Assessment of the Acute and Subchronic Toxicity and Mutagenicity of Sideritis scardica Griseb. Extracts
Abstract
1. Introduction
2. Results
2.1. Phytochemical Analyses
2.2. Acute Oral Toxicity
2.3. Repeated-Dose Oral Toxicity
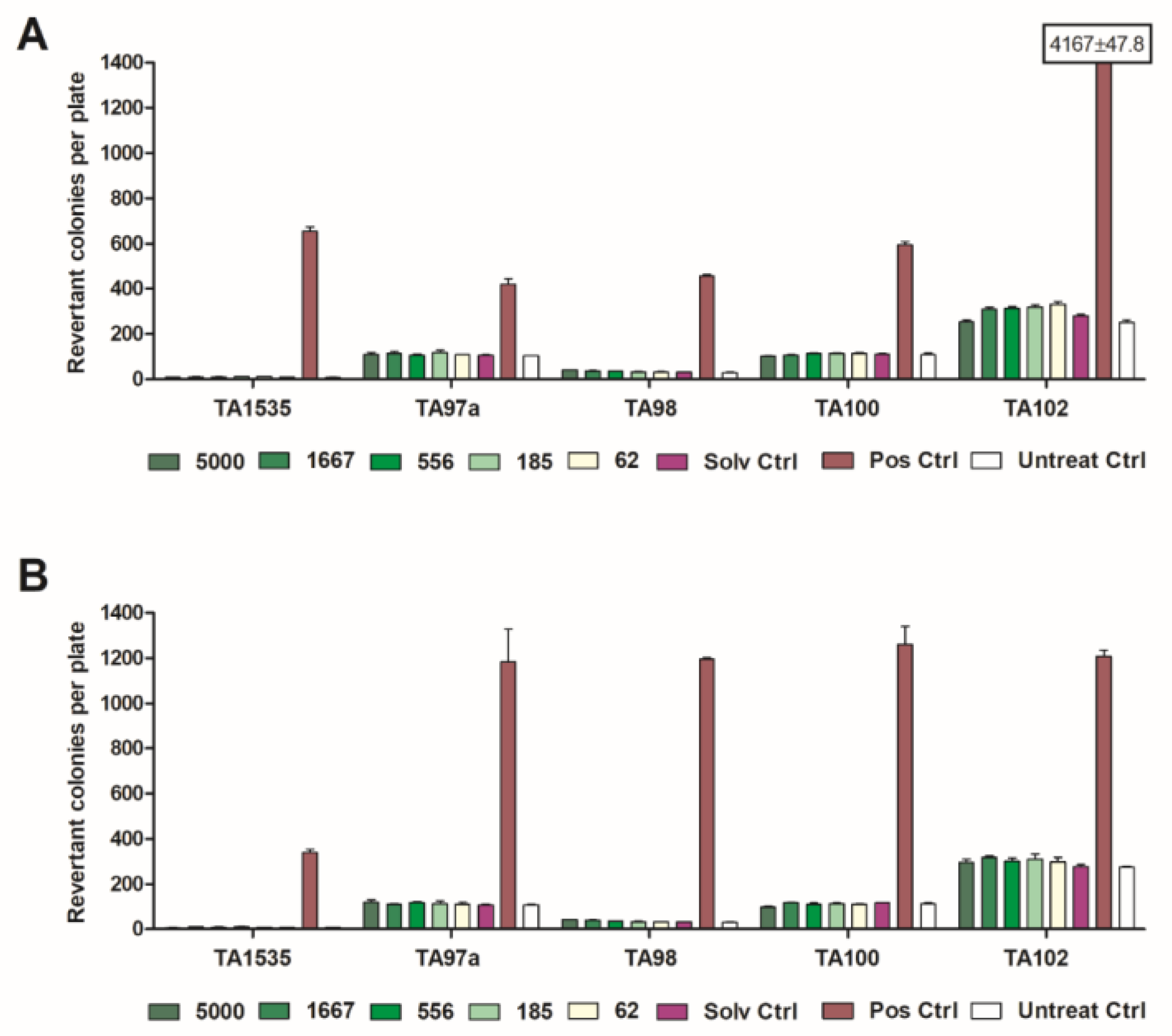
2.4. Mutagenicity Tests
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Selection of Extracts
- extract I (used for test of acute and repeated-dose toxicity and mutagenicity tests): dry extract made with 20% v/v ethanol as extraction solvent, drug/extract ratio (DER) native 5–9:1, 70% native extract, adjustment with 30% maltodextrin;
- extract II (used for mutagenicity test): dry extract made with water as extraction solvent, drug/extract ratio (DER) native 4–8:1, 70% native extract, adjustment with 30% maltodextrin;
- extract III (used for mutagenicity test): dry extract made 50% v/v ethanol as extraction solvent, drug/extract ratio (DER) native 6:1, 70% native extract, adjustment with 15% maltodextrin (MD) and 15% silica;
- extract IV (used for mutagenicity test): dry extract made with n-heptane as extraction solvent, drug/extract ratio (DER) native 83:1, 50% native extract, adjustment with 50% silica.
5.2. Phytochemical Analysis
5.3. Acute Oral Toxicity Tests
5.4. Repeated-Dose Oral Toxicity Tests
5.5. Mutagenicity Test (Salmonella typhimurium Reverse Mutation Assay)
5.6. Statistics
Author Contributions
Funding
Conflicts of Interest
References
- Todorova, M.; Trendafilova, A. Sideritis scardica Griseb., an endemic species of Balkan peninsula: Traditional uses, cultivation, chemical composition, biological activity. J. Ethnopharmacol. 2014, 152, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Heywood, V. Sideritis L. In Flora Europaea; Tutin, T., Heywood, V., Burges, N., Moore, D., Valentine, S., Walters, S., Webb, D., Eds.; Cambridge University Press: Cambridge, UK, 1972; pp. 134–138. [Google Scholar]
- Knörle, R. Extracts of Sideritis scardica as triple monoamine reuptake inhibitors. J. Neural Transm. 2012, 119, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Feistel, B.; Walbroel, B.; Pahnke, J. Extract preparation from Sideritis scardica enhances memorizing skills of mice in Morris water maze. Planta Med. 2013. [Google Scholar] [CrossRef]
- Hofrichter, J.; Krohn, M.; Schumacher, T.; Lange, C.; Feistel, B.; Walbroel, B.; Pahnke, J. Sideritis spp. Extracts Enhance Memory and Learning in Alzheimer’s β-Amyloidosis Mouse Models and Aged C57Bl/6 Mice. J. Alzheimers Dis. 2016, 53, 967–980. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency; Committee on Herbal Medicinal Products (HMPC). European Union Herbal Monograph on Sideritis scardica Griseb., Sideritis clandestina (Bory & Chaub.) Hayek, Sideritis raeseri Boiss. & Heldr., Sideritis syriaca L., Herba, Draft; EMA/HMPC/39453/2015; European Union: Brussels, Belgium, 7 July 2015. [Google Scholar]
- European Medicines Agency; Committee on Herbal Medicinal Products (HMPC). Assessment Report on Sideritis scardica Griseb., Sideritis clandestina (Bory & Chaub.) Hayek, Sideritis raeseri Boiss. & Heldr., Sideritis syriaca L., Herba; EMA/HMPC/39455/2015; European Union: Brussels, Belgium, 2015. [Google Scholar]
- Fraga, B.M. Phytochemistry and chemotaxonomy of Sideritis species from the Mediterranean region. Phytochemistry 2012, 76, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Stanoeva, P.J.; Stefova, M. Assay of urinary excretion of polyphenols after ingestion of a cup of mountain tea (Sideritis scardica) measured by HPLC-DAD-ESI-MS/MS. J. Agric. Food Chem. 2013, 61, 10488–10497. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EU) No 1161/2011 of 14 November 2011 Amending Directive 2002/46/EC of the European Parliament and of the Council, Regulation (EC) No 1925/2006 of the European Parliament and of the Council and Commission Regulation (EC) No 953/2009 as Regards the Lists of Mineral Substances that Can Be Added to Foods Text with EEA Relevance; European Union: Brussels, Belgium, 2011. [Google Scholar]
- Zarrelli, A.; Sgambato, A.; Petito, V.; De Napoli, L.; Previtera, L.; Di Fabio, G. New C-23 modified of silybin and 2,3-dehydrosilybin: Synthesis and preliminary evaluation of antioxidant properties. Bioorg. Med. Chem. Lett. 2011, 21, 4389–4392. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Jaśkiewicz, M. Microalgal food supplements from the perspective of Polish consumers: Patterns of use, adverse events, and beneficial effects. J. Appl. Phycol. 2017, 29, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Kelber, O.; Wegener, T.; Steinhoff, B.; Staiger, C.; Wiesner, J.; Knöss, W.; Kraft, K. Assessment of genotoxicity of herbal medicinal products: Application of the “bracketing and matrixing” concept using the example of Valerianae radix (valerian root). Phytomedicine 2014, 21, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Fabio, G.D.; Romanucci, V.; De Marco, A.; Zarrelli, A. Triterpenoids from Gymnema sylvestre and their pharmacological activities. Molecules 2014, 19, 10956–10981. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Maes, M.; Corbi, G.; Willcox, D.C.; Scapagnini, G. Dietary phytochemicals and neuro-inflammaging: From mechanistic insights to translational challenges. Immun. Ageing 2016, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency; Committee on Herbal Medicinal Products (HMPC). Guideline on Non-Clinical Documentation for Herbal Medicinal Products in Applications for Marketing Authorisation (Bibliographical and Mixed Applications) and in Applications for Simplified Registration; EMEA/HMPC/32116/2005; European Union: Brussels, Belgium, 2005. [Google Scholar]
- European Medicines Agency; Committee on Herbal Medicinal Products (HMPC). Guideline on the Assessment of Genotoxicity of Herbal Substances/Preparations; EMEA/HMPC/107079/2007; European Union: Brussels, Belgium, 2007. [Google Scholar]
- Tadić, V.M.; Jeremic, I.; Dobric, S.; Isakovic, A.; Markovic, I.; Trajkovic, V.; Bojovic, D.; Arsic, I. Anti-inflammatory, gastroprotective, and cytotoxic effects of Sideritis scardica extracts. Planta Med. 2012, 78, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-Operation and Development. OECD Guideline for Testing of Chemicals. Repeated Dose 28-Day Oral Toxicity Study in Rodents; Document 407, Adopted by the Council on 27th July 1995; Organisation for Economic Co-operation and Development: Paris, France, 1995. [Google Scholar]
- Organisation for Economic Co-Operation and Development. OECD Guideline for Testing of Chemicals, Bacterial Reverse Mutation Test; Document No. 471, Adopted 21 July; Organisation for Economic Co-Operation and Development: Paris, France, 1997. [Google Scholar]
- Organisation for Economic Co-Operation and Development. OECD Guideline for Testing of Chemicals. Acute Oral Toxicity—Acute Toxic Class Method; Document 423, Adopted on 17 December 2001; Organisation for Economic Co-Operation and Development: Paris, France, 2001. [Google Scholar]
- Qari, S.H.; Adbel-Fattaj, N.A.H. Genotoxic studies of selected plant oil extracts on Rhyzopertha dominica (Coleoptera: Bostrichidae). J. Taibah Univ. Sci. 2017, 11, 478–486. [Google Scholar] [CrossRef]
- Kim, J.C.; Yun, H.I.; Lim, K.H.; Suh, J.E.; Chung, M.-K. Haematological values during normal pregnancy in Sprague–Dawley rats. Comp. Haematol. Int. 2000, 10, 74–79. [Google Scholar] [CrossRef]
- Lillie, L.E.; Temple, N.J.; Florence, L.Z. Reference values for young normal Sprague-Dawley rats: Weight gain, hematology and clinical chemistry. Hum. Exp. Toxicol. 1996, 15, 612–616. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Committee on Herbal Medicinal Products (HMPC). Guideline on Selection of Test Materials for Genotoxicity Testing for Traditional Herbal Medicinal Products/Herbal Medicinal Products; EMEA/HMPC/67644/2009; European Union: Brussels, Belgium, 2009. [Google Scholar]
- Council of Europe. European Pharmacopeia, 9th ed.; European Directorate for the Quality of Medicines & HealthCare, Council of Europe: London, UK, 2017. [Google Scholar]
- Claxton, L.D.; Umbuzeiro Gde, A.; DeMarini, D.M. The Salmonella mutagenicity assay: The stethoscope of genetic toxicology for the 21st century. Environ. Health Perspect. 2010, 118, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- European Union. Commission Regulation (EC) No. 440/2008 8.13/14 (2008) Mutagenicity—Reverse Mutation Test Using Bacteria; European Union: Brussels, Belgium, 2008. [Google Scholar]
- Environmental Protection Agency. Health Effects Test Guidelines, OPPTS 870.5100. Bacterial Reverse Mutation Assay; EPA 712-C-98-247; Environmental Protection Agency: Washington, DC, USA, 1998.
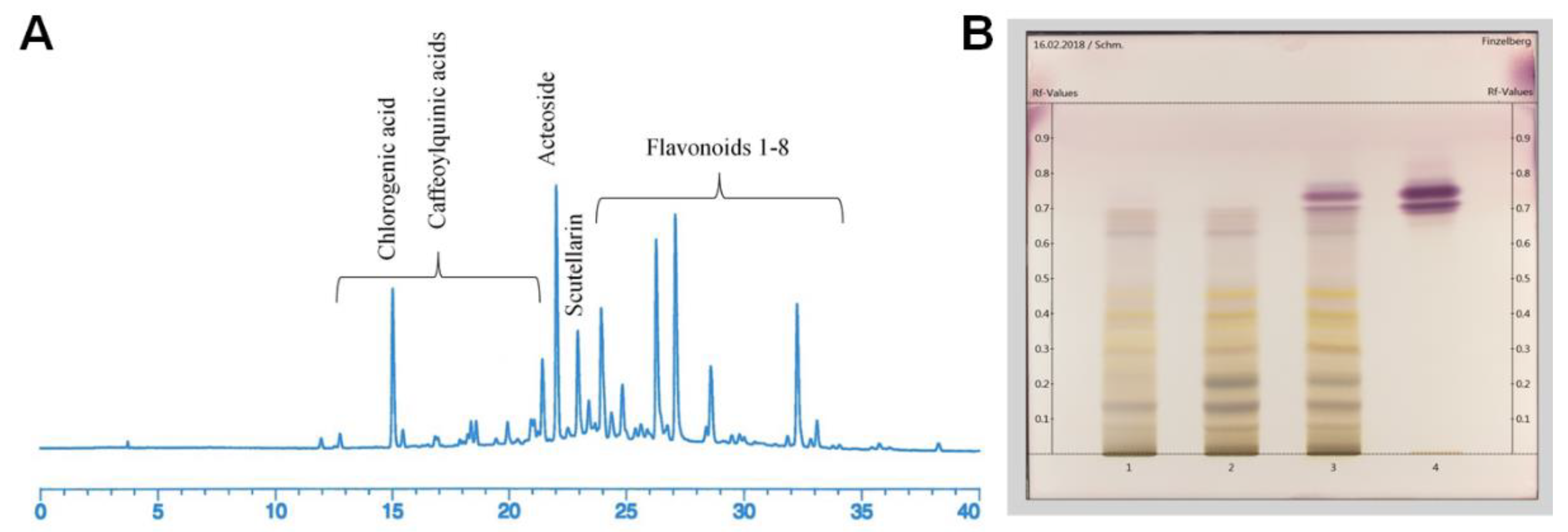
| Extract no. | I | II | III | IV |
|---|---|---|---|---|
| Extraction solvent | Ethanol 20% v/v | Water | Ethanol 50% v/v | Heptane |
| Total polyphenols (%) | 6.25 | 5.07 | 6.23 | 0.18 |
| Flavonoids (%) | 1.18 | 0.59 | 2.03 | n.d. |
| Acteoside (%) | 0.41 | 0.12 | 0.94 | n.d. |
| Caffeoylquinic acids (%) | 0.47 | 0.24 | 0.38 | n.d. |
| Parameter | Sex M/F | Group | |||||
|---|---|---|---|---|---|---|---|
| 0 (Control) | 250 | 500 | 1000 | 0 (Reversal) | 1000 (Reversal) | ||
| Hb (g/dL) | M | 15.0 ± 1.1 | 15.2 ± 0.4 | 16.5 ± 1.5 | 14.5 ± 1.6 | 16.3 ± 0.5 | 16.7 ± 2.2 |
| F | 15.6 ± 1.0 | 16.1 ± 0.8 | 15.5 ± 1.4 | 16.3 ± 0.7 | 15.8 ± 0.6 | 15.3 ± 0.5 | |
| RBC (× 106/µL) | M | 7.36 ± 0.67 | 7.15 ± 0.52 | 8.02 ± 0.65 | 6.65 ± 1.06 | 8.42 ± 0.25 | 8.48 ± 1.19 |
| F | 7.62 ± 0.72 | 7.88 ± 0.20 | 7.51 ± 1.05 | 7.84 ± 0.33 | 7.97 ± 0.25 | 8.03 ± 0.29 | |
| HCT (%) | M | 44.5 ± 3.1 | 45.1 ± 1.06 | 48.8 ± 4.2 | 43.4 ± 4.3 | 69.0 ± 2.5 | 70.0 ± 9.4 |
| F | 45.3 ± 3.0 | 47.0 ± 2.0 | 45.6 ± 4.3 | 46.5 ± 2.4 | 65.6 ± 3.0 | 64.0 ± 1.0 | |
| MCV (fL) | M | 60.6 ± 2.7 | 63.4 ± 3.6 | 60.1 ± 2.4 | 66.5 ± 3.7 | 81.9 ± 2.0 | 82.6 ± 1.0 |
| F | 59.5 ± 1.8 | 59.7 ± 3.3 | 61.1 ± 4.1 | 59.4 ± 2.1 | 82.2 ± 1.6 | 79.6 ± 2.1 | |
| MCH (pg) | M | 20.5 ± 1.3 | 21.3 ± 1.2 | 20.5 ± 0.7 | 21.9 ± 1.0 | 19.4 ± 0.5 | 19.8 ± 0.7 |
| F | 20.5 ± 0.7 | 20.4 ± 1.3 | 20.8 ± 1.5 | 20.8 ± 0.8 | 19.9 ± 0.3 | 19.1 ± 0.7 | |
| MCHC (g/dL) | M | 33.8 ± 0.6 | 33.7 ± 0.4 | 33.7 ± 0.4 | 32.9 ± 0.8 | 23.7 ± 0.3 | 23.9 ± 0.5 |
| F | 34.5 ± 0.2 | 34.1 ± 0.3 | 34.0 ± 0.2 | 35.0 ± 0.3 | 24.2 ± 0.2 | 24.0 ± 0.5 | |
| PLT (× 103/µL) | M | 311.2 ± 29.9 | 370.6 ± 57.2 | 367.2 ± 100.4 | 358.8 ± 45.7 | 291.2 ± 128.0 | 254.4 ± 62.6 |
| F | 316.6 ± 89.3 | 314.0 ± 61.7 | 328.8 ± 31.1 | 426.8 ± 56.0 | 348.8 ± 20.6 | 306.8 ± 82.7 | |
| WBC (× 103/µL) | M | 16.18 ± 1.49 | 12.36 ± 3.96 | 11.66 ± 3.11 * | 18.30 ± 1.57 | 15.30 ± 2.41 | 17.04 ± 5.47 |
| F | 18.50 ± 4.97 | 17.32 ± 3.37 | 13.32 ± 3.00 | 11.82 ± 7.46 | 12.7 ± 1.96 | 15.28 ± 2.89 | |
| NEU (%) | M | 19.4 ± 3.0 | 20.8 ± 4.2 | 19.8 ± 4.8 | 20.2 ± 4.1 | 21.0 ± 3.5 | 19.2 ± 4.2 |
| F | 21.2 ± 4.5 | 19.2 ± 3.8 | 20.4 ± 3.9 | 20.8 ± 3.3 | 21.2 ± 3.6 | 21.6 ± 4.3 | |
| LYMPH (%) | M | 77.0 ± 2.5 | 76.0 ± 2.7 | 77.0 ± 4.8 | 76.6 ± 3.6 | 76.2 ± 2.6 | 77.4 ± 3.8 |
| F | 76.0 ± 4.7 | 77.6 ± 3.6 | 76.6 ± 4.1 | 75.8 ± 2.6 | 75.0 ± 3.4 | 75.4 ± 4.0 | |
| EOS (%) | M | 1.0 ± 0.7 | 0.8 ± 0.8 | 0.8 ± 0.8 | 1.0 ± 0.7 | 0.6 ± 0.9 | 1.0 ± 1.0 |
| F | 0.6 ± 0.5 | 0.8 ± 0.8 | 0.8 ± 0.8 | 1.2 ± 0.8 | 1.2 ± 0.8 | 1.0 ± 0.7 | |
| MONO (%) | M | 2.6 ± 1.1 | 2.4 ± 1.1 | 2.4 ± 0.5 | 2.2 ± 0.8 | 2.2 ± 0.8 | 2.4 ± 0.5 |
| F | 2.2 ± 0.8 | 2.4 ± 0.5 | 2.2 ± 0.8 | 2.2 ± 0.8 | 2.6 ± 0.5 | 2.0 ± 0.7 | |
| BASO (%) | M | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| F | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| PT (sec) | M | 15.4 ± 3.9 | 15.0 ± 3.6 | 15.6 ± 2.3 | 16.0 ± 2.5 | 14.8 ± 3.7 | 14.4 ± 3.0 |
| F | 15.4 ± 3.8 | 15.0 ± 2.9 | 15.4 ± 4.0 | 15.2 ± 3.6 | 17.2 ± 4.2 | 14.2 ± 3.0 | |
| Parameter | Sex M/F | Group | |||||
|---|---|---|---|---|---|---|---|
| 0 (Control) | 250 | 500 | 1000 | 0 (Reversal) | 1000 (Reversal) | ||
| Total protein (g/dL) | M | 7.44 ± 0.39 | 7.60 ± 0.60 | 7.61 ± 0.50 | 7.50 ± 0.46 | 7.52 ± 0.26 | 7.49 ± 0.28 |
| F | 7.47 ± 0.47 | 7.48 ± 0.45 | 7.58 ± 0.57 | 7.36 ± 0.36 | 7.72 ± 0.52 | 7.38 ± 0.35 | |
| Urea (mg/dL) | M | 40.4 ± 8.2 | 40.0 ± 2.9 | 40.2 ± 6.6 | 35.8 ± 5.8 | 40.2 ± 5.5 | 39.8 ± 4.9 |
| F | 40.8 ± 2.6 | 41.8 ± 6.8 | 42.4 ± 5.7 | 41.4 ± 5.2 | 39.2 ± 5.6 | 40.0 ± 6.9 | |
| ALT (IU/L) | M | 36.2 ± 7.7 | 40.8 ± 7.7 | 38.0 ± 4.8 | 36.8 ± 9.6 | 37.6 ± 7.6 | 36.6 ± 4.3 |
| F | 36.0 ± 6.6 | 37.0 ± 6.0 | 37.6 ± 7.9 | 38.6 ± 6.6 | 34.4 ± 8.8 | 34.4 ± 8.5 | |
| AST (IU/L) | M | 60.2 ± 6.2 | 59.0 ± 7.3 | 61.6 ± 5.7 | 58.2 ± 7.0 | 58.6 ± 4.7 | 63.0 ± 4.2 |
| F | 60.4 ± 6.7 | 65.6 ± 3.6 | 61.8 ± 7.7 | 57.0 ± 8.1 | 60.4 ± 7.5 | 63.4 ± 5.9 | |
| AP (IU/L) | M | 74.2 ± 4.4 | 72.6 ± 7.5 | 73.8 ± 8.9 | 68.0 ± 8.7 | 69.2 ± 6.8 | 71.8 ± 6.2 |
| F | 73.4 ± 4.3 | 77.6 ± 3.7 | 72.8 ± 8.7 | 68.6 ± 9.2 | 70.2 ± 7.6 | 70.2 ± 10.4 | |
| Glucose (mg/dL) | M | 88.8 ± 11.8 | 87.6 ± 15.1 | 85.8 ± 6.5 | 89.0 ± 13.2 | 95.4 ± 80 | 92.8 ± 12.8 |
| F | 85.4 ± 13.1 | 97.2 ± 5.9 | 95.2 ± 16.4 | 92.4 ± 10.7 | 87.2 ± 15.8 | 94.8 ± 10.5 | |
| Albumin (g/dL) | M | 3.50 ± 0.33 | 3.69 ± 0.37 | 3.56 ± 0.27 | 3.63 ± 0.33 | 3.48 ± 0.44 | 3.44 ± 0.12 |
| F | 3.57 ± 0.29 | 3.47 ± 0.36 | 3.49 ± 0.38 | 3.51 ± 0.17 | 3.50 ± 0.35 | 3.38 ± 0,17 | |
| Globulin (g/dL) | M | 3.94 ± 0.50 | 3.91 ± 0.49 | 4.05 ± 0.35 | 3.87 ± 0.51 | 4.04 ± 0.41 | 4.05 ± 0.28 |
| F | 3.90 ± 0.45 | 4.01 ± 0.70 | 4.09 ± 0.59 | 3.85 ± 0.44 | 4.30 ± 0.76 | 4.0 ± 0.44 | |
| Creatinine (mg/dL) | M | 1.07 ± 0.13 | 0.97 ± 0.13 | 0.96 ± 0.14 | 0.96 ± 0.10 | 0.97 ± 0.16 | 0.97 ± 0.09 |
| F | 1.05 ± 0.18 | 0.99 ± 0.16 | 0.90 ± 0.04 | 1.05 ± 0.11 | 0.97 ± 0.09 | 0.93 ± 0.10 | |
| Total bilirubin (mg/dL) | M | 0.63 ± 0.04 | 0.62 ± 0.12 | 0.62 ± 0.04 | 0.64 ± 0.11 | 0.65 ± 0.08 | 0.61 ± 0.10 |
| F | 0.61 ± 0.11 | 0.58 ± 0.08 | 0.59 ± 0.08 | 0.59 ± 0.08 | 0.61 ± 0.07 | 0.67 ± 0.16 | |
| Total cholesterol (mg/dL) | M | 58.6 ± 7.7 | 59.4 ± 6.3 | 57.6 ± 6.7 | 55.8 ± 5.3 | 60.2 ± 6.3 | 60.8 ± 4.6 |
| F | 60.4 ± 7.4 | 61.0 ± 6.5 | 57.4 ± 5.7 | 57.6 ± 5.3 | 63.2 ± 5.4 | 58.4 ± 5.7 | |
| Triglycerides (mg/dL) | M | 110.0 ± 8.4 | 106.8 ± 10.5 | 109.0 ± 8.7 | 108.8 ± 8.8 | 108.6 ± 10.3 | 111.2 ± 11.2 |
| F | 107.4 ± 11.6 | 103.6 ± 7.7 | 103.0 ± 8.5 | 102.0 ± 12.0 | 108.0 ± 13.0 | 114.0 ± 8.7 | |
| Phosphorus (mg/dL) | M | 4.10 ± 0.49 | 4.16 ± 0.70 | 4.34 ± 0.43 | 3.84 ± 0.71 | 3.86 ± 0.59 | 3.92 ± 0.61 |
| F | 4.10 ± 0.63 | 3.88 ± 0.73 | 4.08 ± 0.54 | 3.66 ± 0.49 | 4.02 ± 0.66 | 3.76 ± 0.42 | |
| Calcium (mmol/L) | M | 2.34 ± 0.11 | 2.88 ± 0.13 ** | 3.00 ± 0.38 ** | 2.99 ± 0.13 ** | 2.99 ± 0.13 | 4.15 ± 0.14 * |
| F | 3.95 ± 0.1 | 3.72 ± 0.12 ** | 3.86 ± 0.09 | 3.63 ± 0.08 ** | 3.63 ± 0.2 | 3.67 ± 0.13 | |
| Sodium (mmol/L) | M | 144.5 ± 1.6 | 151.5 ± 1.4 | 150.7 ± 2.8 | 154.4 ± 2.2 | 161.8 ± 1.0 | 162.1 ± 1.6 |
| F | 150.5 ± 1.2 | 148.7 ± 1.7 | 147.9 ± 1.1 * | 147.8 ± 1.4 * | 151.8 ± 1.1 | 151.9 ± 1.5 | |
| Potassium (mmol/L) | M | 4.74 ± 0.34 | 4.73 ± 0.4 | 4.94 ± 0.2 | 5.00 ± .25 | 3.98 ± 0.3 | 4.05 ± 0.3 |
| F | 4.95 ± 0.7 | 4.67 ± 0.3 | 4.66 ± 0.2 | 4.52 ± 0.4 | 4.08 ± 0.4 | 3.90 ± 0.3 | |
| Chloride (mmol/L) | M | 100.7 ± 2.9 | 91.5 ± 2.1 | 91.8 ± 2.9 | 95.6 ± 3.5 | 109.7 ± 1.9 | 110.9 ± 1.6 |
| F | 89.6 ± 2.9 | 91.1 ± 2.4 | 89.7 ± 1.0 | 89.3 ± 1.4 | 98.4 ± 1.4 | 99.2 ± 1.3 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feistel, B.; Wegener, T.; Rzymski, P.; Pischel, I. Assessment of the Acute and Subchronic Toxicity and Mutagenicity of Sideritis scardica Griseb. Extracts. Toxins 2018, 10, 258. https://doi.org/10.3390/toxins10070258
Feistel B, Wegener T, Rzymski P, Pischel I. Assessment of the Acute and Subchronic Toxicity and Mutagenicity of Sideritis scardica Griseb. Extracts. Toxins. 2018; 10(7):258. https://doi.org/10.3390/toxins10070258
Chicago/Turabian StyleFeistel, Björn, Tankred Wegener, Piotr Rzymski, and Ivo Pischel. 2018. "Assessment of the Acute and Subchronic Toxicity and Mutagenicity of Sideritis scardica Griseb. Extracts" Toxins 10, no. 7: 258. https://doi.org/10.3390/toxins10070258
APA StyleFeistel, B., Wegener, T., Rzymski, P., & Pischel, I. (2018). Assessment of the Acute and Subchronic Toxicity and Mutagenicity of Sideritis scardica Griseb. Extracts. Toxins, 10(7), 258. https://doi.org/10.3390/toxins10070258






