Diel Variations in Cell Abundance and Trophic Transfer of Diarrheic Toxins during a Massive Dinophysis Bloom in Southern Brazil
Abstract
1. Introduction
2. Results
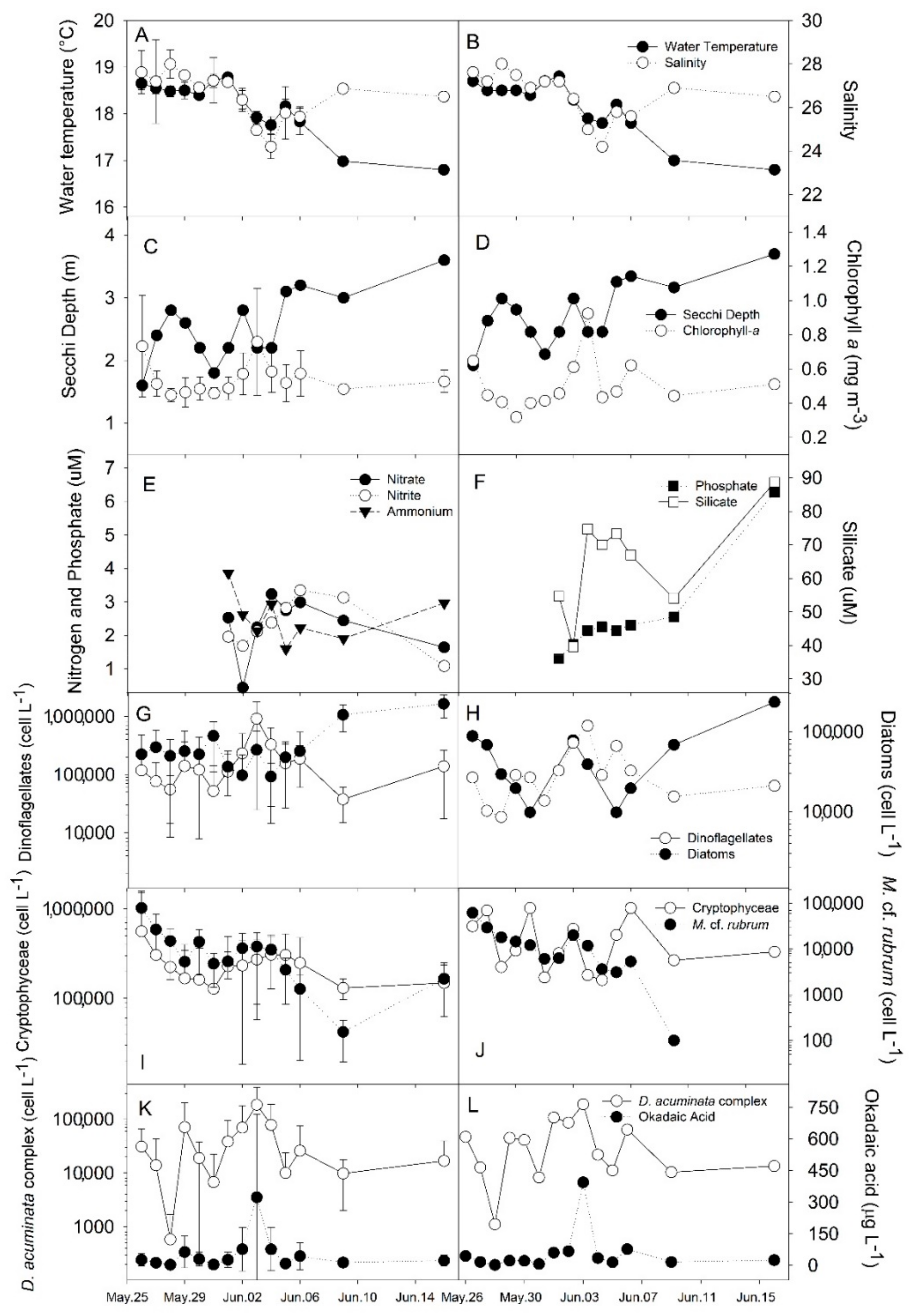
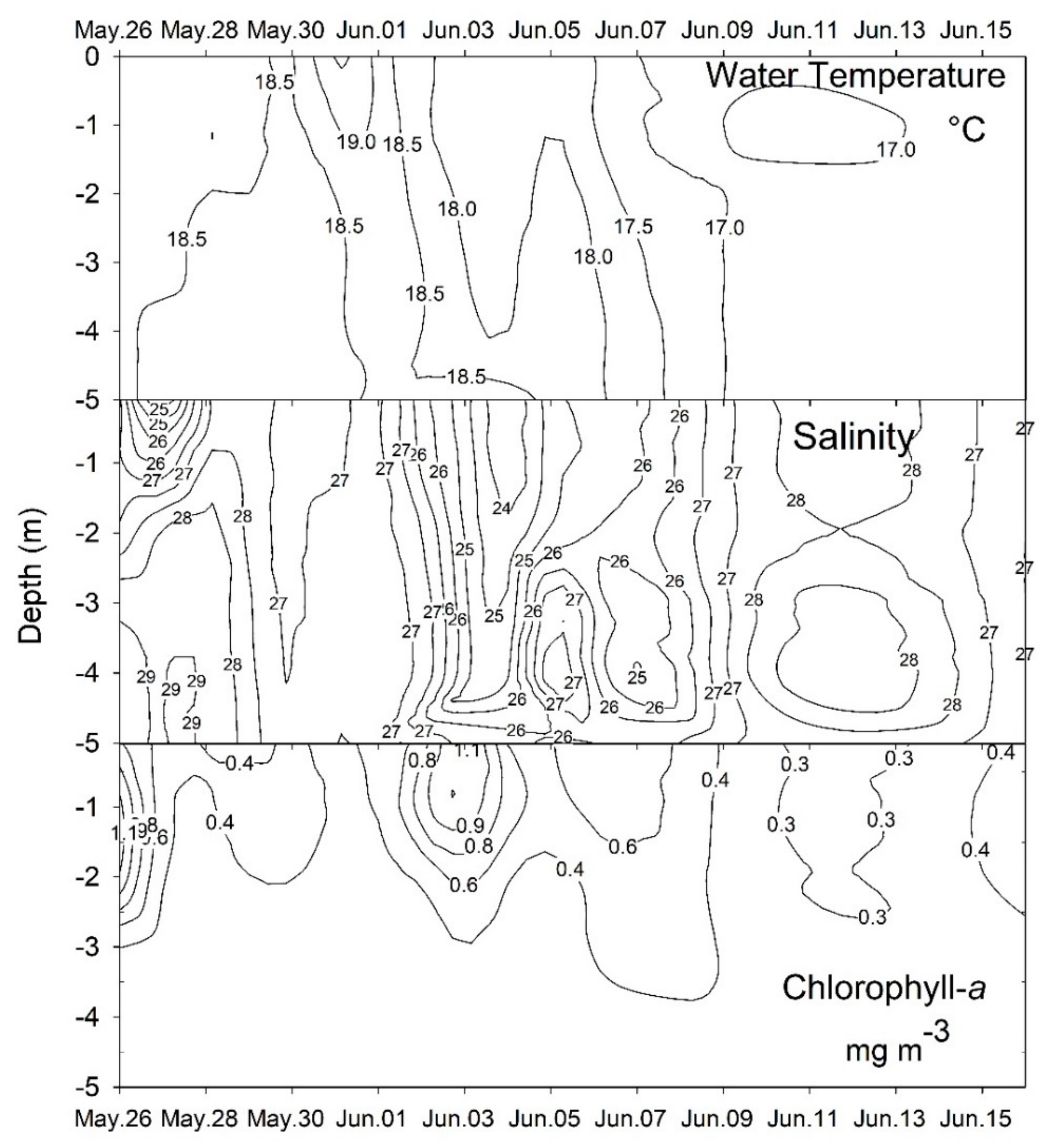
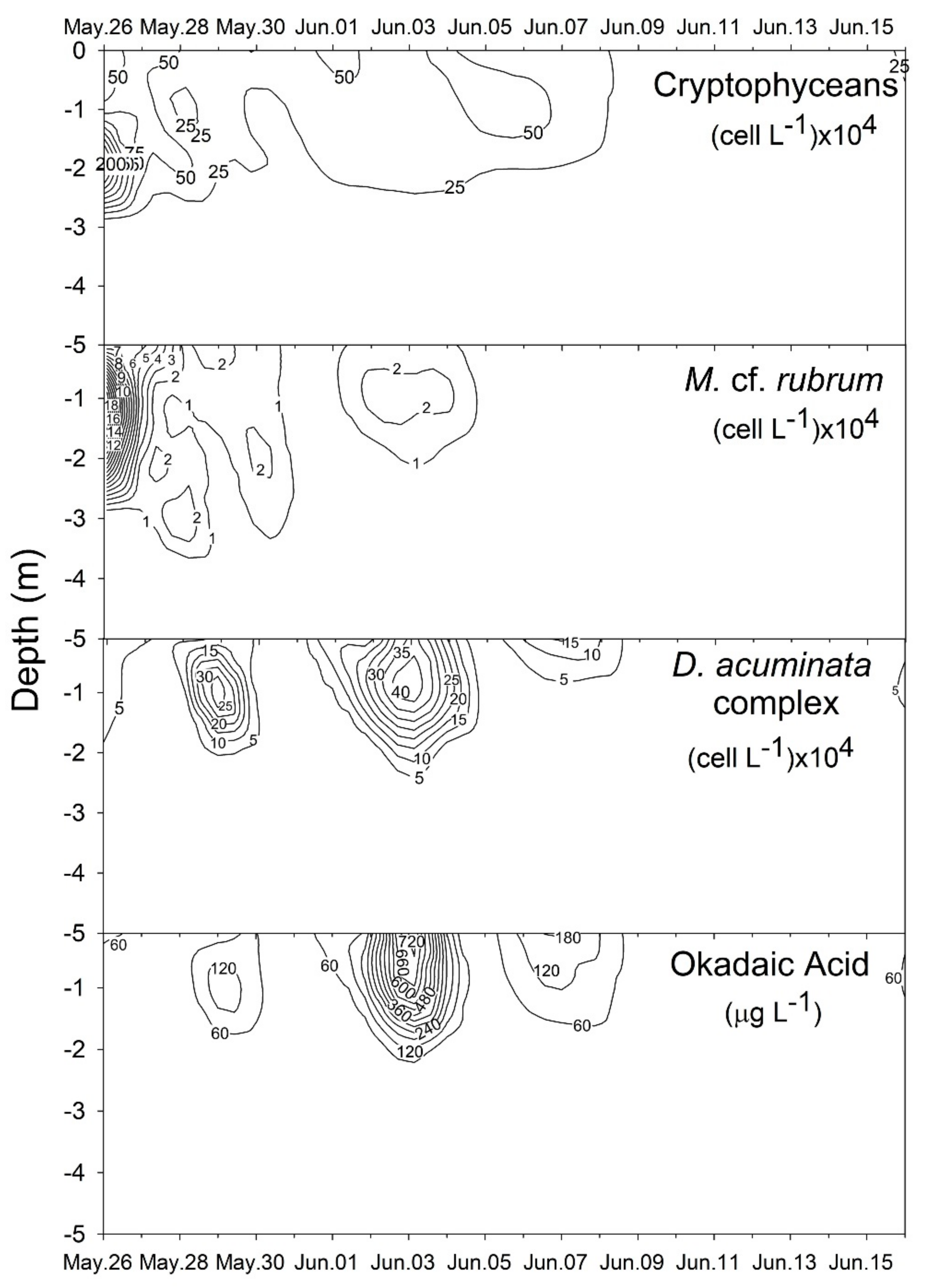
2.1. Plankton and Toxins in the Water Column
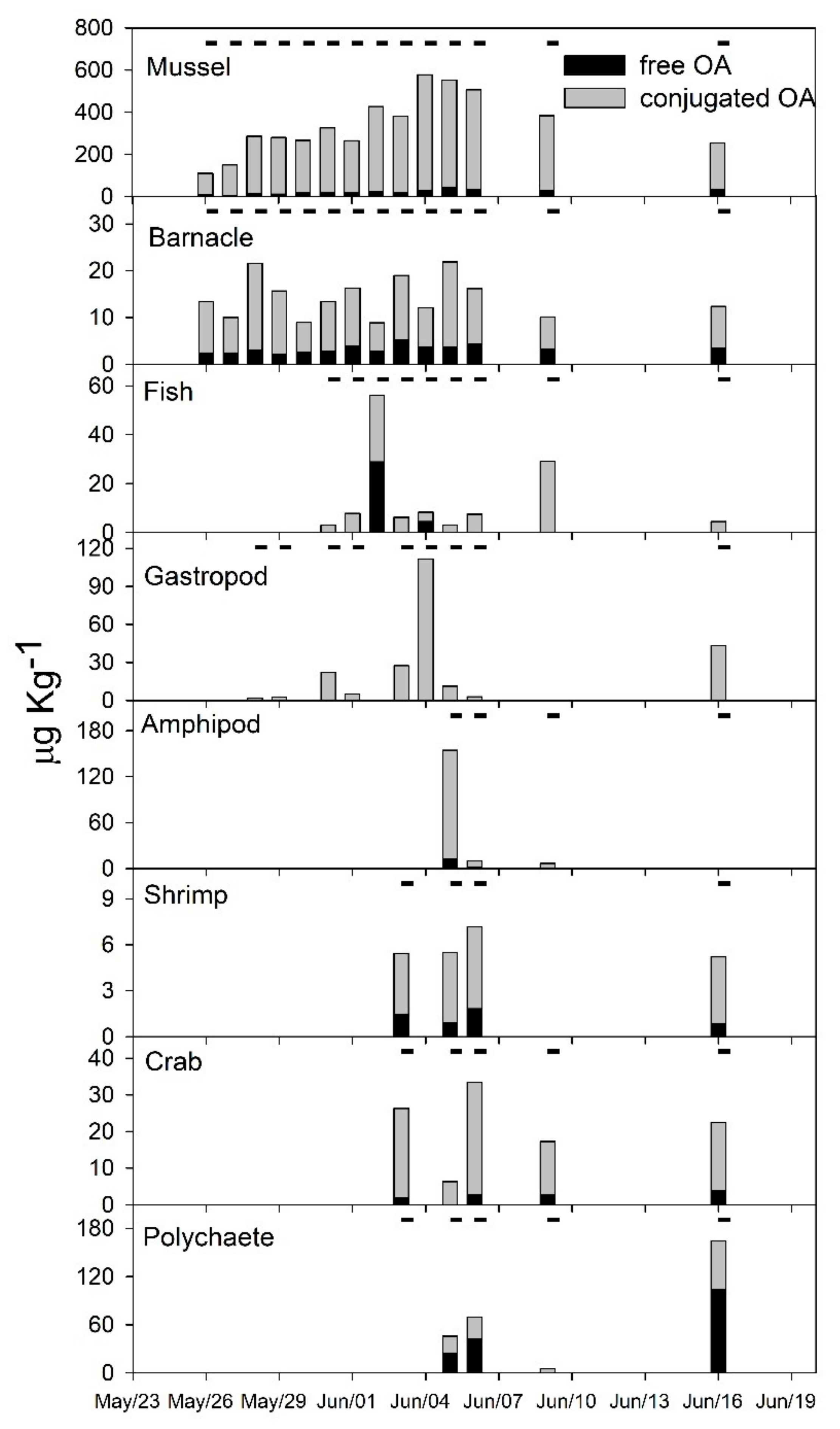
2.2. Diarrheic Toxins in Marine Fauna
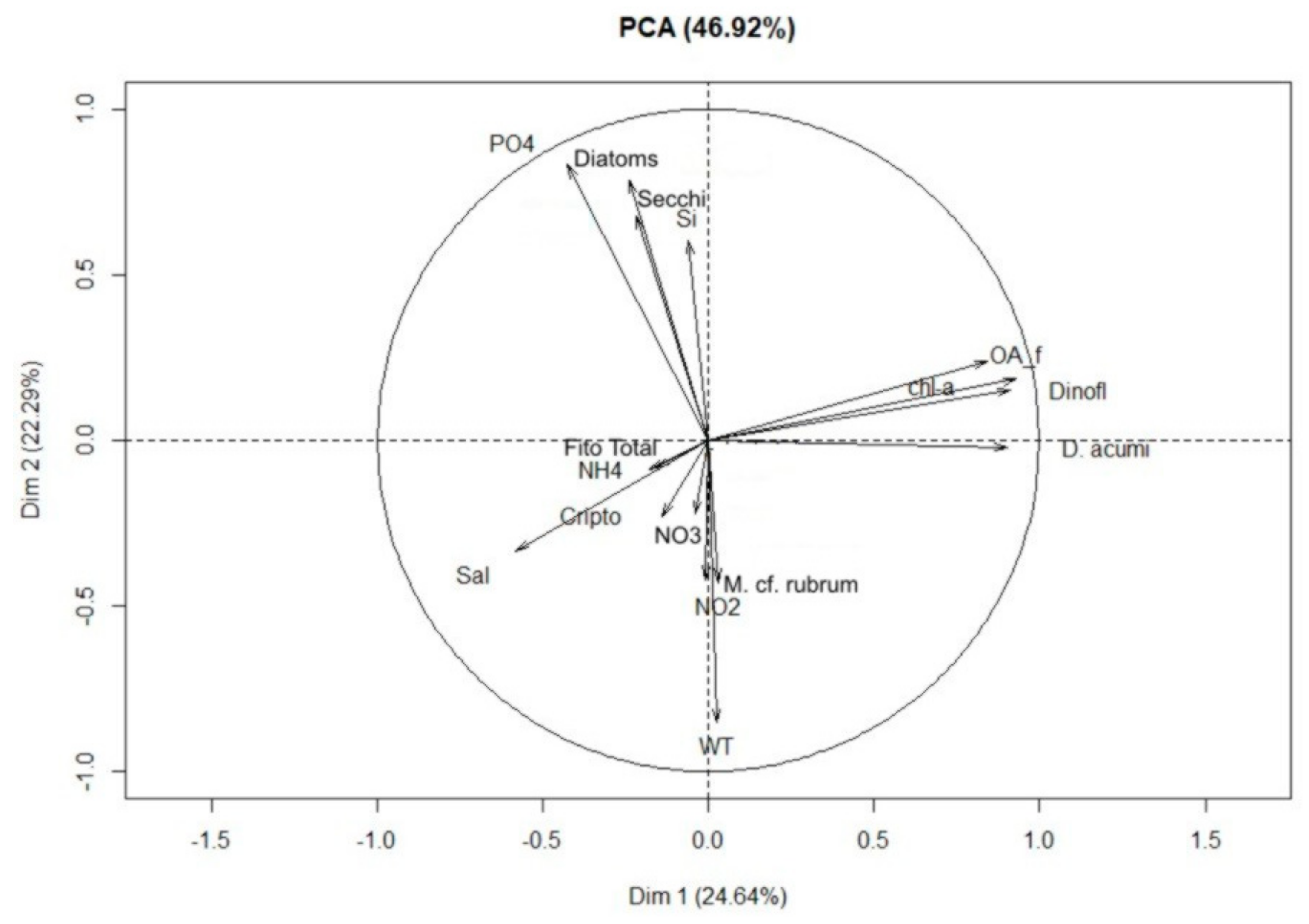
2.3. Correlations
3. Discussion
3.1. Bloom Development and Trophic Relationships
3.2. Fate of Diarrheic Toxins during the Bloom
4. Materials and Methods
4.1. Study Area
4.2. Sampling Design
4.3. Processing of Samples
4.4. Phytoplankton Identification and Enumeration
4.5. Spectrophotometric Analysis of Dissolved Inorganic Nutrients
4.6. Analysis of Photosynthetic Pigments by LC-DAD
4.7. Analysis of Diarrheic Toxins by LC-MS/MS
4.8. Data Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Reguera, B.; Velo-Suárez, L.; Raine, R.; Park, M.G. Harmful Dinophysis species: A review. Harmful Algae 2012, 14, 87–106. [Google Scholar] [CrossRef]
- García-Altares, M.; Casanova, A.; Fernández-Tejedor, M.; Diogène, J.; de la Iglesia, P. Bloom of Dinophysis spp. dominated by D. sacculus and its related diarrhetic shellfish poisoning (DSP) outbreak in Alfacs Bay (Catalonia, NW Mediterranean Sea): Identification of DSP toxins in phytoplankton, shellfish and passive samplers. Reg. Stud. Mar. Sci. 2016, 6, 19–28. [Google Scholar] [CrossRef]
- Whyte, C.; Swan, S.; Davidson, K. Changing wind patterns linked to unusually high Dinophysis blooms around the Shetland Islands, Scotland. Harmful Algae 2014, 39, 365–373. [Google Scholar] [CrossRef]
- Pitcher, G.C.; Krock, B.; Cembella, A.D. Accumulation of diarrhetic shellfish poisoning toxins in the oyster Crassostrea gigas and the mussel Choromytilus meridionalis in the southern Benguela ecosystem. Afr. J. Mar. Sci. 2011, 33, 273–281. [Google Scholar] [CrossRef]
- Aissaoui, A.; Dhib, A.; Reguera, B.; Ben Hassine, O.K.; Turki, S.; Aleya, L. First evidence of cell deformation occurrence during a Dinophysis bloom along the shores of the Gulf of Tunis (SW Mediterranean Sea). Harmful Algae 2014, 39, 191–201. [Google Scholar] [CrossRef]
- Li, A.; Sun, G.; Qiu, J.; Fan, L. Lipophilic shellfish toxins in Dinophysis caudata picked cells and in shellfish from the East China Sea. Environ. Sci. Pollut. Res. 2015, 22, 3116–3126. [Google Scholar] [CrossRef] [PubMed]
- Hattenrath-Lehmann, T.K.; Marcoval, M.A.; Berry, D.L.; Fire, S.; Wang, Z.; Morton, S.L.; Gobler, C.J. The emergence of Dinophysis acuminata blooms and DSP toxins in shellfish in New York waters. Harmful Algae 2013, 26, 33–44. [Google Scholar] [CrossRef]
- Martínez, A.; Nacional, D.; Acuáticos, D.R.; Fabre, A. Intensification of marine dinoflagellates blooms in Uruguay Intensificación de floraciones de dinoflagelados marinos en Uruguay Intensification of marine dinoflagellates blooms in Uruguay. Rev. Lab. Tecnol. Urug. 2017, 13, 19–25. [Google Scholar]
- Sar, E.A.; Sunesen, I.; Goya, A.B.; Lavigne, A.S.; Tapia, E.; García, C.; Lagos, N. First Report of Diarrheic Shellfish Toxins in Mollusks from Buenos Aires Province (Argentina) associated with Dinophysis spp.: Evidence os okadaic acid, dinophysistoxin-1 and their acyl-derivates. Biol. Soc. Argent. Bot. 2012, 47, 5–14. [Google Scholar]
- Villalobos, L.G.; Santinelli, N.; Sastre, V.; Krock, B.; Esteves, J.L. Dinophysis Species Associated with Diarrhetic Shellfish Poisoning Episodes in North Patagonian Gulfs (Chubut, Argentina). J. Shellfish Res. 2015, 34, 1141–1149. [Google Scholar] [CrossRef]
- Smayda, T.J. Complexity in the eutrophication–harmful algal bloom relationship, with comment on the importance of grazing. Harmful Algae 2008, 8, 140–151. [Google Scholar] [CrossRef]
- Lewitus, A.J.; Brock, L.M.; Burke, M.K.; DeMattio, K.A.; Wilde, S.B. Lagoonal stormwater detention ponds as promoters of harmful algal blooms and eutrophication along the South Carolina coast. Harmful Algae 2008, 8, 60–65. [Google Scholar] [CrossRef]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.; et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Buskey, E.J. How does eutrophication affect the role of grazers in harmful algal bloom dynamics? Harmful Algae 2008, 8, 152–157. [Google Scholar] [CrossRef]
- Hallegraeff, G.M. Ocean climate change, phytoplankton community responses, and harmful algal blooms: A formidable predictive challenge. J. Phycol. 2010, 46, 220–235. [Google Scholar] [CrossRef]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Haraguchi, L.; Odebrecht, C. Dinophysiales (Dinophyceae) no extremo Sul do Brasil (inverno de 2005, verão de 2007). Biota Neotrop. 2010, 10, 101–114. [Google Scholar] [CrossRef]
- Proença, L.A.O.; Schramm, M.A.; da Silva Tamanaha, M.; Alves, T.P. Diarrhoetic shellfish poisoning (DSP) outbreak in Subtropical Southwest Atlantic. Harmful Algae News 2007, 1–28. [Google Scholar]
- Rosa, C.M.A.; Philippi, J.M.S. Perfil epidemiologico de surtos de DTA por moluscos bivalves em Santa Catarina, Brasil, em 2007 e 2008. Hig. Aliment. 2009, 23, 570. [Google Scholar]
- Mafra, L.L.; Lopes, D.; Bonilauri, V.C.; Uchida, H.; Suzuki, T. Persistent Contamination of Octopuses and Mussels with Lipophilic Shellfish Toxins during Spring Dinophysis Blooms in a Subtropical Estuary. Mar. Drugs. 2015, 13, 3920–3935. [Google Scholar] [CrossRef] [PubMed]
- Mafra, L.L.; Ribas, T.; Alves, T.P.; Proença, L.A.O.; Schramm, M.A.; Uchida, H.; Suzuki, T. Differential okadaic acid accumulation and detoxification by oysters and mussels during natural and simulated Dinophysis blooms. Fish. Sci. 2015, 81, 749–762. [Google Scholar] [CrossRef]
- Proença, L.A.; Schmitt, F.; Costa, T.; Rorig, L. Evidences of diarrhetic shellfish poisoning in Santa Catarina—Brazil. J. Braz. Assoc. Advant. Sci. 1998, 50, 459–462. [Google Scholar]
- BRASIL. Instrução Normativa Interministerial 7 de 8 de Maio de 2012; Governo do Estado do Tocantins: Brasília, Brasil, 2012; pp. 55–59.
- Reguera, B.; Riobó, P.; Rodríguez, F.; Díaz, P.A.; Pizarro, G.; Paz, B.; Franco, J.M.; Blanco, J. Dinophysis toxins: Causative organisms, distribution and fate in shellfish. Mar. Drugs. 2014, 12, 394–461. [Google Scholar] [CrossRef] [PubMed]
- Moita, M.T.; Pazos, Y.; Rocha, C.; Nolasco, R.; Oliveira, P.B. Toward predicting Dinophysis blooms off NW Iberia: A decade of events. Harmful Algae 2016, 53, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Nam, S.W.; Shin, W.; Coats, D.W.; Park, M.G. Dinophysis caudata (dinophyceae) sequesters and retains plastids from the mixotrophic ciliate prey Mesodinium rubrum. J. Phycol. 2012, 48, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Giménez Papiol, G.; Beuzenberg, V.; Selwood, A.I.; MacKenzie, L.; Packer, M.A. The use of a mucus trap by Dinophysis acuta for the capture of Mesodinium rubrum prey under culture conditions. Harmful Algae 2016, 58, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mafra, L.L.; Nagai, S.; Uchida, H.; Tavares, C.P.S.; Escobar, B.P.; Suzuki, T. Harmful effects of Dinophysis to the ciliate Mesodinium rubrum: Implications for prey capture. Harmful Algae 2016, 59, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Ojamäe, K.; Hansen, P.J.; Lips, I. Mass entrapment and lysis of Mesodinium rubrum cells in mucus threads observed in cultures with Dinophysis. Harmful Algae 2016, 55, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.J.; Wang, D.Z.; Niu, T.; Xu, Y.X. Trophic transfer of paralytic shellfish toxins from the cladoceran (Moina mongolica) to larvae of the fish (Sciaenops ocellatus). Toxicon 2007, 50, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Reizopoulou, S.; Strogyloudi, E.; Giannakourou, A.; Pagou, K.; Hatzianestis, I.; Pyrgaki, C.; Granéli, E. Okadaic acid accumulation in macrofilter feeders subjected to natural blooms of Dinophysis acuminata. Harmful Algae 2008, 7, 228–234. [Google Scholar] [CrossRef]
- Mafra, L.L.; dos Santos Tavares, C.P.; Schramm, M.A. Diarrheic toxins in field-sampled and cultivated Dinophysis spp. cells from southern Brazil. J. Appl. Phycol. 2014, 26, 1727–1739. [Google Scholar] [CrossRef]
- Sipiä, V.; Kankaanpää, H.; Meriluoto, J.; Høisæter, T. The first observation of okadaic acid in flounder in the Baltic Sea. Sarsia. 2000, 85, 471–475. [Google Scholar] [CrossRef]
- Jiang, T.J.; Niu, T.; Xu, Y.X. Transfer and metabolism of paralytic shellfish poisoning from scallop (Chlamys nobilis) to spiny lobster (Panulirus stimpsoni). Toxicon 2006, 48, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Vale, P.; Sampayo, M.A.D.M. First confirmation of human diarrhoeic poisonings by okadaic acid esters after ingestion of razor clams (Solen marginatus) and green crabs (Carcinus maenas) in Aveiro lagoon, Portugal and detection of okadaic acid esters in phytoplankton. Toxicon 2002, 40, 989–996. [Google Scholar] [CrossRef]
- Hernández, M.; Robinson, I.; Aguilar, A.; González, L.M.; López-Jurado, L.F.; Reyero, M.I.; Cacho, E.; Franco, J.; López-Rodas, V.; Costas, E. Did algal toxins cause monk seal mortality? Nature 1998, 393, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Mafra, L.L.; Nolli, P.K.; Luz, L.F.; Leal, J.G.; Sobrinho, B.F.; Pimenta, B.; Escobar; Juraczky, L.; Gonzalez, A.R.M.; Mota, L.E.; et al. Okadaic acid contamination during an exceptionally massive Dinophysis cf. acuminata bloom in southern Brazil. In Proceedings of the Abstracts of the 17th International Conference of Harmful Algae, Florianópolis, Brazil, 9–14 October 2016; p. 212. [Google Scholar]
- Díaz, P.; Molinet, C.; Cáceres, M.A.; Valle-Levinson, A. Seasonal and intratidal distribution of Dinophysis spp. in a Chilean fjord. Harmful Algae 2011, 10, 155–164. [Google Scholar] [CrossRef]
- Alves-de-Souza, C.; Varela, D.; Contreras, C.; de La Iglesia, P.; Fernández, P.; Hipp, B.; Hernández, C.; Riobó, P.; Reguera, B.; Franco, J.M.; et al. Seasonal variability of Dinophysis spp. and Protoceratium reticulatum associated to lipophilic shellfish toxins in a strongly stratified Chilean fjord. Deep. Res. Part II Top. Stud. Oceanogr. 2014, 101, 152–162. [Google Scholar] [CrossRef]
- Aissaoui, A.; Armi, Z.; Turki, S.; Hassine, O.K. Ben Seasonal dynamic and in situ division rates of the dominant Dinophysis species in Punic harbors of Carthage (Gulf of Tunis, South Mediterranean). Environ. Monit. Assess. 2013, 185, 9361–9384. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoff, J.; Bell, E. The ciliate Mesodinium rubrum and its cryptophyte prey in Antarctic aquatic environments. Polar Biol. 2015, 38, 1305–1310. [Google Scholar] [CrossRef]
- Crawford, D.W.; Lindholm, T. Some observations on vertical distribution and migration of the phototrophic ciliate Mesodinium rubrum (=Myrionecta rubra) in a stratified brackish inlet. Aquat. Microb. Ecol. 1997, 13, 267–274. [Google Scholar] [CrossRef]
- Johnson, M.D.; Stoecker, D.K.; Marshall, H.G. Seasonal dynamics of Mesodinium rubrum in Chesapeake Bay. J. Plankton Res. 2013, 35, 877–893. [Google Scholar] [CrossRef]
- Kim, M.; Kim, S.; Yih, W.; Park, M.G. The marine dinoflagellate genus Dinophysis can retain plastids of multiple algal origins at the same time. Harmful Algae 2012, 13, 105–111. [Google Scholar] [CrossRef]
- Stern, R.F.; Amorim, A.L.; Bresnan, E. Diversity and plastid types in Dinophysis acuminata complex (Dinophyceae) in Scottish waters. Harmful Algae 2014, 39, 223–231. [Google Scholar] [CrossRef]
- Wisecaver, J.H.; Hackett, J.D. Transcriptome analysis reveals nuclear-encoded proteins for the maintenance of temporary plastids in the dinoflagellate Dinophysis acuminata. BMC Genom. 2010, 11, 366. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Nam, S.W.; Shin, W.; Coats, D.W.; Park, M.G. Fate of green plastids in Dinophysis caudata following ingestion of the benthic ciliate Mesodinium coatsi: Ultrastructure and psbA gene. Harmful Algae 2015, 43, 66–73. [Google Scholar] [CrossRef]
- Lindahl, O.; Lundve, B.; Johansen, M. Toxicity of Dinophysis spp. in relation to population density and environmental conditions on the Swedish west coast. Harmful Algae 2007, 6, 218–231. [Google Scholar] [CrossRef]
- Raine, R. A review of the biophysical interactions relevant to the promotion of HABs in stratified systems: The case study of Ireland. Deep. Res. Part II Top. Stud. Oceanogr. 2014, 101, 21–31. [Google Scholar] [CrossRef]
- Koukaras, K. Dinophysis blooms in Greek coastal waters (Thermaikos Gulf, NW Aegean Sea). J. Plankton Res. 2004, 26, 445–457. [Google Scholar] [CrossRef]
- Fabro, E.; Almandoz, G.O.; Ferrario, M.E.; Hoffmeyer, M.S.; Pettigrosso, R.E.; Uibrig, R.; Krock, B. Co-occurrence of Dinophysis tripos and pectenotoxins in Argentinean shelf waters. Harmful Algae 2015, 42, 25–33. [Google Scholar] [CrossRef]
- Möller, O.O.; Piola, A.R.; Freitas, A.C.; Campos, E.J.D. The effects of river discharge and seasonal winds on the shelf off southeastern South America. Cont. Shelf Res. 2008, 28, 1607–1624. [Google Scholar] [CrossRef]
- Piola, A.R.; Romero, S.I.; Zajaczkovski, U. Space–time variability of the Plata plume inferred from ocean color. Cont. Shelf Res. 2008, 28, 1556–1567. [Google Scholar] [CrossRef]
- Méndez, S.; Martínez, A.; Fabre, A. Extreme abundant bloom of Dinophysis ovum associated to positive SST anomalies in Uruguay. In Proceedings of the 17th International Conference of Harmful Algae, Florianópolis, Brazil, 9–14 October 2016; Proença, L.A.O., Hallegraeff, G.M., Eds.; International Society for the Study of Harmful Algae: Florianópolis, Brazil, 2016; pp. 22–25. [Google Scholar]
- Méndez, S.; Rodriguez, F.; Reguera, B.; Franco, J.M.; Riobo, P.; Fabre, A. Characterization of Dinophysis ovum as the causative agent of the exceptional DSP event in Uruguay during 2015. In Proceedings of the 17th International Conference on Harmful Algae, Florianópolis, Brazil, 9–14 October 2016; Proença, L.A.O., Hallegraeff, G.M., Eds.; International Society for the Study of Harmful Algae: Florianópolis, Brazil, 2016; pp. 26–29. [Google Scholar]
- Proença, L.A.O. Clorofila a do fitoplâncton em seis enseadas utilizadas para o cultivo de moluscos bivalves no litoral de Santa Catarina. Braz. J. Aquat. Sci. Technol. 2002, 6, 33–44. [Google Scholar] [CrossRef]
- Ferreira, J.F.; Besen, K.; Wormsbecher, A.G.; Santos, R.F. Physical-Chemical Parameters of Seawater Mollusc Culture Sites in Santa Catarina—Brazil. J. Coast. Res. 2006, 1122–1126. [Google Scholar]
- Escalera, L.; Pazos, Y.; Doval, M.D.; Reguera, B. A comparison of integrated and discrete depth sampling for monitoring toxic species of Dinophysis. Mar. Pollut. Bull. 2012, 64, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Farrell, H.; Gentien, P.; Fernand, L.; Lunven, M.; Reguera, B.; González-Gil, S.; Raine, R. Scales characterising a high density thin layer of Dinophysis acuta Ehrenberg and its transport within a coastal jet. Harmful Algae 2012, 15, 36–46. [Google Scholar] [CrossRef]
- Aune, T.; Larsen, S.; Aasen, J.A.B.; Rehmann, N.; Satake, M.; Hess, P. Relative toxicity of dinophysistoxin-2 (DTX-2) compared with okadaic acid, based on acute intraperitoneal toxicity in mice. Toxicon 2007, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Álvarez, G.; Uribe, E. Identification of pectenotoxins in plankton, filter feeders, and isolated cells of a Dinophysis acuminata with an atypical toxin profile, from Chile. Toxicon 2007, 49, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Froese, R.; Pauly, D. FishBase. Available online: http://fishbase.org (accessed on 25 January 2018).
- Menezes, N.A.; Figueiredo, J.L. Manual de Peixes Marinhos do Sudeste do Brasil. V. Teleostei (4); University of São Paulo: São Paulo, Brasil, 1985. [Google Scholar]
- Caine, E.A. Caprellid amphipods: fast food for the reproductively active. J. Exp. Mar. Bio. Ecol. 1991, 148, 27–33. [Google Scholar] [CrossRef]
- Brasil. Portaria No 204, de 28 de Junho de 2012; Ministério da Pesca e Aquicultura: Brasília-DF, Brazil, 2012; pp. 2–5.
- Penna, A.; Bertozzini, E.; Battocchi, C.; Galluzzi, L.; Giacobbe, M.G.; Vila, M.; Garces, E.; Luglie, A.; Magnani, M. Monitoring of HAB species in the Mediterranean Sea through molecular methods. J. Plankton Res. 2006, 29, 19–38. [Google Scholar] [CrossRef]
- Higman, W.A.; Algoet, M.; Stubbs, B.; Lees, D. Overview of developments of the algal biotoxin monitoring programme in England, Scotland and Wales. In Proceedings of the 6th International Conference on Molluscan Shellfish Safety, Blenheim, Marlborough, New Zealand, 18–23 March 2007; pp. 41–45. [Google Scholar]
- Vale, P.; Botelho, M.J.; Rodrigues, S.M.; Gomes, S.S.; Sampayo, M.A.D.M. Two decades of marine biotoxin monitoring in bivalves from Portugal (1986–2006): A review of exposure assessment. Harmful Algae 2008, 7, 11–25. [Google Scholar] [CrossRef]
- Brooks, S.; Harman, C.; Soto, M.; Cancio, I.; Glette, T.; Marigómez, I. Integrated coastal monitoring of a gas processing plant using native and caged mussels. Sci. Total Environ. 2012, 426, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Trainer, V.L.; Hardy, F.J. Integrative monitoring of marine and freshwater harmful algae in Washington State for public health protection. Toxins. 2015, 7, 1206–1234. [Google Scholar] [CrossRef] [PubMed]
- Doyle, P.; Mather, A.E.; Bennett, M.R.; Bussell, M.A. Miocene barnacle assemblages from southern Spain and their palaeoenvironmental significance. Lethaia 1996, 29, 267–274. [Google Scholar] [CrossRef]
- Turner, J.T. Planktonic marine copepods and harmful algae. Harmful Algae 2014, 32, 81–93. [Google Scholar] [CrossRef]
- Maneiro, I.; Guisande, C.; Frangópulos, M.; Riveiro, I. Importance of copepod faecal pellets to the fate of the DSP toxins produced by Dinophysis spp. Harmful Algae 2002, 1, 333–341. [Google Scholar] [CrossRef]
- Kozlowsky-Suzuki, B.; Carlsson, P.; Rühl, A.; Granéli, E. Food selectivity and grazing impact on toxic Dinophysis spp. by copepods feeding on natural plankton assemblages. Harmful Algae 2006, 5, 57–68. [Google Scholar] [CrossRef]
- Armi, Z.; Turki, S.; Trabelsi, E.; Ceredi, A.; Riccardi, E.; Milandri, A. Occurrence of diarrhetic shellfish poisoning (DSP) toxins in clams (Ruditapes decussatus) from Tunis north lagoon. Environ. Monit. Assess. 2012, 184, 5085–5095. [Google Scholar] [CrossRef] [PubMed]
- Dumbauld, B.R.; Ruesink, J.L.; Rumrill, S.S. The ecological role of bivalve shellfish aquaculture in the estuarine environment: A review with application to oyster and clam culture in West Coast (USA) estuaries. Aquaculture. 2009, 290, 196–223. [Google Scholar] [CrossRef]
- García-Mendoza, E.; Sánchez-Bravo, Y.A.; Turner, A.; Blanco, J.; O’Neil, A.; Mancera-Flores, J.; Pérez-Brunius, P.; Rivas, D.; Almazán-Becerril, A.; Peña-Manjarrez, J.L. Lipophilic toxins in cultivated mussels (Mytilus galloprovincialis) from Baja California, Mexico. Toxicon 2014, 90, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO); World Health Organization (WHO). Technical Paper on Toxicity Equivalency Factors for Marine Biotoxins Associated with Bivalve Molluscs; FAO: Roman, Italy; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Branco, J.O. Biologia e pesca do camarão sete-barbas Xiphopenaeus kroyeri (Heller) (Crustacea, Penaeidae), na Armação do Itapocoroy, Penha, Santa Catarina, Brasil. Rev. Bras. Zool. 2005, 22, 1050–1062. [Google Scholar] [CrossRef]
- Alves, T.P.; Schramm, M.A.; Proença, L.A.O.; Pinto, T.O.; Mafra, L.L. Interannual variability in Dinophysis spp. abundance and toxin accumulation in farmed mussels (Perna perna) in a subtropical estuary. Environ. Monit. Assess. 2018. [Google Scholar] [CrossRef] [PubMed]
- Trochimczuk-Fo, A.; Schettini, C.A.F. Avaliação da dispersão espacial da pluma do estuário do rio Itajaí-Açu em diferentes períodos de descarga. Braz. J. Aquat. Sci. Technol. 2003, 7, 83–96. [Google Scholar] [CrossRef]
- Schettini, C.A.F.; Carvalho, J.L.B.; Truccolo, E.C. Aspectos Hidrodinâmicos da Enseada da Armação de Itapocoroy, SC. Braz. J. Aquat. Sci. Technol. 1999, 3, 99. [Google Scholar] [CrossRef]
- Schettini, C.A.F.; Resgalla, C., Jr.; Pereira-Fo, J.; Silva, M.A.C.; Truccolo, E.C.; Rörig, L.R. Variabilidade temporal das características oceanográficas e ecológicas da região de influência fluvial do rio Itajaí-Açu. Braz. J. Aquat. Sci. Technol. 2005, 9, 93–102. [Google Scholar] [CrossRef]
- EURLMB EU-Harmonised Standard Operating Procedure for Determination of Lipophilic Marine Biotoxins in Molluscs by LC-MS/MS, Version 5. 2015, pp. 1–31. Available online: www.aesan.msps.es/en/CRLMB/web/home.shtml (accessed on 7 May 2017).
- Edler, L.; Elbrächter, M. The Utermöhl method for quantitative phytoplankton analysis. Microsc. Mol. Methods Quant. Phytoplankt. Anal. 2010, 13–20. [Google Scholar] [CrossRef]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis, 3rd ed.; Grasshoff, K., Kremling, K., Ehrhardt, M., Eds.; Wiley-VCH: Weinheim, Germany; New York, NY, USA; Chiester, UK; Brisbane, Australia; Singapore; Toronto, ON, Canada, 1999; Volume 7, ISBN 3-527-25998-8. [Google Scholar]
- Zapata, M.; Rodríguez, F.; Garrido, J.L. Separation of chlorophylls and carotenoids from marine phytoplankton: A new HPLC method using a reversed phase C8column and pyridine-containing mobile phases. Mar. Ecol. Prog. Ser. 2000, 195, 29–45. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Dinno, A. Dunn’s Test of Multiple Comparisons Using Rank Sums; The Comprehensive R Archive Network: Portland, OR, USA, 2017. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25. [Google Scholar] [CrossRef]

| Toxins | Q1 (m/z) | Q3 (m/z) | DP (v) | EP (v) | CEP (v) | CE (v) | CXP (v) |
|---|---|---|---|---|---|---|---|
| OA | 803.5 | 255.0 | −129 | −10 | −40.1 | −82 | −2 |
| OA | 803.5 | 113.0 | −129 | −10 | −41.5 | −64 | −2 |
| DTX-2 | 803.5 | 255.0 | −129 | −10 | −40.6 | −64 | −2 |
| DTX-2 | 803.5 | 113.0 | −129 | −10 | −41.5 | −84 | −2 |
| DTX-1 | 817.5 | 255.0 | −129 | −10 | −41.5 | −62 | −2 |
| DTX-1 | 817.5 | 113.0 | −120 | −10 | −51.7 | −82 | −2 |
| DTX-3 | 1041.6 | 255.0 | −129 | −10 | −47.9 | −76 | −2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, T.P.; Mafra, L.L., Jr. Diel Variations in Cell Abundance and Trophic Transfer of Diarrheic Toxins during a Massive Dinophysis Bloom in Southern Brazil. Toxins 2018, 10, 232. https://doi.org/10.3390/toxins10060232
Alves TP, Mafra LL Jr. Diel Variations in Cell Abundance and Trophic Transfer of Diarrheic Toxins during a Massive Dinophysis Bloom in Southern Brazil. Toxins. 2018; 10(6):232. https://doi.org/10.3390/toxins10060232
Chicago/Turabian StyleAlves, Thiago Pereira, and Luiz Laureno Mafra, Jr. 2018. "Diel Variations in Cell Abundance and Trophic Transfer of Diarrheic Toxins during a Massive Dinophysis Bloom in Southern Brazil" Toxins 10, no. 6: 232. https://doi.org/10.3390/toxins10060232
APA StyleAlves, T. P., & Mafra, L. L., Jr. (2018). Diel Variations in Cell Abundance and Trophic Transfer of Diarrheic Toxins during a Massive Dinophysis Bloom in Southern Brazil. Toxins, 10(6), 232. https://doi.org/10.3390/toxins10060232




