Botulinum Toxin Injections and Electrical Stimulation for Spastic Paresis Improve Active Hand Function Following Stroke
Abstract
1. Introduction
2. Results
2.1. Primary Outcomes
2.2. Secondary Outcomes
3. Discussion
4. Conclusions
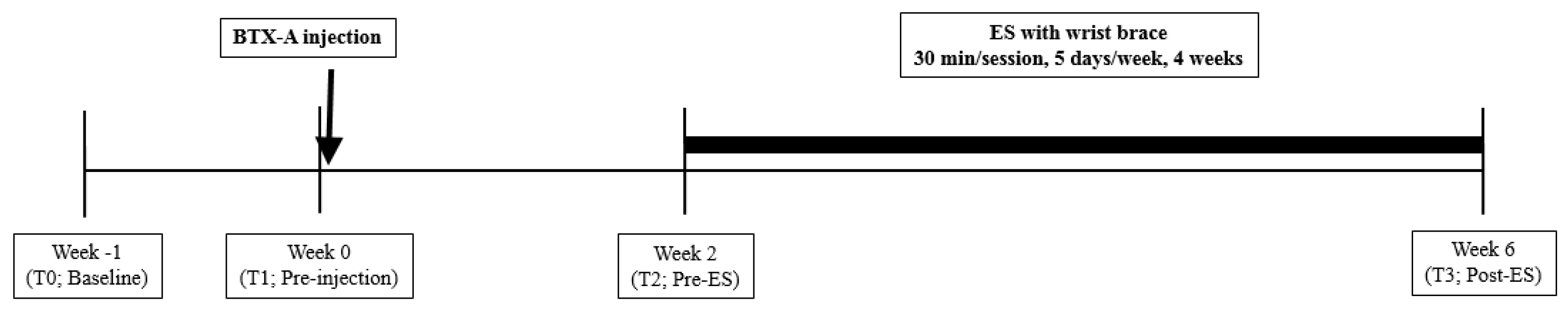
5. Materials and Methods
5.1. Inclusion and Exclusion Criteria
5.2. Intervention
5.2.1. BTX-A Administration
5.2.2. ES with a Wrist Brace
5.3. Outcome Measures
5.3.1. Primary Outcomes
5.3.2. Secondary Outcomes
5.4. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Sommerfeld, D.K.; Eek, E.U.-B.; Svensson, A.-K.; Holmqvist, L.W.; von Arbin, M.H. Spasticity after stroke: Its occurrence and association with motor impairments and activity limitations. Stroke 2004, 35, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Gracies, J.M. Pathophysiology of spastic paresis. I: Paresis and soft tissue changes. Muscle Nerve 2005, 31, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Gracies, J.M. Pathophysiology of spastic paresis. II: Emergence of muscle overactivity. Muscle Nerve 2005, 31, 552–571. [Google Scholar] [CrossRef] [PubMed]
- Foley, N.; Pereira, S.; Salter, K.; Fernandez, M.M.; Speechley, M.; Sequeira, K.; Miller, T.; Teasell, R. Treatment with botulinum toxin improves upper-extremity function post stroke: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2013, 94, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Esquenazi, A.; Albanese, A.; Chancellor, M.B.; Elovic, E.; Segal, K.R.; Simpson, D.M.; Smith, C.P.; Ward, A.B. Evidence-based review and assessment of botulinum neurotoxin for the treatment of adult spasticity in the upper motor neuron syndrome. Toxicon 2013, 67, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Wissel, J.; Ward, A.B.; Erztgaard, P.; Bensmail, D.; Hecht, M.J.; Lejeune, T.M.; Schnider, P. European consensus table on the use of botulinum toxin type A in adult spasticity. J. Rehabil. Med. 2009, 41, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.; Gracies, J.; Graham, H.; Miyasaki, J.; Naumann, M.; Russman, B.; Simpson, L.; So, Y. Assessment: Botulinum neurotoxin for the treatment of spasticity (an evidence-based review) Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2008, 70, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Demetrios, M.; Khan, F.; Turner-Stokes, L.; Brand, C.; Mc-Sweeney, S. Multidisciplinary rehabilitation following botulinum toxin and other focal intramuscular treatment for post-stroke spasticity. Cochrane Database Syst. Rev. 2012, 6, 12. [Google Scholar] [CrossRef]
- Simpson, D.M.; Hallett, M.; Ashman, E.J.; Comella, C.L.; Green, M.W.; Gronseth, G.S.; Armstrong, M.J.; Gloss, D.; Potrebic, S.; Jankovic, J. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016, 86, 1818–1826. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M.; Rossetto, O.; Eleopra, R.; Montecucco, C. Botulinum neurotoxins: Biology, pharmacology, and toxicology. Pharmacol. Rev. 2017, 69, 200–235. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M. Novel Botulinum Neurotoxins: Exploring Underneath the Iceberg Tip. Toxins 2018, 10, 190. [Google Scholar]
- Hallett, M.; Albanese, A.; Dressler, D.; Segal, K.R.; Simpson, D.M.; Truong, D.; Jankovic, J. Evidence-based review and assessment of botulinum neurotoxin for the treatment of movement disorders. Toxicon 2013, 67, 94–114. [Google Scholar] [CrossRef] [PubMed]
- Naumann, M.; Dressler, D.; Hallett, M.; Jankovic, J.; Schiavo, G.; Segal, K.R.; Truong, D. Evidence-based review and assessment of botulinum neurotoxin for the treatment of secretory disorders. Toxicon 2013, 67, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.; Rodgers, H.; Price, C.; van Wijck, F.; Shackley, P.; Steen, N.; Barnes, M.; Ford, G.; Graham, L. BoTULS: A multicentre randomised controlled trial to evaluate the clinical effectiveness and cost-effectiveness of treating upper limb spasticity due to stroke with botulinum toxin type A. Health Technol. Assess. 2010, 14, 1–113. [Google Scholar] [CrossRef] [PubMed]
- Gracies, J.M.; Brashear, A.; Jech, R.; McAllister, P.; Banach, M.; Valkovic, P.; Walker, H.; Marciniak, C.; Deltombe, T.; Skoromets, A.; et al. Safety and efficacy of abobotulinumtoxinA for hemiparesis in adults with upper limb spasticity after stroke or traumatic brain injury: A double-blind randomised controlled trial. Lancet Neurol. 2015, 14, 992–1001. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, T.; Hu, X.; Wang, T. Efficacy and safety of Botulinum Toxin type A for upper limb spasticity after stroke or traumatic brain injury: A systematic review with meta-analysis and trial sequential analysis. Eur. J. Phys. Rehabil. Med. 2016, 53. [Google Scholar] [CrossRef]
- Rosales, R.L.; Efendy, F.; Teleg, E.S.; Santos, M.M.D.; Rosales, M.C.; Ostrea, M.; Tanglao, M.J.; Ng, A.R. Botulinum toxin as early intervention for spasticity after stroke or non-progressive brain lesion: A meta-analysis. J. Neurol. Sci. 2016, 371, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Mills, P.B.; Finlayson, H.; Sudol, M.; O’Connor, R. Systematic review of adjunct therapies to improve outcomes following botulinum toxin injection for treatment of limb spasticity. Clin. Rehabil. 2016, 30, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-F.; Hsu, C.-W.; Sun, H.-P.; Hwang, C.-W.; Yang, C.-L.; Wang, J.-L. Combined botulinum toxin type A with modified constraint-induced movement therapy for chronic stroke patients with upper extremity spasticity: A randomized controlled study. Neurorehabil. Neural Repair 2010, 24, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Meythaler, J.M.; Vogtle, L.; Brunner, R.C. A preliminary assessment of the benefits of the addition of botulinum toxin a to a conventional therapy program on the function of people with longstanding stroke. Arch. Phys. Med. Rehabil. 2009, 90, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Broeks, J.G.; Lankhorst, G.J.; Rumping, K.; Prevo, A.J. The long-term outcome of arm function after stroke: Results of a follow-up study. Disabil. Rehabil. 1999, 21, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Mayer, N.H.; Esquenazi, A.; Childers, M.K. Common patterns of clinical motor dysfunction. Muscle Nerve 1997, 20, 21–35. [Google Scholar] [CrossRef]
- Picelli, A.; Lobba, D.; Midiri, A.; Prandi, P.; Melotti, C.; Baldessarelli, S.; Smania, N. Botulinum toxin injection into the forearm muscles for wrist and fingers spastic overactivity in adults with chronic stroke: A randomized controlled trial comparing three injection techniques. Clin. Rehabil. 2014, 28, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Brashear, A.; Gordon, M.F.; Elovic, E.; Kassicieh, V.D.; Marciniak, C.; Do, M.; Lee, C.-H.; Jenkins, S.; Turkel, C. Intramuscular injection of botulinum toxin for the treatment of wrist and finger spasticity after a stroke. N. Engl. J. Med. 2002, 347, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.J.; Skidmore, E.R.; Niyonkuru, C.; Chang, C.-L.; Huber, L.M.; Munin, M.C. Cyclic functional electrical stimulation does not enhance gains in hand grasp function when used as an adjunct to onabotulinumtoxinA and task practice therapy: A single-blind, randomized controlled pilot study. Arch. Phys. Med. Rehabil. 2010, 91, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Gracies, J.M.; Bayle, N.; Goldberg, S.; Simpson, D.M. Botulinum toxin type B in the spastic arm: A randomized, double-blind, placebo-controlled, preliminary study. Arch. Phys. Med. Rehabil. 2014, 95, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Billian, C.; Gorman, P.H. Upper extremity applications of functional neuromuscular stimulation. Assist. Technol. 1992, 4, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.; Bethoux, F.; Bohinc, T.; Dobos, L.; Davis, T.; Friedl, A. Neuromuscular stimulation for upper extremity motor and functional recovery in acute hemiplegia. Stroke 1998, 29, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.; Alexander, D.; O’brien, C.; Tagliati, M.; Aswad, A.; Leon, J.; Gibson, J.; Mordaunt, J.; Monaghan, E. Botulinum toxin type A in the treatment of upper extremity spasticity A randomized, double-blind, placebo-controlled trial. Neurology 1996, 46, 1306. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Ellis, E.; White, S.; Moore, A. A double-blind placebo-controlled study of botulinum toxin in upper limb spasticity after stroke or head injury. Clin. Rehabil. 2000, 14, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Sheean, G.L. Botulinum treatment of spasticity: Why is it so difficult to show a functional benefit? Curr. Opin. Neurol. 2001, 14, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Kamper, D.; Rymer, W. Impairment of voluntary control of finger motion following stroke: Role of inappropriate muscle coactivation. Muscle Nerve 2001, 24, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Francisco, G.E. New insights into the pathophysiology of post-stroke spasticity. Front. Hum. Neurosci. 2015, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Picelli, A.; Baricich, A.; Cisari, C.; Paolucci, S.; Smania, N.; Sandrini, G. The Italian real-life post-stroke spasticity survey: Unmet needs in the management of spasticity with botulinum toxin type A. Funct. Neurol. 2017, 32, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.M.; Patel, A.T.; Alfaro, A.; Ayyoub, Z.; Charles, D.; Dashtipour, K.; Esquenazi, A.; Graham, G.D.; McGuire, J.R.; Odderson, I. OnabotulinumtoxinA Injection for Poststroke Upper-Limb Spasticity: Guidance for Early Injectors from a Delphi Panel Process. PM R 2017, 9, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Filippi, G.M.; Errico, P.; Santarelli, R.; Bagolini, B.; Manni, E. Botulinum A toxin effects on rat jaw muscle spindles. Acta Oto-Laryngol. 1993, 113, 400–404. [Google Scholar] [CrossRef]
- Manni, E.; Bagolini, B.; Pettorossi, V.E.; Errico, P. Effect of botulinum toxin on extraocular muscle proprioception. Doc. Ophthalmol. 1989, 72, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Wöber, C.; Schnider, P.; Steinhoff, N.; Trattnig, S.; Zebenholzer, K.; Auff, E. Posturographic findings in patients with idiopathic cervical dystonia before and after local injections with botulinum toxin. Eur. Neurol. 1999, 41, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Kaňovský, P.; Rosales, R.L. Debunking the pathophysiological puzzle of dystonia–with special reference to botulinum toxin therapy. Parkinsonism Relat. Disord. 2011, 17, S11–S14. [Google Scholar] [CrossRef] [PubMed]
- Veverka, T.; Hluštík, P.; Hok, P.; Otruba, P.; Zapletalová, J.; Tüdös, Z.; Krobot, A.; Kaňovský, P. Sensorimotor modulation by botulinum toxin A in post-stroke arm spasticity: Passive hand movement. J. Neurol. Sci. 2016, 362, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Dresel, C.; Bayer, F.; Castrop, F.; Rimpau, C.; Zimmer, C.; Haslinger, B. Botulinum toxin modulates basal ganglia but not deficient somatosensory activation in orofacial dystonia. Mov. Disord. 2011, 26, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Zakin, E.; Simpson, D. Evidence on botulinum toxin in selected disorders. Toxicon 2018, 147, 134–140. [Google Scholar] [CrossRef] [PubMed]
- McCrory, P.; Turner-Stokes, L.; Baguley, I.J.; De Graaff, S.; Katrak, P.; Sandanam, J.; Davies, L.; Munns, M.; Hughes, A. Botulinum toxin A for treatment of upper limb spasticity following stroke: A multi-centre randomized placebo-controlled study of the effects on quality of life and other person-centred outcomes. J. Rehabil. Med. 2009, 41, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Fheodoroff, K.; Ashford, S.; Jacinto, J.; Maisonobe, P.; Balcaitiene, J.; Turner-Stokes, L. Factors influencing goal attainment in patients with post-stroke upper limb spasticity following treatment with botulinum toxin A in real-life clinical practice: Sub-analyses from the Upper Limb International Spasticity (ULIS)-II Study. Toxins 2015, 7, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.C.; Price, C.I.; van Wijck, F.M.; Shackley, P.; Steen, N.; Barnes, M.P.; Ford, G.A.; Graham, L.A.; Rodgers, H. Botulinum Toxin for the Upper Limb after Stroke (BoTULS) Trial: Effect on impairment, activity limitation, and pain. Stroke 2011, 42, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Emerson, E.T.; Krizek, T.J.; Greenwald, D.P. Anatomy, physiology, and functional restoration of the thumb. Ann. Plast. Surg. 1996, 36, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Asutay, F.; Atalay, Y.; Asutay, H.; Acar, A.H. The Evaluation of the Clinical Effects of Botulinum Toxin on Nocturnal Bruxism. Pain Res. Manag. 2017, 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Desrosiers, J.; Bravo, G.; Hébert, R.; Dutil, É.; Mercier, L. Validation of the Box and Block Test as a measure of dexterity of elderly people: Reliability, validity, and norms studies. Arch. Phys. Med. Rehabil. 1994, 75, 751–755. [Google Scholar] [PubMed]
- Van der Lee, J.H.; Beckerman, H.; Lankhorst, G.J.; Bouter, L.M. The responsiveness of the Action Research Arm test and the Fugl-Meyer Assessment scale in chronic stroke patients. J. Rebab. Med. 2001, 33, 110–113. [Google Scholar]
- Rodriquez, A.A.; McGinn, M.; Chappell, R. Botulinum toxin injection of spastic finger flexors in hemiplegic patients. Am. J. Phys. Med. Rehabil. 2000, 79, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Ring, H.; Rosenthal, N. Controlled study of neuroprosthetic functional electrical stimulation in sub-acute post-stroke rehabilitation. J. Rehabil. Med. 2005, 37, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Gummesson, C.; Ward, M.M.; Atroshi, I. The shortened disabilities of the arm, shoulder and hand questionnaire (Quick DASH): Validity and reliability based on responses within the full-length DASH. BMC Musculoskelet. Disord. 2006, 7, 44. [Google Scholar] [CrossRef] [PubMed]
| Outcome | p† | ||||||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | p * | T1–T2 | T2–T3 | T1–T3 | |
| BB test | 3.07 ± 3.85 | 3.60 ± 4.91 | 4.67 ± 5.25 | 0.039 | 0.473 | 0.120 | 0.028 |
| ARAT-total | 11.33 ± 8.03 | 11.27 ± 7.71 | 12.73 ± 7.67 | 0.043 | 1.000 | 0.036 | 0.044 |
| ARAT-grasp | 2.87 ± 3.82 | 3.00 ± 3.98 | 3.27 ± 3.75 | 0.276 | 0.854 | 0.334 | 0.276 |
| ARAT-grip | 2.13 ± 2.07 | 1.67 ± 1.92 | 2.53 ± 1.92 | 0.120 | 0.141 | 0.059 | 0.257 |
| ARAT-gross movement | 5.67 ± 2.16 | 5.93 ± 2.15 | 6.27 ± 2.37 | 0.013 | 0.046 | 0.096 | 0.021 |
| ARAT-pinch | 0.67 ± 1.23 | 0.67 ± 0.98 | 0.67 ± 0.98 | 1.000 | 1.000 | 1.000 | 1.000 |
| Outcome | p† | ||||||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | p * | T1–T2 | T2–T3 | T1–T3 | |
| Active-FE | 1.73 ± 0.88 | 2.00 ± 0.85 | 2.20 ± 0.94 | 0.060 | 0.102 | 0.180 | 0.053 |
| Distance-FP (cm) | 2.58 ± 3.12 | 3.80 ± 3.02 | 3.67 ± 2.58 | 0.212 | 0.023 | 0.655 | 0.027 |
| Repeat-FE | 2.07 ± 1.58 | 2.27 ± 1.16 | 3.13 ± 1.77 | 0.007 | 0.558 | 0.017 | 0.008 |
| Thumb opposition | 0.07 ± 0.26 | 0.13 ± 0.35 | 0.33 ± 0.62 | 0.223 | 0.317 | 0.180 | 0.102 |
| MAS-WF | 2.13 ± 0.35 | 1.80 ± 0.41 | 1.73 ± 0.46 | 0.015 | 0.025 | 0.317 | 0.014 |
| MAS-WE | 0.20 ± 0.41 | 0.13 ± 0.35 | 0.07 ± 0.26 | 0.223 | 0.317 | 0.317 | 0.157 |
| MAS-FF | 2.33 ± 0.49 | 1.67 ± 0.49 | 1.60 ± 0.51 | <0.001 | 0.002 | 0.317 | 0.001 |
| MAS-FE | 0.07 ± 0.26 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.368 | 0.317 | 1.000 | 0.317 |
| MRC-WF | 2.40 ± 0.91 | 2.40 ± 0.91 | 2.53 ± 0.83 | 0.135 | 1.000 | 0.157 | 0.157 |
| MRC-WE | 2.27 ± 0.88 | 2.40 ± 0.74 | 2.53 ± 0.83 | 0.050 | 0.157 | 0.157 | 0.046 |
| MRC-FF | 2.60 ± 0.51 | 2.60 ± 0.51 | 2.67 ± 0.49 | 0.717 | 1.000 | 0.317 | 0.564 |
| MRC-FE | 1.47 ± 0.64 | 1.60 ± 0.63 | 1.67 ± 0.62 | 0.097 | 0.157 | 0.317 | 0.083 |
| AROM-WF (°) | 46.67 ± 26.37 | 46.67 ± 26.37 | 49.33 ± 23.44 | 0.050 | 1.000 | 0.102 | 0.102 |
| AROM-WE (°) | 32.67 ± 26.04 | 33.67 ± 25.53 | 38.00 ± 24.55 | 0.011 | 0.396 | 0.024 | 0.023 |
| AROM-RD (°) | 4.67 ± 7.42 | 5.33 ± 6.40 | 6.67 ± 7.24 | 0.174 | 0.564 | 0.157 | 0.083 |
| AROM-UD (°) | 4.67 ± 6.40 | 5.33 ± 6.40 | 5.33 ± 6.40 | 0.368 | 0.317 | 1.000 | 0.317 |
| Grip strength (kg) | 0.27 ± 1.03 | 0.13 ± 0.52 | 0.33 ± 1.05 | 0.223 | 0.317 | 0.180 | 0.317 |
| QDASH | 56.88 ± 17.22 | 54.65 ± 14.88 | 53.87 ± 16.54 | 0.162 | 0.220 | 0.529 | 0.058 |
| Patient | Stroke Type | Lesion Side | Time from Stroke (Months) | Dominant Hand | Injection Site (Dosage in Units) |
|---|---|---|---|---|---|
| 1 | Ischemic | Rt. | 11.5 | Rt. | FCU (25), FDP 2/3/4/5 (10/20/20/10), FDS 2/3/4/5 (20/30/25/15), FPL (25) |
| 2 | Ischemic | Rt. | 10.9 | Rt. | FCU (25), FDP 2/3/4 (15/20/15), FDS 2/3/4/5 (25/25/30/15), FPL (30) |
| 3 | Hemorrhagic | Rt. | 13.2 | Rt. | FCU (40), FDP 2/3/4 (10/15/15), FDS 2/3/4/5 (25/30/35/20), FPB (10), FPL (30), PT (50) |
| 4 | Hemorrhagic | Rt. | 17.4 | Rt. | AP (10), BB (40), brachialis (50), FDP 2/3/4/5 (10/15/15/5), FDS 2/3/4/5 (25/35/40/15), FPL (40) |
| 5 | Ischemic | Rt. | 18.9 | Rt. | AP (10), brachialis (55), FDP 2/3/4 (10/10/10), FDS 2/3/4/5 (30/40/35/20), FPL (40), PT (40) |
| 6 | Ischemic | Rt. | 10.2 | Rt. | FCU (30), FDP 2/3/4/5 (10/20/10/10), FDS 2/3/4/5 (25/25/20/15), FPB (5), FPL (25), PT (20) |
| 7 | Hemorrhagic | Lt. | 12.6 | Rt. | Brachialis (60), FCU (40), FDP 2/3/4 (10/10/15), FDS 2/3/4/5 (25/35/30/15), FPL (20), PT (40) |
| 8 | Ischemic | Lt. | 13 | Rt. | Brachialis (60), deltoid (20), FDP 2/3/4/5 (15/15/10/10), FDS 2/3/4/5 (30/40/30/10), FPB (10), FPL (30), PT (20) |
| 9 | Hemorrhagic | Rt. | 10.3 | Lt. | Brachialis (60), deltoid (20), FDP 2/3/4/5 (15/15/10/10), FDS 2/3/4/5 (30/40/30/10), FPB (10), FPL (30), PT (20) |
| 10 | Hemorrhagic | Rt. | 20.3 | Rt. | AP (10), FCU (30), FDS 2/3/4 (10/15/10), FPB (10), FPL (20), lumbricals (10) |
| 11 | Ischemic | Rt. | 8.6 | Rt. | AP (10), FCU (30), FDP 2/3/4/5 (5/10/10/5), FDS 2/3/4/5 (20/30/20/10), FPL (20) |
| 12 | Ischemic | Rt. | 7.2 | Rt. | AP (10), BB (40), FDP 2/3/5 (10/10/5), FDS 2/3/4/5 (30/35/20/10), FPL (30) |
| 13 | Hemorrhagic | Rt. | 9.8 | Rt. | AP (15), FDP 2/3/4 (5/10/5), FDS 2/3/4/5 (10/20/15/5), FPL (15) |
| 14 | Hemorrhagic | Rt. | 12.8 | Rt. | FDP 2/3/4/5 (30/30/35/25), FDS 2/3/4 (10/15/15), FPL (30), opponens (5) |
| 15 | Hemorrhagic | Rt. | 14.9 | Rt. | Brachialis (50), brachioradialis (20), FCU (30), FDP 2/3/4 (15/15/15), FDS 2/3/4/5 (35/40/30/20), FPL (30) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-M.; Gracies, J.-M.; Park, S.-B.; Lee, K.H.; Lee, J.Y.; Shin, J.-H. Botulinum Toxin Injections and Electrical Stimulation for Spastic Paresis Improve Active Hand Function Following Stroke. Toxins 2018, 10, 426. https://doi.org/10.3390/toxins10110426
Lee J-M, Gracies J-M, Park S-B, Lee KH, Lee JY, Shin J-H. Botulinum Toxin Injections and Electrical Stimulation for Spastic Paresis Improve Active Hand Function Following Stroke. Toxins. 2018; 10(11):426. https://doi.org/10.3390/toxins10110426
Chicago/Turabian StyleLee, Jong-Min, Jean-Michel Gracies, Si-Bog Park, Kyu Hoon Lee, Ji Yeong Lee, and Joon-Ho Shin. 2018. "Botulinum Toxin Injections and Electrical Stimulation for Spastic Paresis Improve Active Hand Function Following Stroke" Toxins 10, no. 11: 426. https://doi.org/10.3390/toxins10110426
APA StyleLee, J.-M., Gracies, J.-M., Park, S.-B., Lee, K. H., Lee, J. Y., & Shin, J.-H. (2018). Botulinum Toxin Injections and Electrical Stimulation for Spastic Paresis Improve Active Hand Function Following Stroke. Toxins, 10(11), 426. https://doi.org/10.3390/toxins10110426






