Aptamers and Aptasensors for Highly Specific Recognition and Sensitive Detection of Marine Biotoxins: Recent Advances and Perspectives
Abstract
1. Introduction
2. Aptamers Targeting Marine Biotoxins
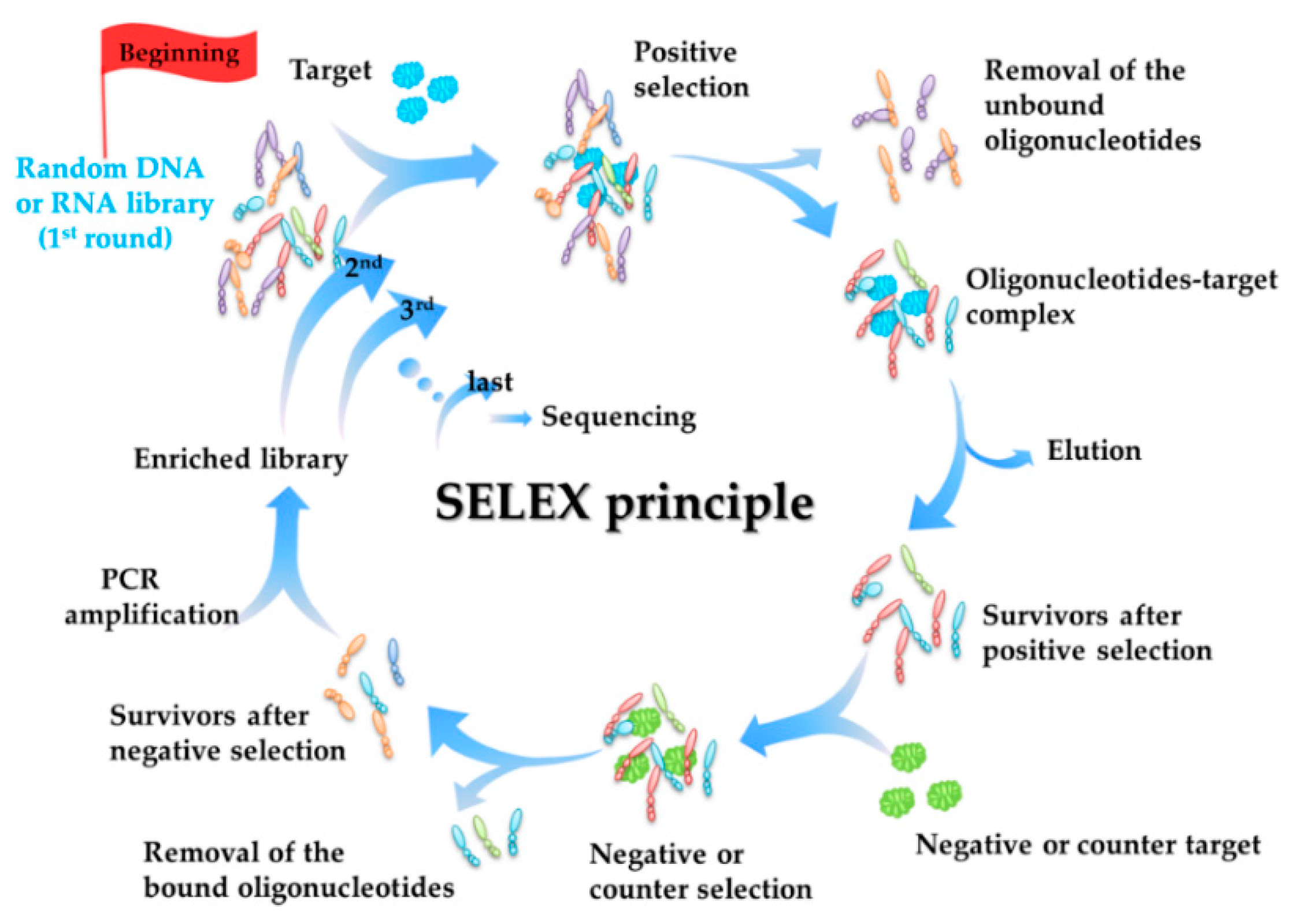
2.1. Selection Principle of Aptamers (SELEX Principle)
2.2. Detailed Information of Aptamers Targeting for Marine Biotoxins
- (a)
- Firstly, aptamers have drawn much attention in the field of marine biotoxins in recent years. According to our investigation, there have been 15 novel aptamers reported, covering all of the three categories of marine biotoxins. Among the targets shown in Table 1, palytoxin (PTX) and okadaic acid (OA) are polyether toxins; brevetoxin-2 (BTX-2) and microcystin (MC) are polypeptide toxins; and tetrodotoxin (TTX), saxitoxin (STX), anatoxin-a (ATX-a), and gonyautoxin1/4 (GTX1/4) are alkaloid toxins. And all of the aptamers were reported after 2012, and 50% of them were successfully selected after 2015. This indicates a high level of academic attention on the aptamers for marine biotoxins.
- (b)
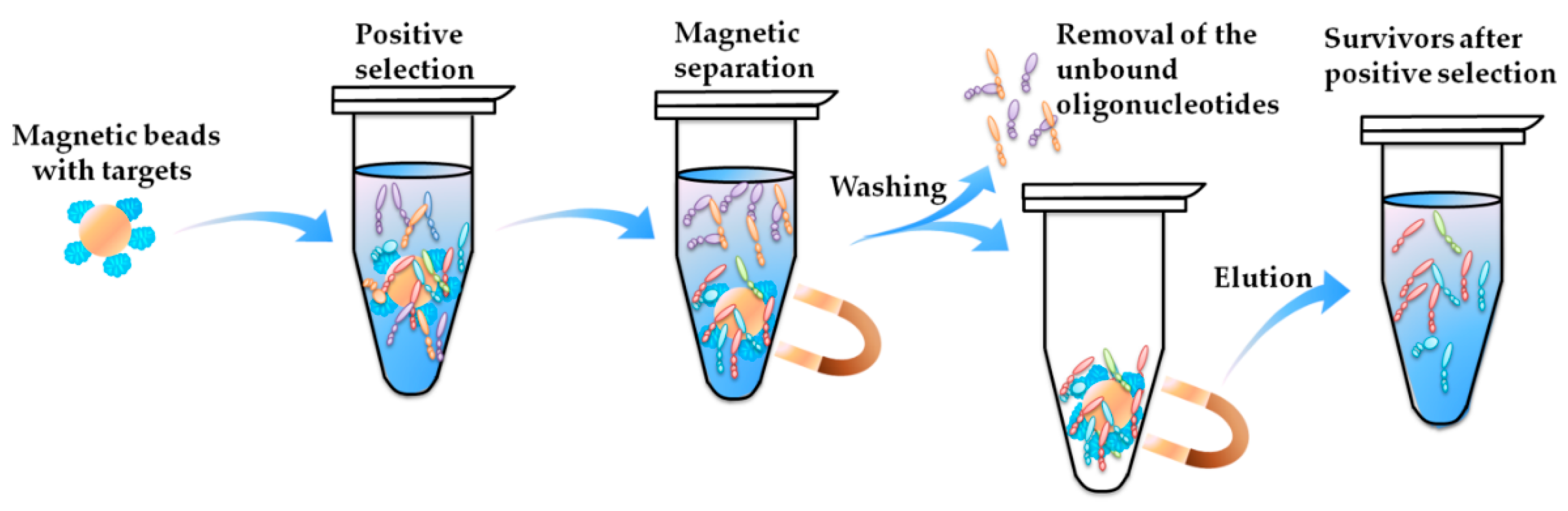
- Secondly, most aptamers targeting marine biotoxins were selected using beads-SELEX or magnetic-beads-SELEX (Mag-beads-SELEX), and most of the selection were finished within no more than 20 rounds. The marine biotoxins were immobilized onto the surface of beads or magnetic-beads. The surface with a spherical shape facilitates the full display of the targets on the beads and beads facilitate the convenient separation [59,60]. Figure 2 illustrates the partition and elution process of the positive selection part in the Mag-beads-SELEX. After the incubation of the target-immobilized magnetic beads with the oligonucleotides in the library, the partition of the oligonucleotides-beads complex from the unbound oligonucleotides and the elution of bound oligonucleotides from the oligonucleotides-beads are both achieved using the magnetic separation. The procedure of the beads-SELEX is similar, and the only difference is the partition of the beads and the supernatant is based on centrifugal separation.
- (c)
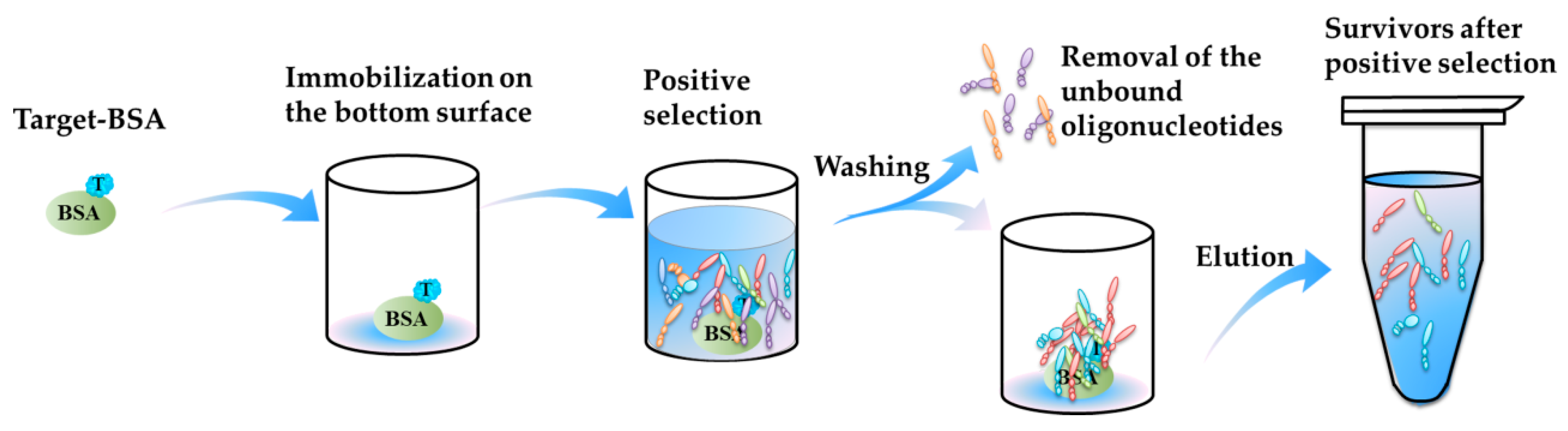
- Thirdly, some new selection methods promote efficient aptamer selection for marine biotoxins. In 2016, Tian et al. [64] completed a selection of aptamers binding to BTX-2 based on microwell-SELEX. The positive selection process is illustrated in Figure 3. The BTX-2 was coupled with a carrier protein, BSA (bovine serum albumin), and immobilized onto the inner bottom surface of the microwells, and the oligonucleotides were incubated with the immobilized BTX-2. Using the microwells as a matrix, no other special separation instruments were needed for the partition of oligonucleotides-beads complex and the unbound oligonucleotides or the elution of the bound oligonucleotides.
- (d)
- Fourthly, some post optimization greatly improved the affinity of the selected aptamers. Two of the aptamers in Table 1 were derived from truncation study. One is M-30f, and the other is GO-18-T-d. Zheng et al. [70] improved the APTSTX selected by Handy et al. [69] to be shorter and to have higher affinity towards STX. The authors analyzed the sequence of APTSTX and adopted rational site-directed mutagenesis, on the basis of secondary structure prediction to improve the conformational stability and thus to strengthen its interaction with STX. Then the authors adopted truncation to remove the unnecessary nucleotides and to remain the key binding structure, and M-30f was obtained with a 30-fold improved affinity. The other sample is the GO-18-T-d. It is truncated by Gao et al. [72] after they obtained the GO-18 using GO-SELEX. GO-18 was truncated based on the secondary structure prediction, and the GO-18-T-d has an 8-fold improved affinity.
3. Developed Aptasensors Targeting Marine Biotoxins
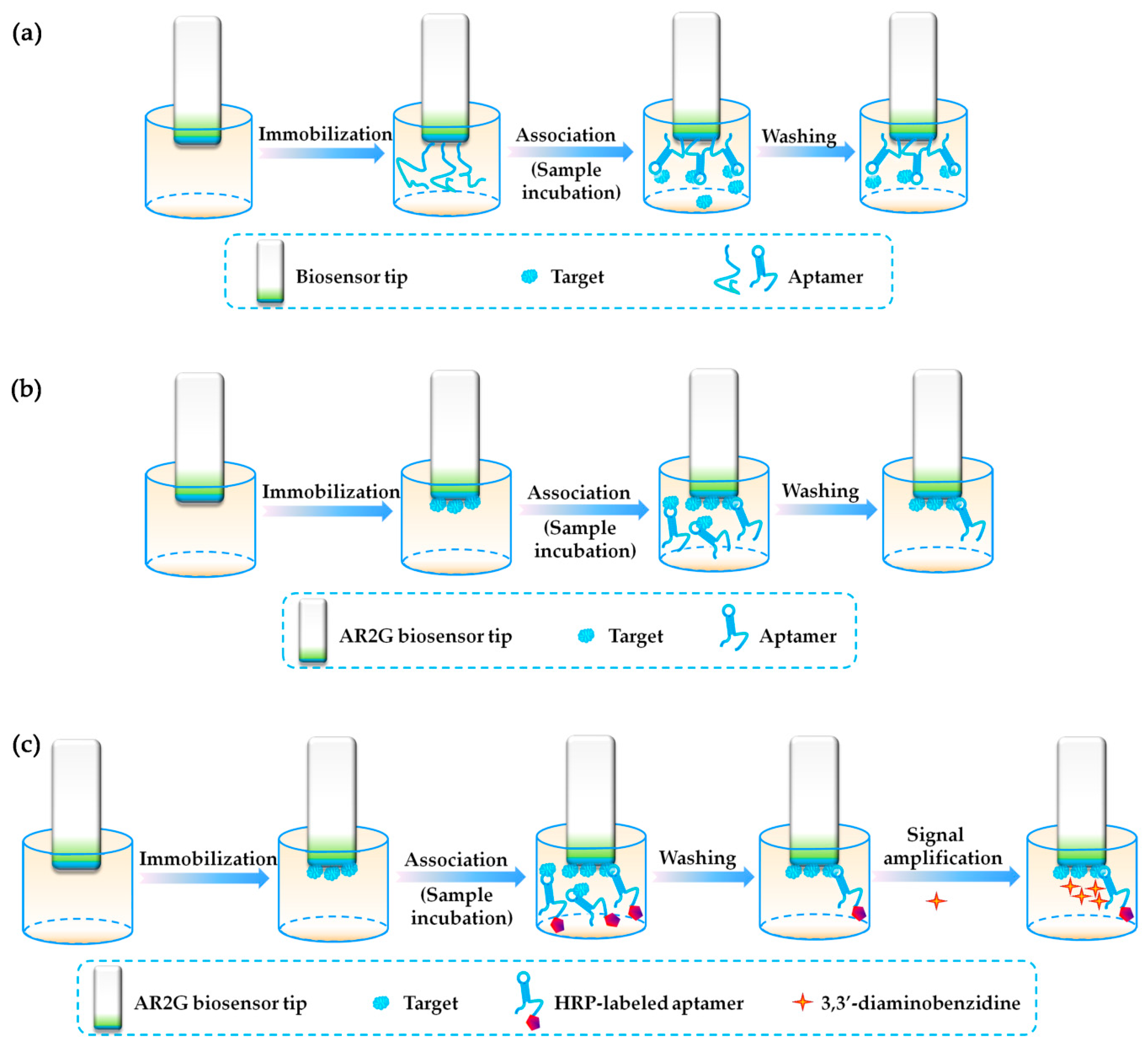
3.1. Biolayer Interferometry (BLI)-Based Aptasensors
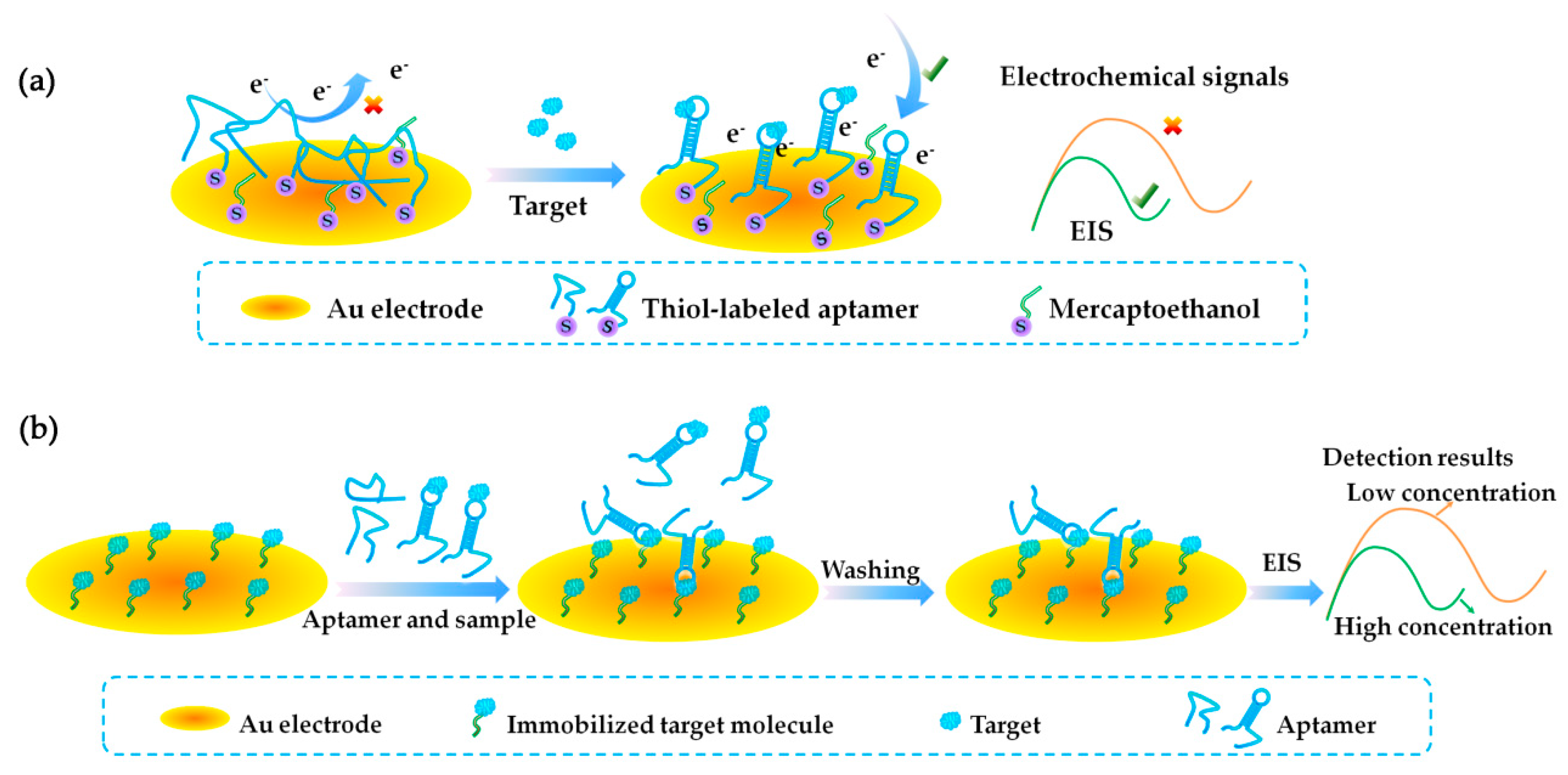
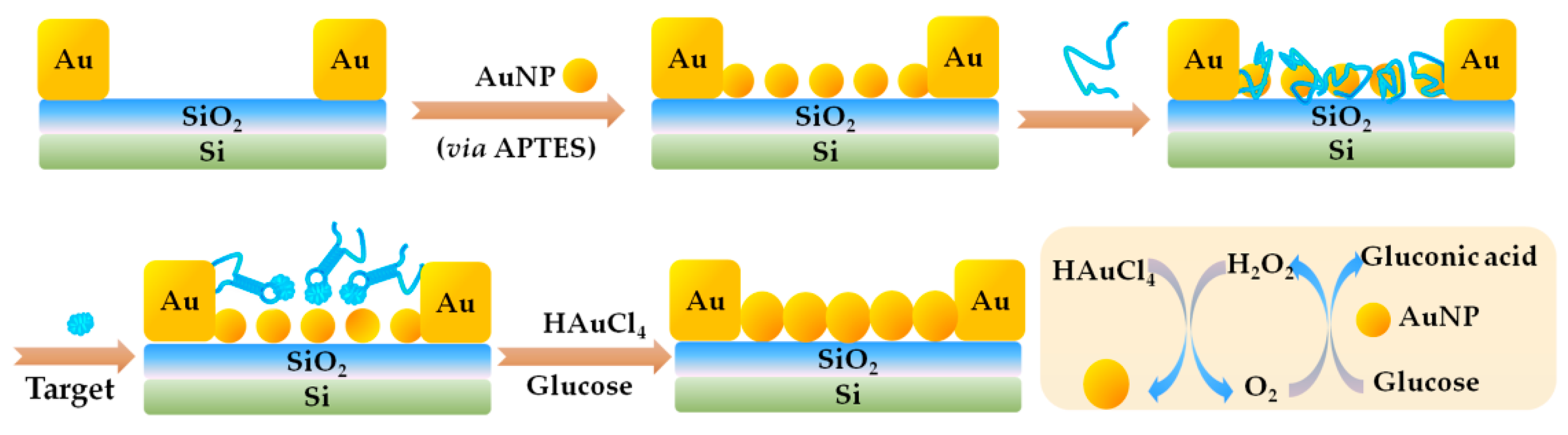
3.2. Electrochemistry (EC)-Based Aptasensor
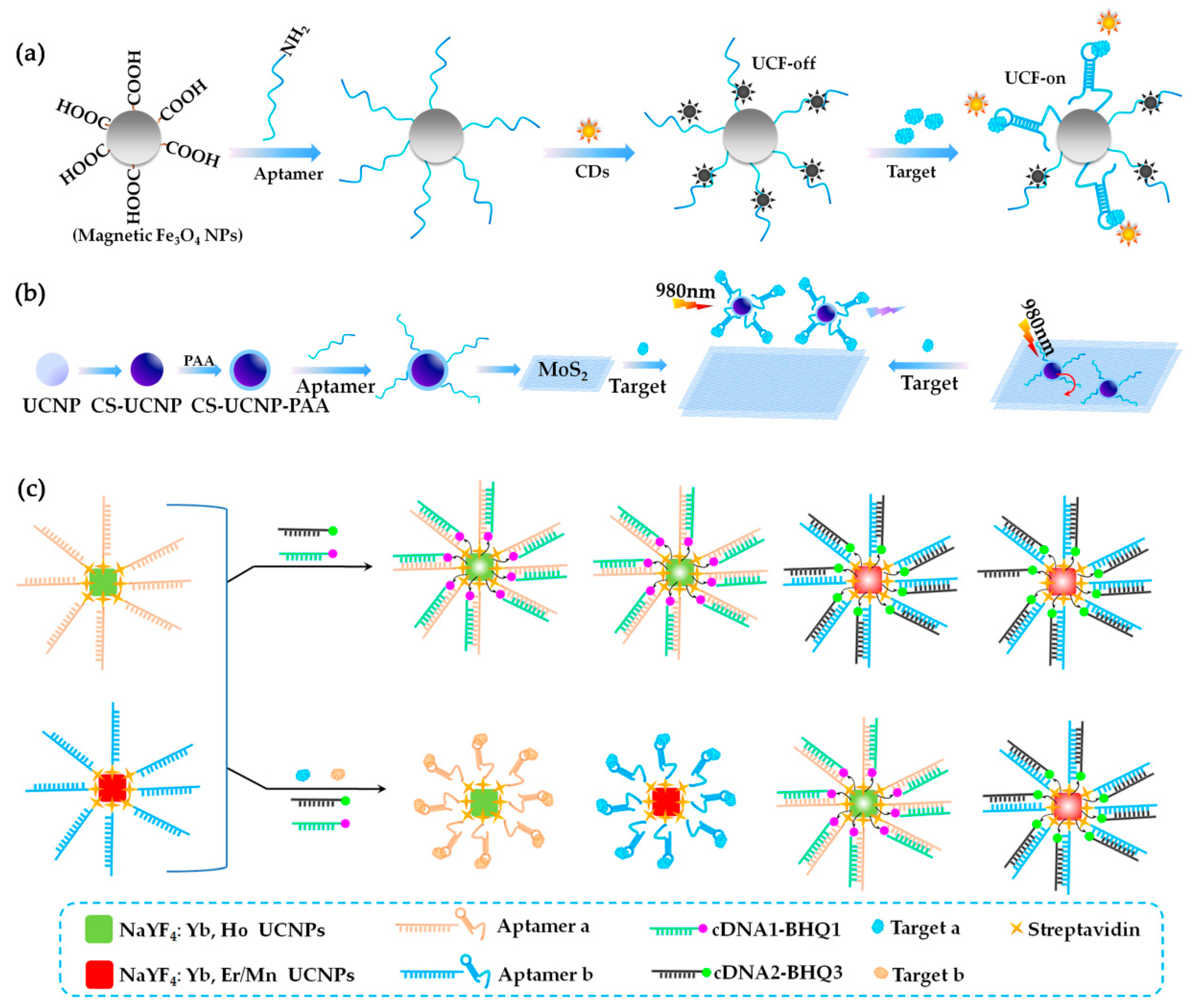
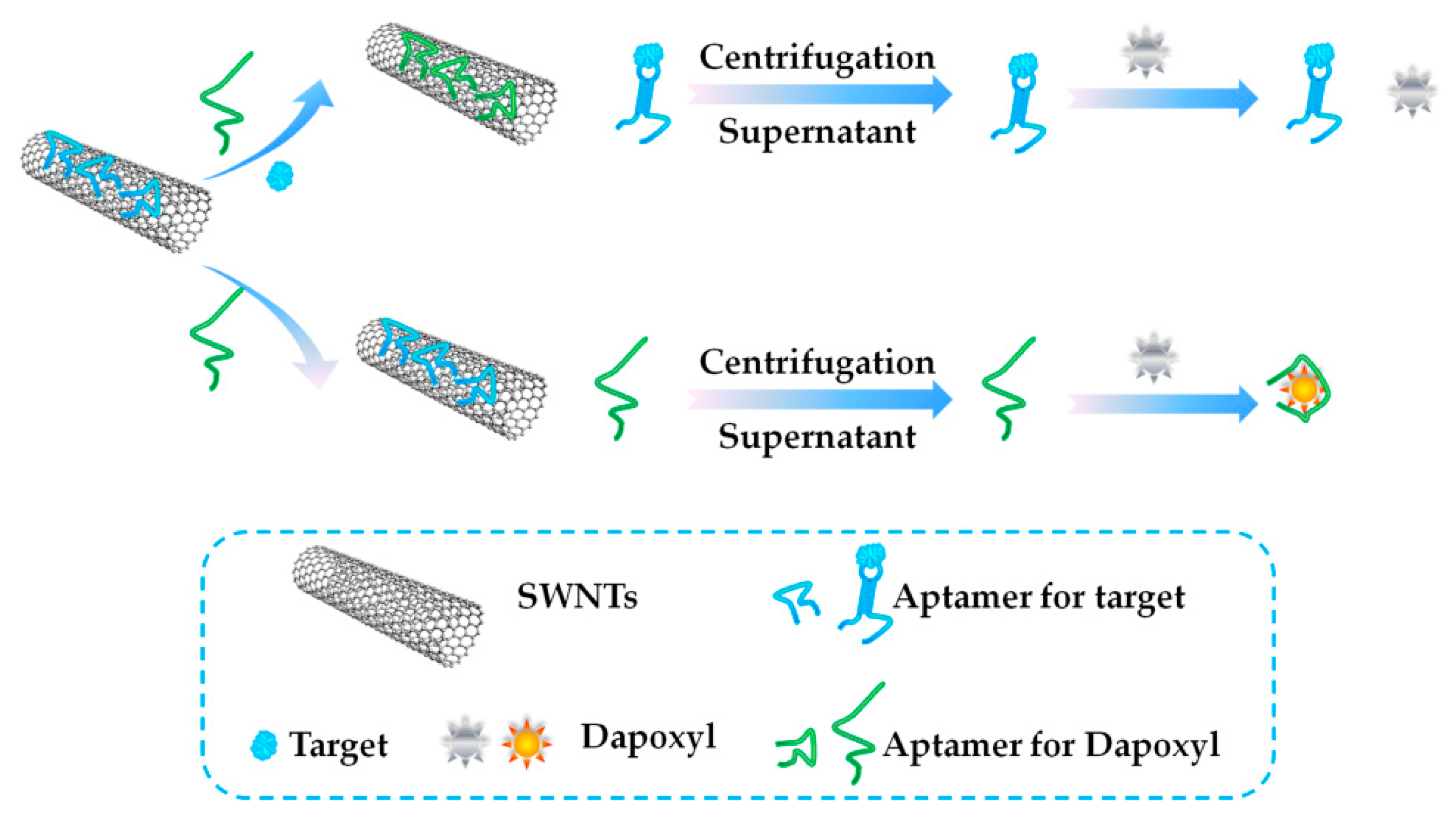
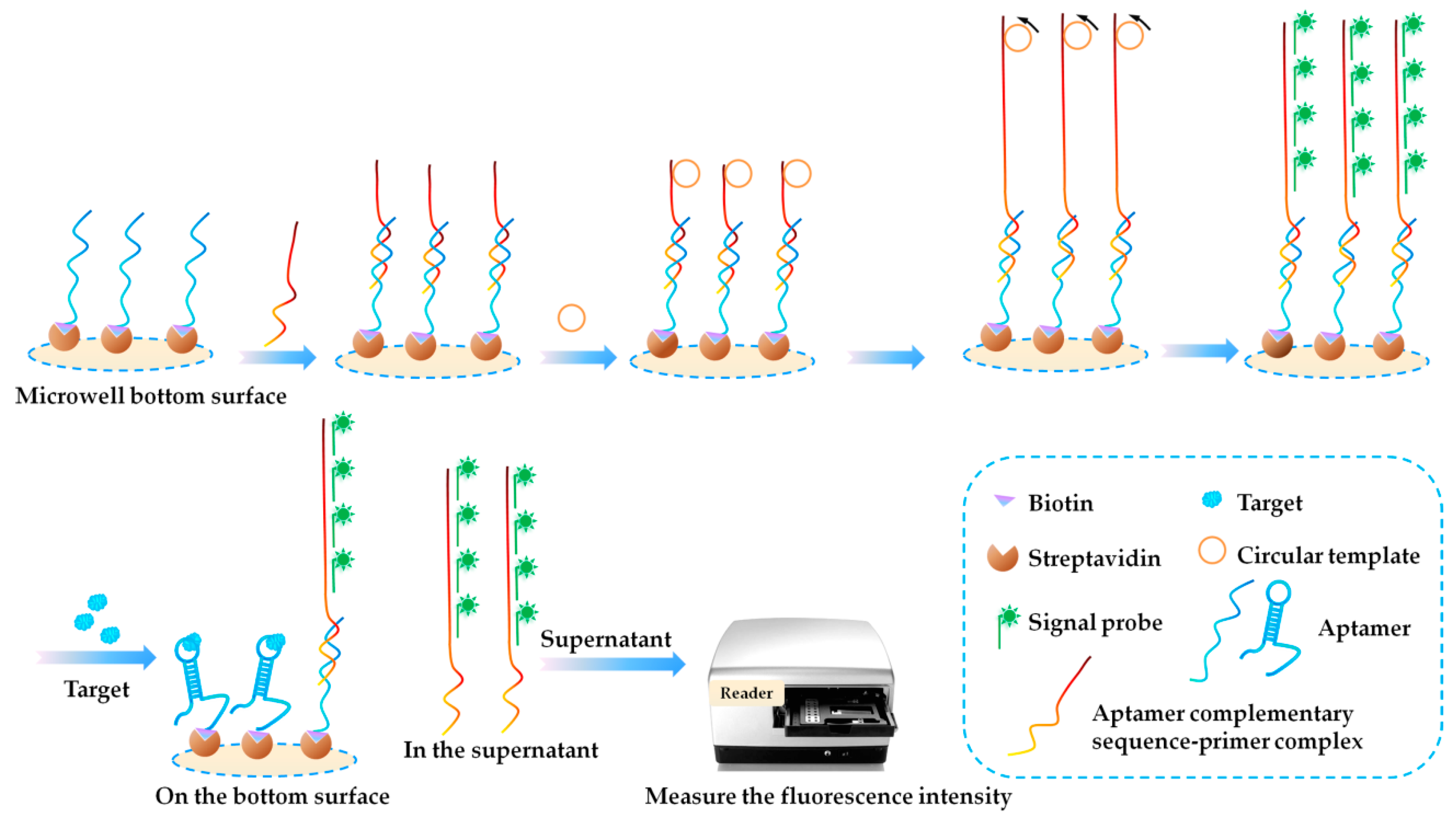
3.3. Fluorescence (FL)-Based Aptasensors
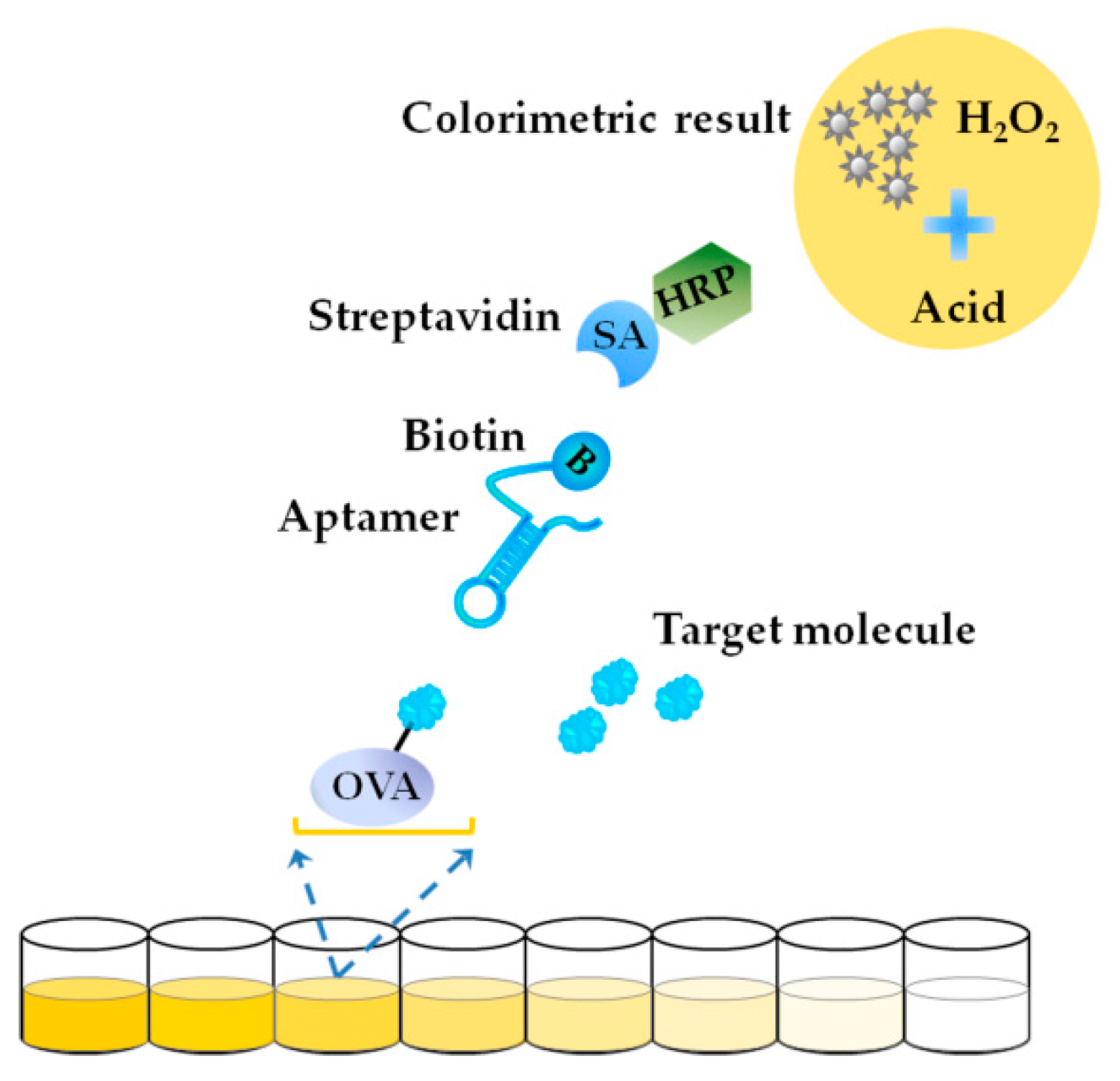
3.4. Enzyme Linked Aptamer Assay (ELAA)-Based Aptasensors
3.5. Detailed Information of the Reported Aptasensors for Marine Biotoxin Detection
- (a)
- Firstly, all of the reported aptasensors achieved high sensitivity, and almost all of them have been validated by real samples. Aptasensors show obvious advantages for sensitive and ultrasensitive detection of marine biotoxins in the real world, compared with the HPLC or MS method. The LODs of the all the reported aptasensors are low enough for the marine biotxins monitoring. LOD of 80% of the reported aptasensors is lower than or equal to 1 ng/mL, LOD of 73% of the aptasensors is lower than or equal to 0.5 ng/mL, LOD of 33% of the aptasensors is lower than or equal to 0.05 ng/mL, and some LOD is even as low as 0.00004 ng/mL. While LOD methods are based on HPLC or MS, they can only achieve LOD at a 1 ng/mL level [23,24,25,26,27,28]. For example, in 2015, Bragg et al. [25] developed an online solid phase extraction hydrophilic interaction liquid chromatography (HILIC) method for the analysis of STX and neosaxitoxin (NEO) in human urine with tandem mass spectrometry, and obtained a LOD at 1.01 ng/mL and 2.62 ng/mL, respectively. A newly reported study in 2018 by Dom et al. [96], which uses liquid chromatography coupled with high resolution mass spectrometry (LC-HRMS) can only achieve a LOD at 1.1~337 ng/g for 18 kinds of marine biotoxins detection. Rey et al. used an improved liquid chromatography coupled with mass spectrometry (LC-MS) for the detection of paralytic shellfish toxins [97]. They tested 15 kinds of biotoxins in four kinds of real samples, and the LOD of the 15 × 14 samples ranged from 0.387 to 55.844 ng/g.
- (b)
- Secondly, the sensitivity of the BLI-based aptasensor is relatively higher, showing great advantages in sensitive detection. However, the linear detection range of BLI-based aptasensors was relatively narrow. This may be caused by the limited chip surface space and limited number of immobilized molecules.
- (c)
- Thirdly, when compared with other biological alternative methods, the reported aptasensors showed great advantages, as most of the aptasensors achieved LOD below 1 ng/mL. In recent years (from 2014 to now), there are some other alternative methods reported for marine biotoxin detection, such as the cell-based impedance biosensor [98], the SPR (surface plasmon resonance) immunosensor [99], the immunochromatographic sensor [8], and so on. However, most of these alternatives only obtained LOD at about 5 ng/mL. The obvious difference may be due to the higher affinity of the aptamers and the superiority of the aptamers to be easily combined with advanced sensitive transducers.
4. Perspectives
- (a)
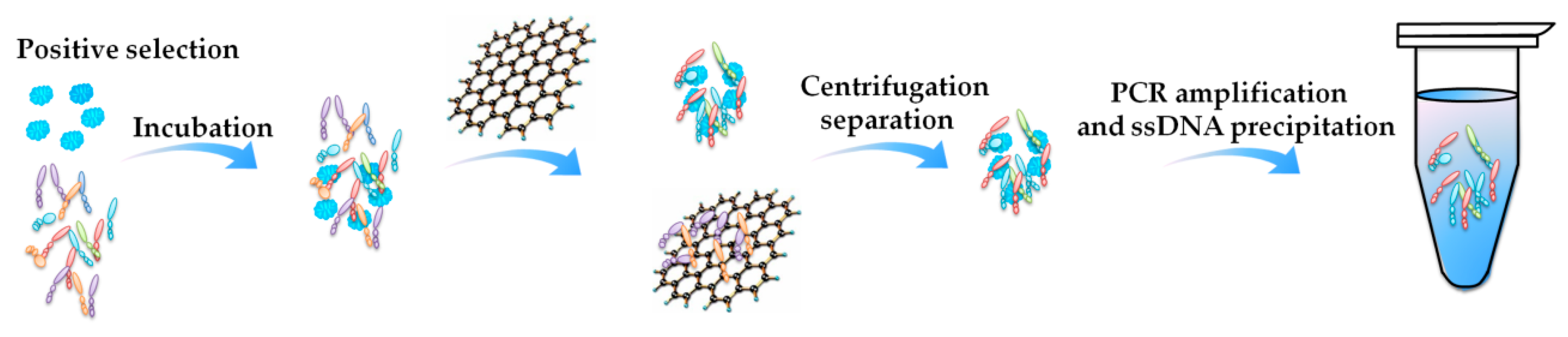
- Firstly, more efforts need to be made to select more aptamers for marine biotoxins. There are only 15 aptamers selected, while there are more than 1000 kinds of marine biotoxins identified in the world. A large number of aptamers is urgently needed. The beads-SELEX and Mag-beads-SELEX can be widely used, referring to the success in the reported selections. The GO-SELEX can be referred, especially for those marine biotoxins that are very small or hard to be immobilized. In addition, some other frontier methods achieve efficient selection [35,100,101,102], such as capillary electrophoresis-SELEX (CE-SELEX) and microfluidic SELEX. CE-SELEX has high-efficiency separation capabilities, and does not need immobilization [103,104,105]. Microfluidic SELEX combines microfluidic chip technology into the aptamer screening process, and can achieve rapid automated selection [106,107].
- (b)
- Secondly, the binding mechanism of each marine biotoxin and its aptamer needs to be further studied. Although many aptamers and aptasensors have been developed, the binding mechanism is not clear. So far, most studies concerning aptamer structures stop with Mfold prediction. However, as shown in Table 1, some of the aptamers have one stem and some of them have more than two. Information from secondary structures is not enough for the mechanism study. Further study should be explored, such as the tertiary structure and molecule docking. Only one study concerning the binding format of the aptamer and marine biotoxin was reported. In 2018, Cheng et al. reported their study about binding the way between STX and its aptamer, M-30f [108]. The authors used the circular dichroism spectra, fluorophore and quencher labeled aptamer, and crystal violet based assays to identify the binding way between STX and aptamer. The results show that the conformation of the aptamer is stabilized in PBS buffer (10 mM phosphate buffer, 2.7 mM KCl, 137 mM NaCl, pH 7.4) and K+ plays an important role in conformation stability, and this conformation may provide a suitable cave for STX binding. We have been conducting research on the recognition mechanism. We once analyzed the binding between tetracycline and its aptamer [109], using the computational prediction and isothermal titration calorimetry (ITC) experiment. The conformational tertiary structure and the potential binding sites of the aptamer were predicted by computational study and proved by chemical experiment. Our study provides a reference, and other methods [43,89,110] can be further referred.
- (c)
- Thirdly, more kinds of aptasensors can be developed. The present aptasensors for marine biotoxins are mainly BLI-based, EC-based, FL-based, and ELAA-based aptasensors. Aptamers show great advantages in terms of easy labeling and easy fabrication. Many other methods can be explored so as to achieve on-site detection with high throughput, visual characters, and high portability. In recent years, various aptasensors have been used to detect various kinds of small molecules, such as optical, mass-dependent, lateral flow chromatography-based aptasensors [75,86,111,112,113], and so on. We also developed several kinds of aptasensors for small molecules, such as the indirect competitive [114] and direct competitive [115] ELAA-based aptasensors, AuNPs-based aptasensor, [116] and a SPR-based aptasensor [117]. All of these three kinds of aptasensors performed well for highly sensitive and specific detection. And all of these reported aptasensors can be used to develop more aptasensors for marine biotoxin detection.
Author Contributions
Funding
Conflicts of Interest
References
- Vilariño, N.; Fonfría, E.S.; Louzao, M.C.; Botana, L.M. Use of biosensors as alternatives to current regulatory methods for marine biotoxins. Sensors 2009, 9, 9414–9443. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.D.; Higgins, C.; Davidson, K.; Veszelovszki, A.; Payne, D.; Hungerford, J.; Higman, W. Potential threats posed by new or emerging marine biotoxins in UK waters and examination of detection methodology used in their control: Brevetoxins. Mar. Drugs 2015, 13, 1224–1254. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, I.; Vieytes, M.R.; Alfonso, A. Analytical challenges for regulated marine toxins. Detection methods. Curr. Opin. Food Sci. 2017, 18, 29–36. [Google Scholar] [CrossRef]
- Volpe, G.; Cozzi, L.; Migliorelli, D.; Croci, L.; Palleschi, G. Development of a haemolytic–enzymatic assay with mediated amperometric detection for palytoxin analysis: Application to mussels. Anal. Bioanal. Chem. 2014, 406, 2399–2410. [Google Scholar] [CrossRef] [PubMed]
- Alsabi, A.; Mcarthur, J.; Ostroumov, V.; French, R.J. Marine toxins that target voltage-gated sodium channels. Mar. Drugs 2006, 4, 157–192. [Google Scholar] [CrossRef]
- Louzao, M.C.; Ares, I.R.; Cagide, E. Marine toxins and the cytoskeleton: A new view of palytoxin toxicity. FEBS J. 2008, 275, 6067–6074. [Google Scholar] [CrossRef] [PubMed]
- Hinder, S.L.; Hays, G.C.; Brooks, C.J.; Davies, A.P.; Edwards, M.; Walne, A.W.; Gravenor, M.B. Toxic marine microalgae and shellfish poisoning in the British Isles: History, review of epidemiology, and future implications. Environ. Health 2011, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Hung, C.; Lu, C.; Chou, H.; Yu, F. Production of monoclonal antibody for okadaic acid and its utilization in an ultrasensitive enzyme-linked immunosorbent assay and one-step immunochromatographic strip. J. Agric. Food Chem. 2014, 62, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Gallardorodríguez, J.; Sánchezmirón, A.; Garcíacamacho, F.; Lópezrosales, L.; Chisti, Y.; Molinagrima, E. Bioactives from microalgal dinoflagellates. Biotechnol. Adv. 2012, 30, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Noguchi, T. Distribution and origin of tetrodotoxin. J. Toxicol. Toxin Rev. 2001, 20, 11–33. [Google Scholar] [CrossRef]
- Narahashi, T. Pharmacology of tetrodotoxin. J. Toxicol. Toxin Rev. 2001, 20, 67–84. [Google Scholar] [CrossRef]
- Shang, F.; Liu, Y.; Wang, S. Simple electrochemiluminescence sensor based on nafion-graphene-ru(bpy) 3 2+ modified electrode for the ultrasensitive detection of tetrodotoxin. J. Electrochem. Soc. 2016, 163, B280–B285. [Google Scholar] [CrossRef]
- Park, D.L.; Adams, W.N.; Graham, S.L.; Jackson, R.C. Variability of mouse bioassay for determination of paralytic shellfish poisoning toxins. J. Assoc. Off. Anal. Chem. 1986, 69, 547–550. [Google Scholar] [PubMed]
- Etheridge, S.M. Paralytic shellfish poisoning: Seafood safety and human health perspectives. Toxicon 2010, 56, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Vale, P.; Taleb, H. Assessment of the quantitative determination of paralytic shellfish poisoning toxins by pre-column derivatization and elimination of interfering compounds by solid-phase extraction. Food Addit. Contam. 2005, 22, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Turrell, E.A.; Stobo, L. A comparison of the mouse bioassay with liquid chromatography-mass spectrometry for the detection of lipophilic toxins in shellfish from Scottish waters. Toxicon 2007, 50, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Kralovec, J.A.; Laycock, M.V.; Richards, R.; Usleber, E. Immobilization of small molecules on solid matrices: A novel approach to enzyme-linked immunosorbent assay screening for saxitoxin and evaluation of anti-saxitoxin antibodies. Toxicon 1996, 34, 1127–1140. [Google Scholar] [CrossRef]
- Malaguti, C.; Milandri, A.; Poletti, R.; Rossini, G.P. Cytotoxic responses to unfractionated extracts from digestive glands of mussels. Toxicon 2002, 40, 573–578. [Google Scholar] [CrossRef]
- Quilliam, M.A.; Thomas, K.; Wright, J.L.C. Analysis of domoic acid in shellfish by thin-layer chromatography. Nat. Toxins 1998, 6, 147–152. [Google Scholar] [CrossRef]
- Prassopoulou, E.; Katikou, P.; Georgantelis, D.; Kyritsakis, A. Detection of okadaic acid and related esters in mussels during diarrhetic shellfish poisoning (DSP) episodes in greece using the mouse bioassay, the pp2a inhibition assay and hplc with fluorimetric detection. Toxicon 2009, 53, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Ikehara, T.; Imamura, S.; Yoshino, A.; Yasumoto, T. Pp2a inhibition assay using recombinant enzyme for rapid detection of okadaic acid and its analogs in shellfish. Toxins 2010, 2, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Poli, M.A.; Rein, K.S.; Baden, D.G. Radioimmunoassay for pbtx-2-type brevetoxins: Epitope specificity of two anti-pbtx sera. J. AOAC Int. 1995, 78, 538–542. [Google Scholar] [PubMed]
- Huang, C.; Xiao-Jing, L.I.; Peng, R.F.; Hong, Y.U. Determination of 7 lipophilic shellfish toxins in shellfish by spe and high performance liquid chromatography-tandem mass spectrometry. Chin. J. Health Lab. Technol. 2011, 21, 1075–1077. [Google Scholar]
- Jen, H.C.; Nguyen, A.T.; Wu, Y.J.; Hoang, T.; Arakawa, O.; Lin, W.F.; Hwang, D.F. Tetrodotoxin and paralytic shellfish poisons in gastropod species from Vietnam analyzed by high-performance liquid chromatography and liquid chromatography-tandem mass spectrometry. J. Food Drug Anal. 2014, 22, 178–188. [Google Scholar] [CrossRef]
- Bragg, W.A.; Lemire, S.W.; Coleman, R.M.; Hamelin, E.I.; Johnson, R.C. Detection of human exposure to saxitoxin and neosaxitoxin in urine by online-solid phase extraction-liquid chromatography-tandem mass spectrometry. Toxicon 2015, 99, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.F.; Niedzwiadek, B.; Menard, C. Quantitative determination of paralytic shellfish poisoning toxins in shellfish using prechromatographic oxidation and liquid chromatography with fluorescence detection: Interlaboratory study. J. AOAC Int. 2004, 88, 1714–1732. [Google Scholar]
- Plakas, S.M.; Jester, E.L.; El Said, K.R.; Granade, H.R.; Abraham, A.; Dickey, R.W.; Scott, P.S.; Flewelling, L.J.; Henry, M.; Blum, P. Monitoring of brevetoxins in the karenia brevis bloom-exposed eastern oyster (Crassostrea virginica). Toxicon 2008, 52, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ramsdell, J.S. Analysis of interactions of brevetoxin-b and human serum albumin by liquid chromatography/mass spectrometry. Chem. Res. Toxicol. 2011, 24, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Plakas, S.M.; Dickey, R.W. Advances in monitoring and toxicity assessment of brevetoxins in molluscan shellfish. Toxicon 2010, 56, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Wharton, R.E.; Feyereisen, M.C.; Gonzalez, A.L.; Abbott, N.L.; Hamelin, E.I.; Johnson, R.C. Quantification of saxitoxin in human blood by ELISA. Toxicon 2017, 133, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Demoulin, L.; Charlier, C.; Singh, G.; Godefroy, S.B.; Campbell, K.; Elliott, C.T.; Delahaut, P. Development of ELISAs for detecting domoic acid, okadaic acid, and saxitoxin and their applicability for the detection of marine toxins in samples collected in Belgium. Food Addit. Contam. 2010, 27, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Garthwaite, I.; Ross, K.M.; Miles, C.O.; Briggs, L.R.; Towers, N.R.; Borrell, T.; Busby, P. Integrated enzyme-linked immunosorbent assay screening system for amnesic, neurotoxic, diarrhetic, and paralytic shellfish poisoning toxins found in New Zealand. J. AOAC Int. 2001, 84, 1643–1648. [Google Scholar] [PubMed]
- Szkola, A.; Linares, E.M.; Worbs, S.; Dorner, B.G.; Dietrich, R.; Märtlbauer, E.; Niessner, R.; Seidel, M. Rapid and simultaneous detection of ricin, staphylococcal enterotoxin b and saxitoxin by chemiluminescence-based microarray immunoassay. Analyst 2014, 139, 5885–5892. [Google Scholar] [CrossRef] [PubMed]
- Haughey, S.A.; Campbell, K.; Yakes, B.J.; Prezioso, S.M.; Degrasse, S.L.; Kawatsu, K.; Elliott, C.T. Comparison of biosensor platforms for surface plasmon resonance based detection of paralytic shellfish toxins. Talanta 2011, 85, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhu, Y.; Feng, X.; Mei, Z.; Li, Y.; Xin, W.; Lei, Z.; Jian, L.; Liu, G.; Peng, C. Recent trends in SELEX technique and its application to food safety monitoring. Microchim. Acta 2014, 181, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Wu, S.; Dai, S.; Gu, H.; Hao, L.; Ye, H.; Wang, Z. Advances in aptasensors for the detection of food contaminants. Analyst 2016, 141, 3942–3961. [Google Scholar] [CrossRef] [PubMed]
- Bostan, H.B.; Danesh, N.M.; Karimi, G.; Ramezani, M.; Shaegh, S.A.M.; Youssefi, K.; Charbgoo, F.; Abnous, K.; Taghdisi, S.M. Ultrasensitive detection of ochratoxin a using aptasensors. Biosens. Bioelectron. 2017, 98, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Xu, Y.; Yong, W.; Chu, X.; Wang, D. Aptamer and its potential applications for food safety. Crit. Rev. Food Sci. Nutr. 2014, 54, 1548–1561. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage t4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Amayagonzalez, S.; Delossantosalvarez, N.; Mirandaordieres, A.J.; Lobocastanon, M.J. Aptamer-based analysis: A promising alternative for food safety control. Sensors 2013, 13, 16292–16311. [Google Scholar] [CrossRef] [PubMed]
- Van Dorst, B.; Mehta, J.; Bekaert, K.M.; Rouahmartin, E.; De Coen, W.; Dubruel, P.; Blust, R.; Robbens, J. Recent advances in recognition elements of food and environmental biosensors: A review. Biosens. Bioelectron. 2010, 26, 1178–1194. [Google Scholar] [CrossRef] [PubMed]
- Hermann, T.; Patel, D.J. Adaptive recognition by nucleic acid aptamers. Science 2000, 287, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Pagratis, N.C.; Chang, B.Y.F.; Jennings, S.; Fitzwater, T.; Jellinek, D.; Dang, C. Potent 2′-amino-, 2′-fluoro-2′-deoxyribonucleotide RNA inhibitors of keratinocyte growth factor. Nat. Biotechnol. 1997, 15, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yigit, M.V.; Mazumdar, D.; Lu, Y. Molecular diagnostic and drug delivery agents based on aptamer-nanomaterial conjugates. Adv. Drug Deliv. Rev. 2010, 62, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Weigand, J.E.; Wittmann, A.; Suess, B. RNA-based networks: Using RNA aptamers and ribozymes as synthetic genetic devices. Methods Mol. Boil. 2012, 813, 157–168. [Google Scholar]
- Geiger, A.; Burgstaller, P.; Von, d.E.H.; Roeder, A.; Famulok, M. RNA aptamers that bind l-arginine with sub-micromolar dissociation constants and high enantioselectivity. Nucleic Acids Res. 1996, 24, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- White, R.J.; Phares, N.; Lubin, A.A.; Xiao, Y.; Plaxco, K.W. Optimization of electrochemical aptamer-based sensors via optimization of probe packing density and surface chemistry. Langmuir ACS J. Surf. Colloids 2008, 24, 10513–10518. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Labuza, T.P.; He, L. Development of a single aptamer-based surface enhanced raman scattering method for rapid detection of multiple pesticides. Analyst 2014, 139, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Malekzad, H.; Jouyban, A.; Hasanzadeh, M.; Shadjou, N.; Guardia, M.D.L. Ensuring food safety using aptamer based assays: Electroanalytical approach. TrAC Trends Anal. Chem. 2017, 94, 77–94. [Google Scholar] [CrossRef]
- Bostana, H.B.; Taghdisib, S.M.; Bowenc, J.L.; Demertzisc, N.; Rezaeed, R.; Panahia, Y.; Tsatsakise, A.M.; Karimi, G. Determination of microcystin-LR, employing aptasensors. Biosens. Bioelectron. 2018, 119, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Cunha, I.; Biltes, R.; Sales, M.; Vasconcelos, V. Aptamer-based biosensors to detect aquatic phycotoxins and cyanotoxins. Sensors 2018, 18, 2367. [Google Scholar] [CrossRef] [PubMed]
- Sefah, K.; Shangguan, D.; Xiong, X.; O’Donoghue, M.B.; Tan, W. Development of DNA aptamers using Cell-SELEX. Nat. Protoc. 2010, 5, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. Selection in vitro of single-stranded DNA molecules that fold into specific ligand-binding structures. Nature 1992, 355, 850–852. [Google Scholar] [CrossRef] [PubMed]
- Sekella, P.T.; Rueda, D.; Walter, N.G. A biosensor for theophylline based on fluorescence detection of ligand-induced hammerhead ribozyme cleavage. RNA-A Publ. RNA Soc. 2002, 8, 1242–1252. [Google Scholar] [CrossRef]
- Stoltenburg, R.; Reinemann, C.; Strehlitz, B. Selex—A (r)evolutionary method to generate high-affinity nucleic acid ligands. Biomol. Eng. 2007, 24, 381–403. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Liu, Z.; Wang, D.; Li, L.; Hu, P.; Gong, S.; Li, Y.; Cui, C.; Wu, Z.; Gao, Y. Generation of internal-image functional aptamers of okadaic acid via magnetic-bead SELEX. Mar. Drugs 2015, 13, 7433–7445. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Kwon, Y.S.; Kim, J.H.; Gu, M.B. Multiple GO-SELEX for efficient screening of flexible aptamers. Chem. Commun. 2014, 50, 10513–10516. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Gao, Z.; Wang, L.; Wang, H.; Zhang, H.; Li, H. Selection and identification of chloramphenicol-specific DNA aptamers by Mag-SELEX. Appl. Biochem. Biotechnol. 2016, 180, 1644–1656. [Google Scholar] [CrossRef] [PubMed]
- Paniel, N.; Istamboulié, G.; Triki, A.; Lozano, C.; Barthelmebs, L.; Noguer, T. Selection of DNA aptamers against penicillin g using capture-selex for the development of an impedimetric sensor. Talanta 2016, 162, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zheng, X.; Hu, B.; Sun, M.; Wu, J.; Jiao, B.; Wang, L. Enzyme-linked, aptamer-based, competitive biolayer interferometry biosensor for palytoxin. Biosens. Bioelectron. 2017, 89, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Ng, A.; Siaj, M.; Tavares, A.C.; Zourob, M. Selection and identification of DNA aptamers against okadaic acid for biosensing application. Anal. Chem. 2013, 85, 11794–11801. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Siaj, M.; Zourob, M. Aptamer-based competitive electrochemical biosensor for brevetoxin-2. Biosens. Bioelectron. 2015, 69, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.Y.; Lin, C.; Yu, S.Y.; Gong, S.; Hu, P.; Li, Y.S.; Wu, Z.C.; Gao, Y.; Zhou, Y.; Liu, Z.S. Preparation of a specific ssDNA aptamer for brevetoxin-2 using SELEX. J. Anal. Methods Chem. 2016, 2016, 9241860. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.; Chinnappan, R.; Eissa, S.; Liu, H.; Tlili, C.; Zourob, M. Selection, characterization, and biosensing application of high affinity congener-specific microcystin-targeting aptamers. Environ. Sci. Technol. 2012, 46, 10697–10703. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Gui, R.; Sun, J.; Wang, Y. Facilely self-assembled magnetic nanoparticles/aptamer/carbon dots nanocomposites for highly sensitive up-conversion fluorescence turn-on detection of tetrodotoxin. Talanta 2017, 176, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Gao, X.; Yang, F.; Chen, W.; Miao, T.; Peng, J. Screening and structure analysis of the aptamer against tetrodotoxin. J. Chin. Inst. Food Sci. Technol. 2012, 12, 137–143. [Google Scholar]
- Shao, B.Y.; Chen, B.; Chen, W.B.; Yang, F.; Miao, T.Y.; Peng, J. Preparation and application of tetrodotoxin DNA aptamer. Food Sci. 2014, 35, 205–208. [Google Scholar]
- Handy, S.M.; Yakes, B.J.; Degrasse, J.A.; Campbell, K.; Elliott, C.T.; Kanyuck, K.M.; Degrasse, S.L. First report of the use of a saxitoxin–protein conjugate to develop a DNA aptamer to a small molecule toxin. Toxicon 2013, 61, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Hu, B.; Gao, S.X.; Liu, D.J.; Sun, M.J.; Jiao, B.H.; Wang, L.H. A saxitoxin-binding aptamer with higher affinity and inhibitory activity optimized by rational site-directed mutagenesis and truncation. Toxicon 2015, 101, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Elshafey, R.; Siaj, M.; Zourob, M. DNA aptamers selection and characterization for development of label-free impedimetric aptasensor for neurotoxin anatoxin-a. Biosens. Bioelectron. 2015, 68, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Hu, B.; Zheng, X.; Cao, Y.; Liu, D.; Sun, M.; Jiao, B.; Wang, L. Gonyautoxin 1/4 aptamers with high-affinity and high-specificity: From efficient selection to aptasensor application. Biosens. Bioelectron. 2016, 79, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.C.; Kouzani, A.Z.; Duan, W. Aptasensors: A review. J. Biomed. Nanotechnol. 2010, 6, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Torreschavolla, E.; Alocilja, E.C. Aptasensors for detection of microbial and viral pathogens. Biosens. Bioelectron. 2009, 24, 3175–3182. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Raston, N.H.A.; Gu, M.B. Aptamer-based nanobiosensors. Biosens. Bioelectron. 2016, 76, 2–19. [Google Scholar] [PubMed]
- Wang, Z.; Yu, J.; Gui, R.; Jin, H.; Xia, Y. Carbon nanomaterials-based electrochemical aptasensors. Biosens. Bioelectron. 2016, 79, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, G.L.; Hytönen, V.P.; Kaguni, L.S. Biolayer Interferometry: A Novel Method to Elucidate Protein-Protein and Protein-DNA Interactions in the Mitochondrial DNA Replisome; Springer: New York, NY, USA, 2016; p. 223. [Google Scholar]
- Kumaraswamy, S.; Tobias, R. Label-Free Kinetic Analysis of an Antibody-Antigen Interaction Using Biolayer Interferometry; Springer: New York, NY, USA, 2015; pp. 165–182. [Google Scholar]
- Concepcion, J.; Witte, K.; Wartchow, C.; Choo, S.; Yao, D.; Persson, H.; Wei, J.; Li, P.; Heidecker, B.; Ma, W. Label-free detection of biomolecular interactions using biolayer interferometry for kinetic characterization. Comb. Chem. High Throughput Screen. 2009, 12, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zheng, X.; Wu, J. A biolayer interferometry-based competitive biosensor for rapid and sensitive detection of saxitoxin. Sens. Actuators B Chem. 2017, 246, 169–174. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef] [PubMed]
- Stradiotto, N.R.; Yamanaka, H.; Zanoni, M.V.B. Electrochemical sensors: A powerful tool in analytical chemistry. J. Braz. Chem. Soc. 2003, 14, 159–173. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Li, H.; Wan, Q.; Li, Y.; Yang, N. Electrochemical properties and sensing applications of nanocarbons: A comparative study. Carbon 2018, 129, 301–309. [Google Scholar] [CrossRef]
- Pan, Y.; Wan, Z.; Zhong, L.; Li, X.; Wu, Q.; Wang, J.; Wang, P. Label-free okadaic acid detection using growth of gold nanoparticles in sensor gaps as a conductive tag. Biomed. Microdevices 2017, 19, 33. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.F.; Chai, Y.; Liu, X.Q.; Li, L.L.; Yang, L.W.; Liu, P.P.; Zhou, Y.M.; Ju, H.X.; Cheng, Y.Z. A photoelectrochemical aptasensor constructed with core-shell CuS-TiO2 heterostructure for detection of microcystin-LR. Biosens. Bioelectron. 2018, 117, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.E.; Zhang, Y.; Cai, J.; Cai, W.; Gao, T. Aptamer-based fluorescent biosensors. Curr. Med. Chem. 2011, 18, 4175–4184. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Wu, N. Fluorescence and sensing applications of graphene oxide and graphene quantum dots: A review. Chem. Asian J. 2017, 12, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Li, Y.; Tang, B.; Zhang, C. Fluorescent biosensors based on single-molecule counting. Acc. Chem. Res. 2016, 49, 1722–1730. [Google Scholar] [CrossRef] [PubMed]
- Pilehvar, S.; Jambrec, D.; Gebala, M.; Schuhmann, W.; De Wael, K. Intercalation of proflavine in ssdna aptamers: Effect on binding of the specific target chloramphenicol. Electroanalysis 2015, 27, 1836–1841. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, X.; Wang, Y.; Zheng, X.; Li, C.M. Aptamer based fluorescence recovery assay for aflatoxin b1 using a quencher system composed of quantum dots and graphene oxide. Microchim. Acta 2015, 182, 571–578. [Google Scholar] [CrossRef]
- Lv, J.J.; Zhao, S.; Wu, S.J.; Wang, Z.P. Upconversion nanoparticles grafted molybdenum disulfide nanosheets platform for microcystin-LR sensing. Biosens. Bioelectron. 2017, 90, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Duan, N.; Zhang, H.; Wang, Z. Simultaneous detection of microcysin-lr and okadaic acid using a dual fluorescence resonance energy transfer aptasensor. Anal. Bioanal. Chem. 2015, 407, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Taghdisi, S.M.; Danesh, N.M.; Ramezani, M.; Ghows, N.; Shaegh, S.A.M.; Abnous, K. A novel fluorescent aptasensor for ultrasensitive detection of microcystin-LR based on single-walled carbon nanotubes and dapoxyl. Talanta 2017, 16, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, K.; Bustos, P.; Sullivan, C.O.; Conejeros, P. Facile and cost-effective detection of saxitoxin exploiting aptamer structural switching. Food Technol. Biotechnol. 2015, 53, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Hao, L.; Duan, N.; Wu, S.; Xia, Y.; Ma, X.; Wang, Z. A competitive fluorescent aptasensor for okadaic acid detection assisted by rolling circle amplification. Microchim. Acta 2017, 184, 2893–2899. [Google Scholar] [CrossRef]
- Dom, I.; Biré, R.; Hort, V.; Lavison-Bompard, G.; Nicolas, M.; Guérin, T. Extended targeted and non-targeted strategies for the analysis of marine toxins in mussels and oysters by (LC-HRMS). Toxins 2018, 10, 375. [Google Scholar] [CrossRef] [PubMed]
- Rey, V.; Botana, A.M.; Antelo, A.; Alvarez, M. Botana, L.M. Rapid analysis of paralytic shellfish toxins and tetrodotoxins by liquid chromatography-tandem mass spectrometry using a porous graphitic carbon column. Food Chem. 2018, 269, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Wu, C.; Wang, Q.; Zhou, J.; Su, K.; Li, H.; Hu, N.; Wang, P. An improved sensitive assay for the detection of psp toxins with neuroblastoma cell-based impedance biosensor. Biosens. Bioelectron. 2015, 67, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Garibo, D.; Campbell, K.; Casanova, A.; Iglesia, P.D.L.; Fernández-Tejedor, M.; Diogène, J.; Elliott, C.T.; Campàs, M. Spr immunosensor for the detection of okadaic acid in mussels using magnetic particles as antibody carriers. Sens. Actuators B Chem. 2014, 190, 822–828. [Google Scholar] [CrossRef]
- Gopinath, S.C.B. Methods developed for SELEX. Anal. Bioanal. Chem. 2006, 387, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Darmostuk, M.; Rimpelova, S.; Gbelcova, H.; Ruml, T. Current approaches in SELEX: An update to aptamer selection technology. Biotechnol. Adv. 2015, 33, 1141–1161. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Z.; Yu, Y.; Wang, M.; Li, J.; Zhang, Z.; Liu, J.; Wu, X.; Lu, A.; Zhang, G.; Zhang, B. Recent advances in selex technology and aptamer applications in biomedicine. Int. J. Mol. Sci. 2017, 18, 2142. [Google Scholar] [CrossRef] [PubMed]
- Mendonsa, S.D.; Bowser, M.T. In vitro evolution of functional DNA using capillary electrophoresis. J. Am. Chem. Soc. 2004, 126, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Bowser, M.T. Capillary electrophoresis–SELEX selection of catalytic DNA aptamers for a small-molecule porphyrin target. Anal. Chem. 2013, 85, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Mosing, R.K.; Bowser, M.T. Isolating aptamers using capillary electrophoresis-SELEX (CE-SELEX). Methods Mol. Boil. 2009, 535, 33–43. [Google Scholar]
- Olsen, T.; Zhu, J.; Kim, J.; Pei, R.; Stojanovic, M.N.; Lin, Q. An integrated microfluidic SELEX approach using combined electrokinetic and hydrodynamic manipulation. J. Lab. Autom. 2017, 22, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, H.; Jia, W.; Chen, Z.; Xu, D. Selection of aptamers based on a protein microarray integrated with a microfluidic chip. Lab Chip 2017, 17, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Zheng, B.; Yao, D.B.; Kuai, S.L.; Tian, J.J.; Liang, H.L.; Ding, Y.S. Study of the binding way between saxitoxin and its aptamer and a fluorescent aptasensor for detection of saxitoxin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 204, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, J.; Dong, Y.; Su, H.; Tan, T. Conformational structure-dependent molecular recognition of two aptamers for tetracycline. RSC Adv. 2015, 5, 53796–53801. [Google Scholar] [CrossRef]
- Cassiday, L.A.; Lebruska, L.L.; Benson, L.M.; Naylor, S.; Owen, W.G.; Maher, L.J. Binding stoichiometry of an rna aptamer and its transcription factor target. Anal. Biochem. 2002, 306, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Gu, M.B. Advances in aptamer screening and small molecule aptasensors. Adv. Biochem. Eng. Biotechnol. 2013, 140, 29–67. [Google Scholar]
- Nguyen, V.T.; Kwon, Y.S.; Gu, M.B. Aptamer-based environmental biosensors for small molecule contaminants. Curr. Opin. Biotechnol. 2017, 45, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, S.; Zhang, S.; Liu, J.; Dong, Y. State of the art: Lateral flow assay (LFA) biosensor for on-site rapid detection. Chin. Chem. Lett. 2018, 29, 1567–1577. [Google Scholar] [CrossRef]
- Wang, S.; Yong, W.; Liu, J.; Zhang, L.; Chen, Q.; Dong, Y. Development of an indirect competitive assay-based aptasensor for highly sensitive detection of tetracycline residue in honey. Biosens. Bioelectron. 2014, 57, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, J.; Yong, W.; Chen, Q.; Zhang, L.; Dong, Y.; Su, H.; Tan, T. A direct competitive assay-based aptasensor for sensitive determination of tetracycline residue in honey. Talanta 2015, 131, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, S.; Sun, S.; Yang, Y.; Zhang, Y.; Liu, J.; Dong, Y.; Su, H.; Tan, T. A molecular recognition assisted colorimetric aptasensor for tetracycline. RSC Adv. 2016, 6, 45645–45651. [Google Scholar] [CrossRef]
- Wang, S.; Dong, Y.; Liang, X. Development of a SPR aptasensor containing oriented aptamer for direct capture and detection of tetracycline in multiple honey samples. Biosens. Bioelectron. 2018, 109, 1–7. [Google Scholar] [CrossRef] [PubMed]

| Num. | Target | Aptamer Name | Selection Method | Year | Sequence (5′–3′) | Affinity (Kd, nM) | Secondary Structure | Folding Reference Condition | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Palytoxin (PTX) C1 | PTX-13 | Mag-beads-SELEX | 2017 | GGAGGTGGTGGGGACTTTGCTTGTACTGGGCGCCCGGTTGAA | 84.3 |  | 20 mM Tris, 100 mM NaCl, 2 mM MgCl2, 5 mM KCl, pH 7.5 | [61] |
| 2 | Okadaic acid (OA) C1 | OA34 | Beads-SELEX | 2013 | GGTCACCAACAACAGGGAGCGCTACGCGAAGGGTCAATGTGACGTCATGCGGATGTGTGG | 77 | 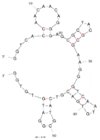 | 50 mM Tris, 150 mM NaCl, 2 mM MgCl2, pH 7.5 | [62] |
| 3 | Brevetoxin 2 (BTX-2) C2 | BT10 | Beads-SELEX | 2015 | GGCCACCAAACCACACCGTCGCAACCGCGAGAACCGAAGTAGTGATCATGTCCCTGCGTG | 92 | 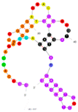 | 50 mM Tris, 10 mM MgCl2, pH 7.5 | [63] |
| 4 | Brevetoxin 2 (BTX-2) C2 | Bap5 | Microwell-SELEX | 2016 | GAGGCAGCACTTCACACGATCTGTGAAGTTTTTGTCATGGTTTGGGGGTGGTAGGGGTGTTGTCTGCGTAATGACTGTAGAGATG | 4830 |  | 20 mM Hepes, 120 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2 | [64] |
| 5 | Microcystin-LR (MC-LR) C2 | AN6 | Beads-SELEX | 2012 | GGCGCCAAACAGGACCACCATGACAATTACCCATACCACCTCATTATGCCCCATCTCCGC | 50 | 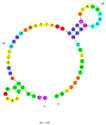 | 50 mM Tris, 150 mM NaCl, 2 mM MgCl2, pH 7.5 | [65] |
| 6 | Microcystin-LA (MC-LA) C2 | RC4 | Beads-SELEX | 2012 | CACGCACAGAAGACACCTACAGGGCCAGATCACAATCGGTTAGTGAACTCGTACGGCGCG | 76 |  | 50 mM Tris, 150 mM NaCl, 2 mM MgCl2, pH 7.5 | [65] |
| 7 | Microcystin-YR (MC-YR) C2 | HC1 | Beads-SELEX | 2012 | GGACAACATAGGAAAAAGGCTCTGCTACCGGATCCCTGTTGTATGGGCATATCTGTTGAT | 193 | 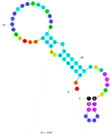 | 50 mM Tris, 150 mM NaCl, 2 mM MgCl2, pH 7.5 | [65] |
| 9 | Tetrodotoxin (TTX) C3 | G11-T ⁑ | Truncation | 2012 | AAAAATTTCACACGGGTGCCTCGGCTGTCC | N/A |  | 250 mM NaCl, 1 mM MgCl2, 0.1 mM EDTA, 1 mM DTT, 20 mM Tris-HCl, pH 7.5 | [66,67] |
| 10 | Tetrodotoxin (TTX) C3 | A3 | Beads-SELEX | 2014 | GGGAGCTCAGAATAA ACGCTCAACCCTGCCGGGGGCTTCTCCTTGCTGCTCTGCTCTGTTCGACATGAGGCCCGGATC | N/A |  | 10 mM PBS, pH 7.5 | [68] |
| 11 | Saxitoxin (STX) C3 | APTSTX | Mag-beads-SELEX | 2013 | GGTATTGAGGGTCGCATCCCGTGGAAACATGTTCATTGG GCGCACTCCGCTTTCTGTAGATGGCTCTAACTCTCCTCT | 3840 | 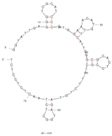 | 10 mM phosphate buffer, 2.7 mM KCl, 140 mM NaCl, 0.05% Tween-20, pH 7.4 | [69] |
| 12 | Saxitoxin(STX) C3 | M-30f | Truncation | 2015 | TTGAGGGTCGCATCCCGTGGAAACAGGTTCATTG | 133 | 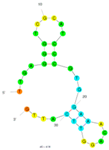 | 10 mM phosphate buffer, 2.7 mM KCl, 140 mM NaCl, 0.05% Tween-20, pH 7.4 | [70] |
| 13 | Anatoxin-a (ATX-a) C3 | ATX8 | Beads-SELEX | 2015 | TGGCGACAAGAAGACGTACAAACACGCACCAGGCCGGAGTGGAGTATTCTGAGGTCGG | 81.378 | 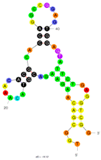 | 50 mM Tris, pH 7.5, 150 mM NaCl, 2 mM MgCl2, pH 7.5 | [71] |
| 14 | Gonyautoxin1/4 (GTX1/4) C3 | GO18-T | GO-SELEX | 2016 | AGCAGCACAGAGGTCAGATGCAATCGGAACGAGTAACCTTTGGTCGGGCAAGGTAGGTTGCCTATGCGTGCTACCGTGAA | 62 |  | 20 mM Tris-HCl, 100 mM NaCl, 2 mM MgCl2, 5 mM KCl, pH 7.5 | [72] |
| 15 | Gonyautoxin1/4 (GTX1/4) C3 | GO18-T-d | Truncation | 2016 | AACCTTTGGTCGGGCAAGGTAGGTT | 8.1 | 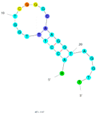 | 20 mM Tris–HCl and 10 mM MgCl2, pH 7.5 | [72] |
| Target | Aptamer | Aptasensor | Year | Linear detection Range (ng/mL) a | LOD (ng/mL) a | Samples | References |
|---|---|---|---|---|---|---|---|
| GTX1/4 | GO18-T-d | BLI-based | 2016 | 0.2~200 | 0.05 | shellfish | [72] |
| STX | M-30f | BLI-based | 2017 | 0.1~0.8 | 0.5 | shellfish | [80] |
| PTX | PTX-13 | BLI-based | 2017 | 0.2~0.7 | 0.00004 | shellfish, seawater | [61] |
| OA | OA34 | EC-based | 2013 | 0.1~60 | 0.07 | shellfish | [62] |
| ATX | ATX8 | EC-based | 2015 | 1~100 | 0.5 | drinking water, certified samples | [71] |
| BTX-2 | BT10 | EC-based | 2015 | 0.01~2000 | 0.106 | shellfish, mussel | [63] |
| OA | OA34 | EC-based | 2017 | 5~100 | 1 | buffer | [84] |
| MC-LR | AN6 | EC-based | 2018 | 5.0 × 10−5~248.8 | 2.0 | water | [85] |
| TTX | G11-T | FL-based | 2017 | 0.1~100,000 | 0.06 | fish | [66] |
| MC-LR | AN6 | FL-based | 2017 | 0.01~50 | 0.002 | water | [91] |
| MC-LR and OA | AN6 for MC-LR and OA34 for OA | FL-based | 2015 | 0.1~50 | 0.025 for MC-LR and 0.05 for OA | water, shrimps, fish | [92] |
| MC-LR | AN6 | FL-based | 2017 | 0.4~1194 | 0.137 | water, serum samples | [93] |
| STX | APTSTX | FL-based | 2015 | 15~3000 | 7.5 | gastric juice, serum, urine | [94] |
| OA | OA34 | FL-based | 2017 | 0.001~100 | 0.001 | shellfish | [95] |
| BTX-2 | Bap5 | ELAA-based | 2016 | 3.125~200 | 3.125 | buffer | [64] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Huang, Y.; Dong, Y.; Han, X.; Wang, S.; Liang, X. Aptamers and Aptasensors for Highly Specific Recognition and Sensitive Detection of Marine Biotoxins: Recent Advances and Perspectives. Toxins 2018, 10, 427. https://doi.org/10.3390/toxins10110427
Zhao L, Huang Y, Dong Y, Han X, Wang S, Liang X. Aptamers and Aptasensors for Highly Specific Recognition and Sensitive Detection of Marine Biotoxins: Recent Advances and Perspectives. Toxins. 2018; 10(11):427. https://doi.org/10.3390/toxins10110427
Chicago/Turabian StyleZhao, Lianhui, Yunfei Huang, Yiyang Dong, Xutiange Han, Sai Wang, and Xingguo Liang. 2018. "Aptamers and Aptasensors for Highly Specific Recognition and Sensitive Detection of Marine Biotoxins: Recent Advances and Perspectives" Toxins 10, no. 11: 427. https://doi.org/10.3390/toxins10110427
APA StyleZhao, L., Huang, Y., Dong, Y., Han, X., Wang, S., & Liang, X. (2018). Aptamers and Aptasensors for Highly Specific Recognition and Sensitive Detection of Marine Biotoxins: Recent Advances and Perspectives. Toxins, 10(11), 427. https://doi.org/10.3390/toxins10110427







