iTRAQ-Based Quantitative Proteomic Analysis Reveals Changes in Metabolite Biosynthesis in Monascus purpureus in Response to a Low-Frequency Magnetic Field
Abstract
1. Introduction
2. Results
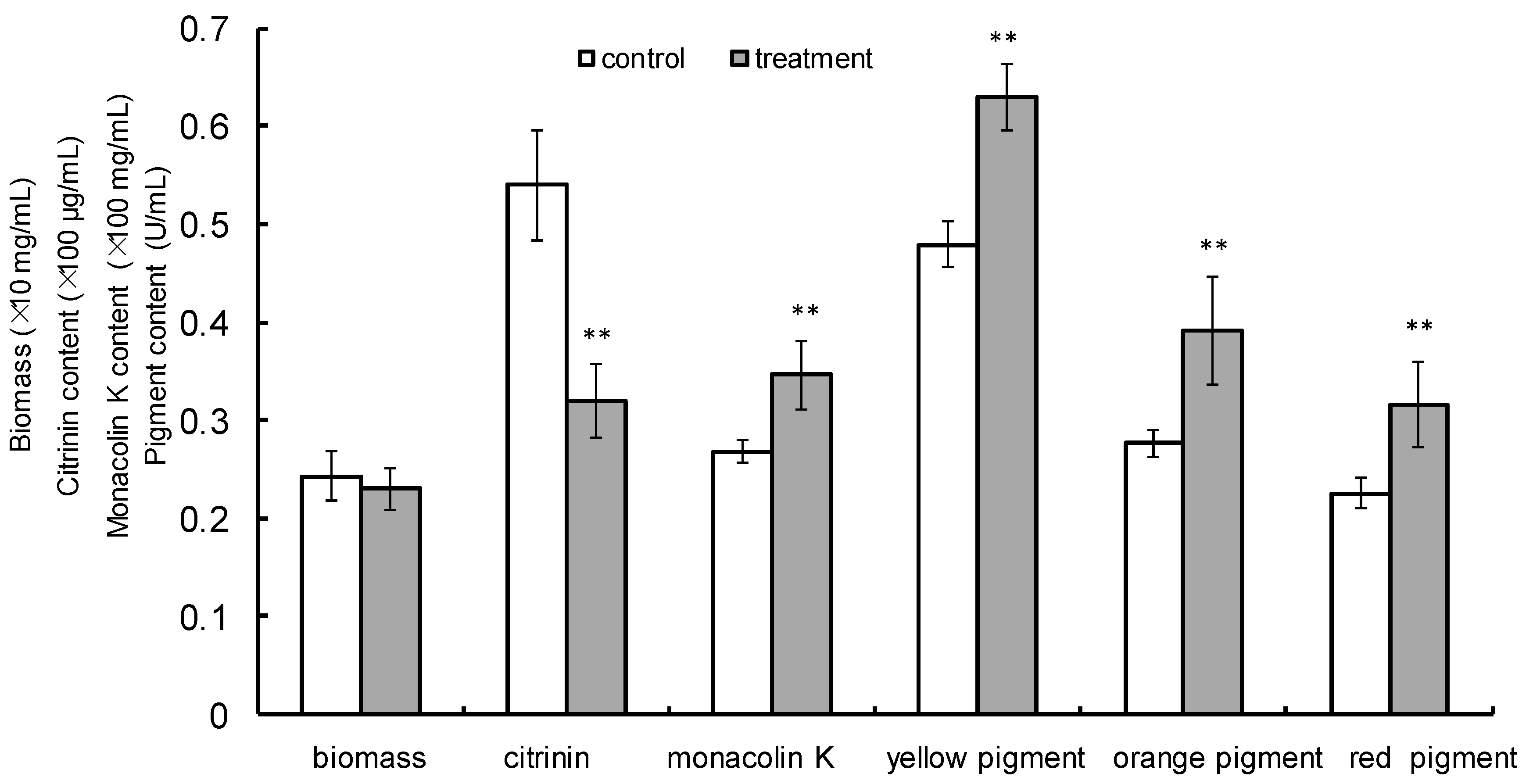
2.1. LF-FM Treatment Influences Biomass, Citrinin, Pigment and Monacolin K Yield
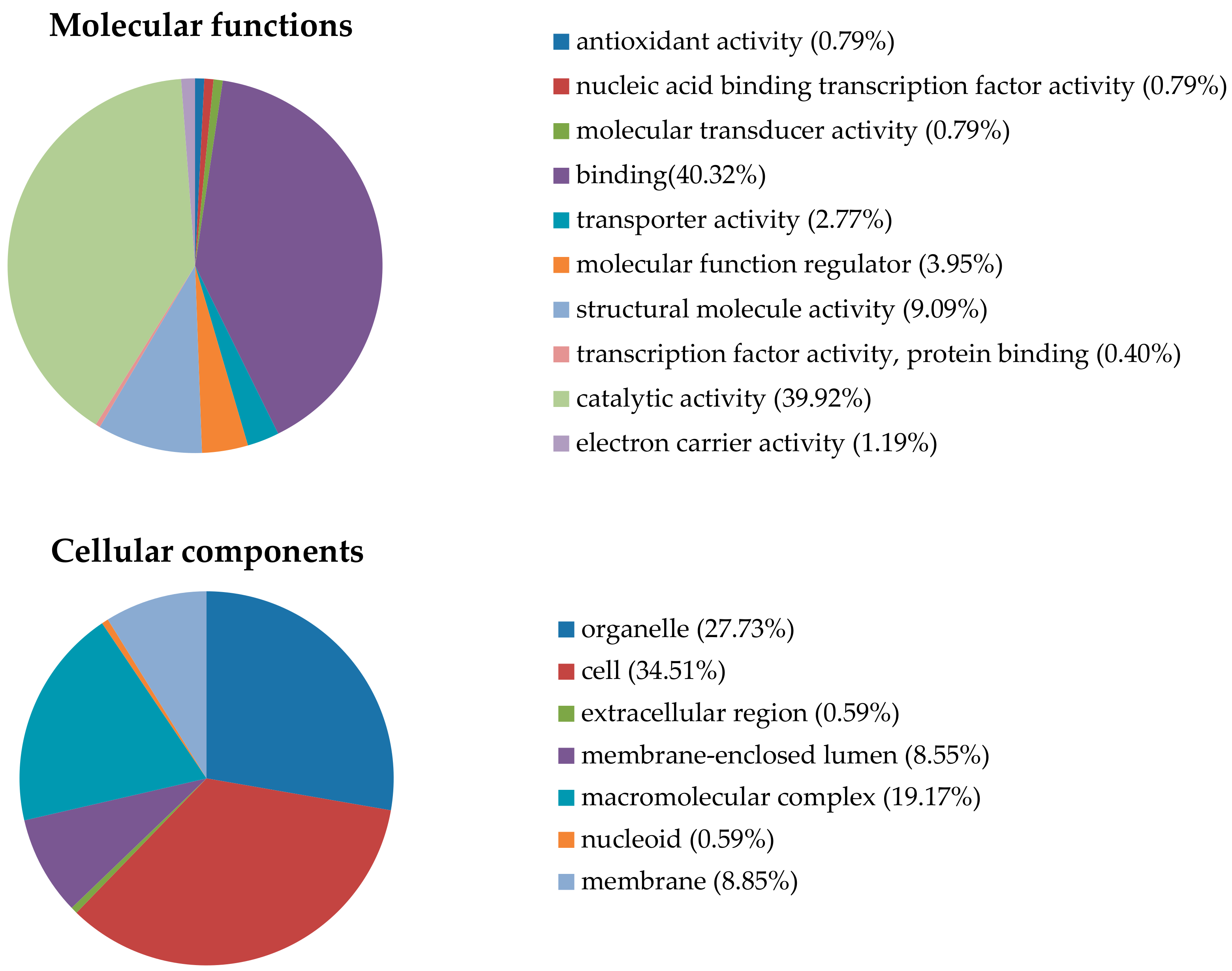
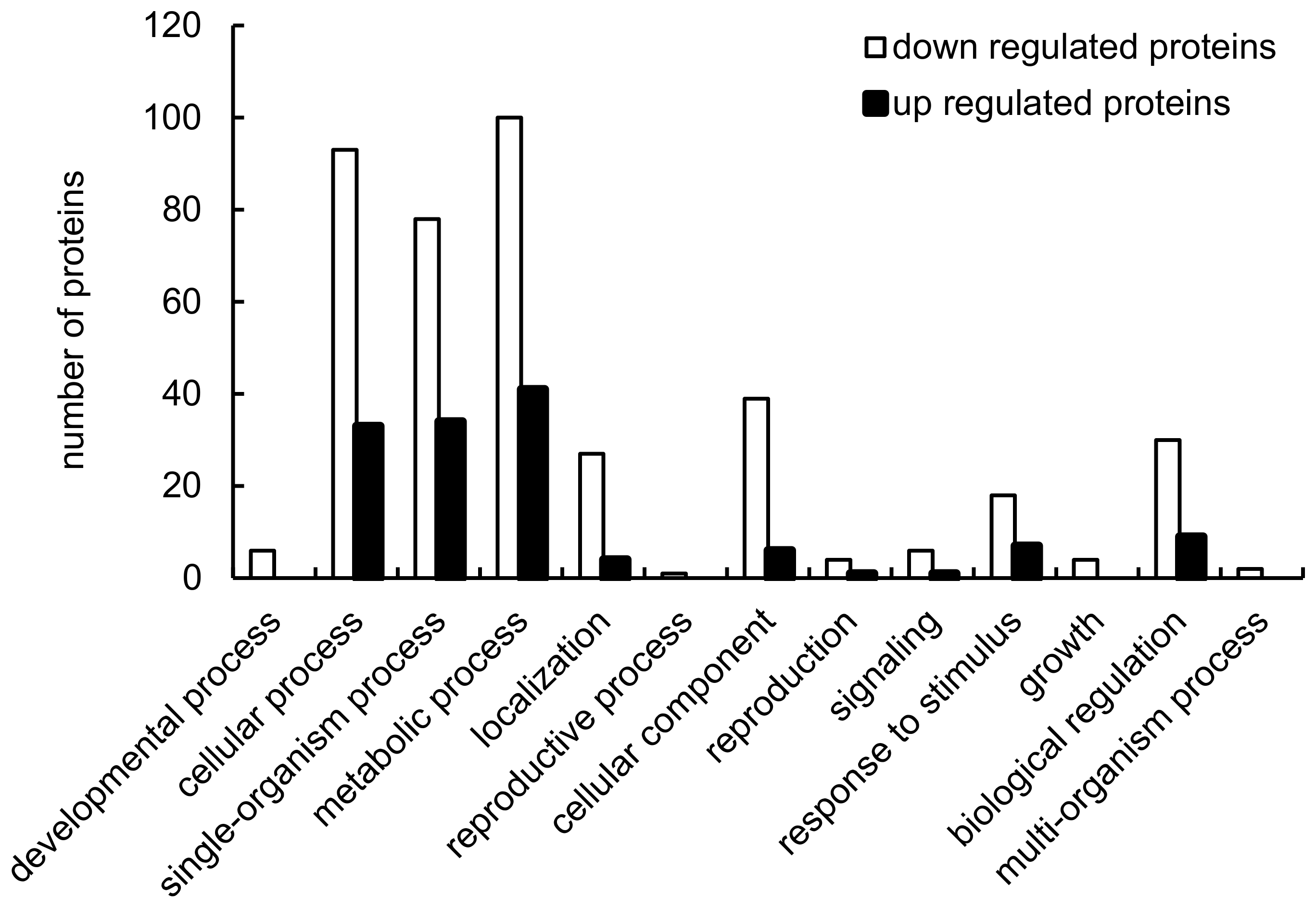
2.2. Identification of Proteins Differentially Expressed in Response to LF-MFs
2.3. Analysis of the Protein Families Associated with Citrinin, Pigment, and Monacolin K Biosynthesis
3. Discussion
4. Materials and Methods
4.1. Inoculum Preparation and LF-MF Treatments
4.2. Determination of Biomass and Secondary Metabolites
4.3. M. purpureus Collection and Protein Extraction
4.4. Protein Quantification and Digestion
4.5. iTRAQ Labeling and Fractionation
4.6. LC-MS/MS Analysis
4.7. iTRAQ Data Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, R.; Dwivedi, P.D.; Dhawan, A.; Das, M.; Ansari, K.M. Citrinin-generated reactive oxygen species cause cell cycle arrest leading to apoptosis via the intrinsic mitochondrial pathway in mouse skin. Toxicol. Sci. 2011, 122, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Patakova, P. Monascus secondary metabolites: Production and biological activity. J. Ind. Microbiol. Biot. 2013, 40, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Diana, M.; Quílez, J.; Rafecas, M. Gamma-aminobutyric acid as a bioactive compound in foods: A review. J. Funct. Foods 2014, 10, 407–420. [Google Scholar] [CrossRef]
- Shao, Y.; Lei, M.; Mao, Z.; Zhou, Y.; Chen, F. Insights into Monascus biology at the genetic level. Appl. Microbiol. Biot. 2014, 98, 3911–3922. [Google Scholar] [CrossRef] [PubMed]
- Endo, A. Monacolin k, a new hypocholesterolemic agent produced by a Monascus species. J. Antibiot. 1979, 32, 852–854. [Google Scholar] [CrossRef] [PubMed]
- Giulia, N.; Alessandro, A.; Francesco, D.P. Development of a new highly standardized and granulated extract from Monascus purpureus with a high content of monacolin K and KA and free of inactive secondary monacolins and citrinin. Nutrafoods 2015, 14, 197–205. [Google Scholar]
- Blanc, P.J.; Laussac, J.P.; Le Bars, J.; Le Bars, P.; Loret, M.O.; Pareilleux, A.; Promé, D.; Promé, J.C.; Santerre, A.L.; Goma, G. Characterization of monascidin a from Monascus as citrinin. Int. J. Food. Microbiol. 1995, 27, 201–213. [Google Scholar] [CrossRef]
- Kalaivani, M.; Rajasekaran, A. Improvement of monacolin K/citrinin production ratio in Monascus purpureus using UV mutagenesis. Nutrafoods 2014, 13, 79–84. [Google Scholar] [CrossRef]
- Kang, B.; Zhang, X.; Wu, Z.; Qi, H.; Wang, Z.; Park, S. Production of citrinin-free Monascus pigments by submerged culture at low pH. Enzyme Microb. Technol. 2014, 55, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Zhang, X.; Wu, Z.; Wang, Z. Optimal selection of agricultural products to inhibit citrinin production during submerged culture of Monascus anka. Biotechnol. Bioproc. Eng. 2014, 19, 1005–1013. [Google Scholar] [CrossRef]
- Wang, J.J.; Lee, C.L.; Pan, T.M. Modified mutation method for screening low citrinin-producing strains of Monascus purpureus on rice culture. J. Agric. Food Chem. 2004, 52, 6977–6982. [Google Scholar] [CrossRef] [PubMed]
- Je, S.K.; Bin, Y.J. Monascus sp. Mutant and Use Thereof. Patent KR20090084074, 5 August 2009. [Google Scholar]
- Balakrishnan, B.; Park, S.H.; Kwon, H.J. A reductase gene mppE controls yellow component production in azaphilone polyketide pathway of Monascus. Biotechnol. Lett. 2017, 39, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Tan, H.L.; Chen, G.; Wang, L.; Wu, Z.Q. Rising temperature stimulates the biosynthesis of water-soluble fluorescent yellow pigments and gene expression in Monascus ruber CGMCC10910. AMB Express 2017, 7, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Tseng, C.P.; Liaw, L.L.; Wang, C.L.; Chen, I.H.; Wu, W.J.; Wu, M.D.; Yuan, G.F. Cloning and characterization of monacolin K biosynthetic cluster from Monascus pilosus. J. Agric. Food Chem. 2008, 56, 5639–5646. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Kinoshita, H.; Nihira, T. Identification of mokB involved in monacolin K biosynthesis in Monascus pilosus. Biotechnol. Lett. 2009, 31, 1911–1916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liang, J.; Yang, L.; Chai, S.Y.; Zhang, C.X.; Sun, B.G.; Wang, C.T. Glutamic acid promotes monacolin K biosynthetic gene cluster expression in Monascus. AMB Express 2017, 7, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kinoshita, H.; Ishihara, S.; Sakai, K.; Nagai, S.; Nihira, T. Polyketide synthase gene responsible for citrinin biosynthesis in Monascus purpureus. Appl. Environ. Microbiol. 2005, 71, 3453–3457. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kinoshita, H.; Nihira, T. Identification and in vivo functional analysis by gene distruption of ctnA, an actiivator gene involved in citrinin biosynthesis in Monascus purpureus. Appl. Environ. Microbiol. 2007, 73, 5097–5103. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Xu, Y.; Huang, Z.B. Isolation and characterization of the citrinin biosynthetic gene cluster from Monascus aurantiacus. Biotechnol. Lett. 2012, 34, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, D.C.; Perez, V.H.; Justo, O.R.; Alegre, R.M. Effect of the extremely low frequency magnetic field on nisin production by Lactococcus lactis subsp. lactis using cheese whey permeate. Process Biochem. 2006, 41, 1967–1973. [Google Scholar] [CrossRef]
- Perez, V.H.; Reyes, A.F.; Justo, O.R.; Alvarez, D.C.; Alegre, R.M. Bioreactor coupled with electromagnetic field generator: Effects of extremely low frequency electromagnetic fields on ethanol production by Saccharomyces cerevisiae. Biotechnol. Prog. 2007, 23, 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- Canli, O.; Erdal, S.; Taskin, M.; Kurbanoglu, E.B. Effects of extremely low magnetic field on the production of invertase by Rhodotorula glutinis. Toxicol. Ind. Health 2011, 27, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.X.; Zhang, J.L.; Feng, H. Extremely low-frequency magnetic field effects on metabolite of Aspergillus niger. Bioelectromagnetic 2015, 32, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Zeng, D.J.; Xu, C.; Gao, M.X. Effect of low-frequency magnetic field on formation of pigments of Monascus purpureus. Eur. Food Res. Technol. 2015, 240, 577–582. [Google Scholar] [CrossRef]
- Zhang, J.L.; Xu, C.; Wan, Y.L.; Gao, M.X. Effects of extremely low frequency magnetic field on production of mannatide by a-Hemolytic Streptococcus. Bioelectromagnetic 2016, 37, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.L.; Zhang, J.L.; Han, H.X.; Li, L.; Liu, Y.B.; Gao, M.X. Citrinin-producing capacity of Monascus purpureus in response to low−frequency magnetic fields. Process Biochem. 2017, 53, 25–29. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Karki, S.; Chiu, S.H.; Kim, H.J.; Suh, J.W.; Nam, B.; Yoon, Y.M.; Chen, C.C.; Kwon, H.J. Genetic localization and in vivo characterization of a Monascus azaphilone polyketide biosynthetic gene cluster. Appl. Microbiol. Biotechnol. 2013, 97, 6337–6345. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wright, S.J.; Krystofova, S.; Park, G.; Borkovich, K.A. Heterotrimeric G protein signaling in filamentous fungi. Annu. Rev. Microbiol. 2007, 61, 423–452. [Google Scholar] [CrossRef] [PubMed]
- Tag, A.; Hicks, J.; Garifullina, G.; Akel, C.; Phillips, T.D.; Beremand, M.; Keller, N. G-protein signalling mediates differential production of toxic secondary metabolites. Mol. Microbiol. 2000, 38, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Y.; Seo, J.A.; Kim, J.E.; Han, K.H.; Shim, W.B.; Yun, S.H.; Lee, Y.W. Functional analyses of heterotrimeric G protein Gα and Gβ subunits in Gibberella zeae. Microbiology 2008, 154, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shao, Y.C.; Li, Q.; Yang, S.; Chen, F.S. Identification of Mga1, a G-protein α-subunit gene involved in regulating citrinin and pigment production in Monascus ruber M7. FEMS Microbiol. Lett. 2010, 308, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Wang, L.; Qing, L.; Chen, F.S. Effects of cyclic AMP on development and secondary metabolites of Monascus ruber M-7. Lett. Appl. Microbiol. 2011, 52, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Li, L.; Li, X.; Shao, Y.C.; Chen, F.S. MrflbA, encoding a putative FlbA, is involved in aerial hyphal development and secondary metabolite production in Monascus ruber M-7. Fungal Biol. 2012, 116, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.Y.; Lee, J.H.; Nam, Y.S.; Lee, Y.J.; Park, W.H.; Lee, B.C.; Kim, D.; Chung, Y.H.; Jeong, J.H. Extremely low frequency magnetic field induces oxidative stress in mouse cerebellum. Gen. Physiol. Biophys. 2011, 30, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. Cloning of GABA and citinin Metabolism-Related Genes of Monascus. Master’s Thesis, Zhenjiang Normal University, Hangzhou, China, 2015. [Google Scholar]
- Hajjaj, H.; Klaébé, A.; Goma, G.; Blanc, P.J.; Barbier, E.; Francois, J. Medium-chain fatty acids affect citrinin production in the filamentous fungus Monascus rubber. Appl. Environ. Microbiol. 2000, 66, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, M.; Rollini, M. Biosynthesis and biotechnological production of statins by filamentous fungi and application of these cholesterol-lowering drugs. Appl. Microbiol. Biotechnol. 2002, 58, 555–564. [Google Scholar] [PubMed]
- Barrios-González, J.; Miranda, R.U. Biotechnological production and applications of statins. Appl. Microbiol. Biotechnol. 2010, 85, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Hajjaj, H.; Klaébé, A.; Loret, M.O.; Goma, G.; Blanc, P.J.; Francois, J. Biosynthetic pathway of citrinin in the filamentous fungus Monascus ruber as revealed by 13C nuclear magnetic resonance. Appl. Environ. Microbiol. 1999, 65, 311–314. [Google Scholar] [PubMed]
- Li, S.J.; Cronan, J.E.J. The gene encoding the biotin carboxylase subunit of Escherichia coli acetyl-CoA carboxylase. J. Biol. Chem. 1992, 267, 855–856. [Google Scholar] [PubMed]
- Xie, H.; Yang, D.H.; Yao, H.; Bai, G.; Zhang, Y.H.; Xiao, B.G. iTRAQ-based quantitative proteomic analysis reveals proteomic changes in leaves of cultivated tobacco (Nocotiana tabacum) in response to drought stress. Biochem. Biophy. Res. Commun. 2016, 469, 768–775. [Google Scholar] [CrossRef] [PubMed]

| Accession | Protein Name | Ratio |
|---|---|---|
| Proteins and genes involved in citrinin biosynthesis | ||
| Aspzol_71435 | CtnI (acyl-CoA synthetase) | 0.629 |
| Q4wyl4 | CtnH (short chain dehydrogenase) | 0.791 |
| Monrupul_468568 | CtnR1 (WD repeat protein) | 0.774 |
| Evm_model_C5_699 | Biotin synthetase | 0.404 |
| Proteins and genes involved in pigment and monacolin biosynthesis | ||
| Pench1_76083 | Polyketide enoylreductase (ER) | 1.219 |
| A0a0a2jfe4 | Ketoreductase | 1.208 |
| A0a0a2lbj2penit | Esterase | 1.229 |
| A0a0a2lbj2 | Esterase | 1.331 |
| Q5bcz6 | MokH (Zn(II)2Cys6 transcription factor) | 1.267 |
| Aspwe1_68221 | Fatty acid elongase | 1.358 |
| Mycgr3_71228 | P450 monooxygenase | 2.031 |
| Mycgr3_98821 | mokC (P450 monooxygenase) | 1.235 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Liu, Y.; Li, L.; Gao, M. iTRAQ-Based Quantitative Proteomic Analysis Reveals Changes in Metabolite Biosynthesis in Monascus purpureus in Response to a Low-Frequency Magnetic Field. Toxins 2018, 10, 440. https://doi.org/10.3390/toxins10110440
Zhang J, Liu Y, Li L, Gao M. iTRAQ-Based Quantitative Proteomic Analysis Reveals Changes in Metabolite Biosynthesis in Monascus purpureus in Response to a Low-Frequency Magnetic Field. Toxins. 2018; 10(11):440. https://doi.org/10.3390/toxins10110440
Chicago/Turabian StyleZhang, Jialan, Yingbao Liu, Li Li, and Mengxiang Gao. 2018. "iTRAQ-Based Quantitative Proteomic Analysis Reveals Changes in Metabolite Biosynthesis in Monascus purpureus in Response to a Low-Frequency Magnetic Field" Toxins 10, no. 11: 440. https://doi.org/10.3390/toxins10110440
APA StyleZhang, J., Liu, Y., Li, L., & Gao, M. (2018). iTRAQ-Based Quantitative Proteomic Analysis Reveals Changes in Metabolite Biosynthesis in Monascus purpureus in Response to a Low-Frequency Magnetic Field. Toxins, 10(11), 440. https://doi.org/10.3390/toxins10110440





