2 |
| 14.0 | 98 | 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Changwa, R.; Abia, W.; Msagati, T.; Nyoni, H.; Ndleve, K.; Njobeh, P. Multi-Mycotoxin Occurrence in Dairy Cattle Feeds from the Gauteng Province of South Africa: A Pilot Study Using UHPLC-QTOF-MS/MS. Toxins 2018, 10, 294. https://doi.org/10.3390/toxins10070294
Changwa R, Abia W, Msagati T, Nyoni H, Ndleve K, Njobeh P. Multi-Mycotoxin Occurrence in Dairy Cattle Feeds from the Gauteng Province of South Africa: A Pilot Study Using UHPLC-QTOF-MS/MS. Toxins. 2018; 10(7):294. https://doi.org/10.3390/toxins10070294
Chicago/Turabian StyleChangwa, Rumbidzai, Wilfred Abia, Titus Msagati, Hlengilizwe Nyoni, Khanyisa Ndleve, and Patrick Njobeh. 2018. "Multi-Mycotoxin Occurrence in Dairy Cattle Feeds from the Gauteng Province of South Africa: A Pilot Study Using UHPLC-QTOF-MS/MS" Toxins 10, no. 7: 294. https://doi.org/10.3390/toxins10070294
APA StyleChangwa, R., Abia, W., Msagati, T., Nyoni, H., Ndleve, K., & Njobeh, P. (2018). Multi-Mycotoxin Occurrence in Dairy Cattle Feeds from the Gauteng Province of South Africa: A Pilot Study Using UHPLC-QTOF-MS/MS. Toxins, 10(7), 294. https://doi.org/10.3390/toxins10070294






