Functional Consequences of Calcium Influx Promoted by Bacterial Pore-Forming Toxins
Abstract
:1. Introduction
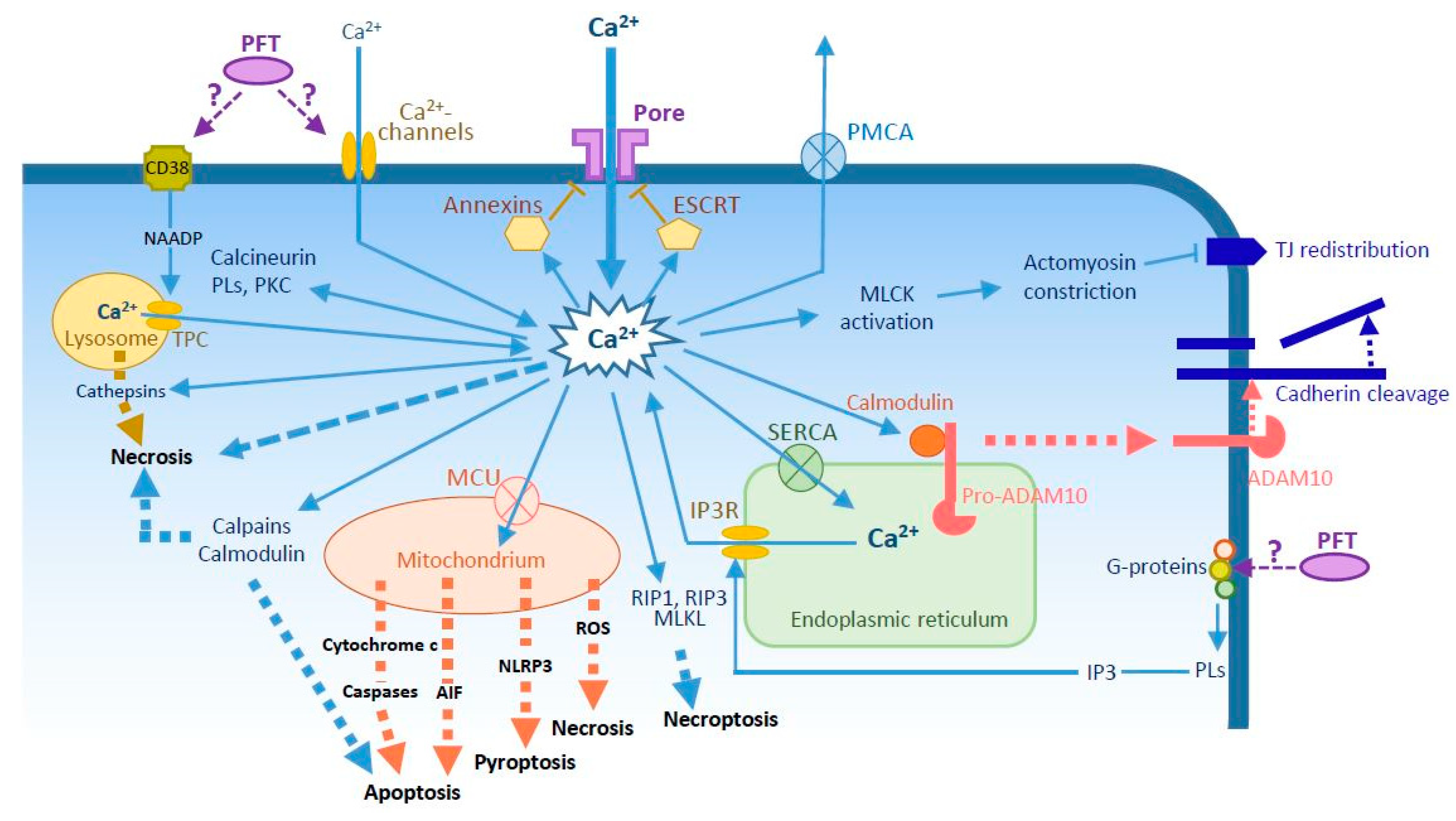
2. How Do PFTs Increase Intracellular Ca2+?
3. Cell Repair Mechanisms
4. Cell Death
5. Intercellular Junction Disruption
6. Other PFT-Mediated Effects
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Bischofberger, M.; Gonzalez, M.R.; van der Goot, F.G. Membrane injury by pore-forming proteins. Curr. Opin. Cell Biol. 2009, 21, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, S.K.; O’Riordan, M.X. More than a pore: The cellular response to cholesterol-dependent cytolysins. Toxins 2013, 5, 618–636. [Google Scholar] [CrossRef] [PubMed]
- Dal Peraro, M.; van der Goot, F.G. Pore-forming toxins: Ancient, but never really out of fashion. Nat. Rev. 2016, 14, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Los, F.C.; Randis, T.M.; Aroian, R.V.; Ratner, A.J. Role of pore-forming toxins in bacterial infectious diseases. Microbiol. Mol. Biol. Rev. 2013, 77, 173–207. [Google Scholar] [CrossRef] [PubMed]
- DuMont, A.L.; Torres, V.J. Cell targeting by the staphylococcus aureus pore-forming toxins: It’s not just about lipids. Trends Microbiol. 2014, 22, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Shaughnessy, L.M.; Hoppe, A.D.; Christensen, K.A.; Swanson, J.A. Membrane perforations inhibit lysosome fusion by altering ph and calcium in listeria monocytogenes vacuoles. Cell. Microbiol. 2006, 8, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Cudd, L.; Clarke, C.; Clinkenbeard, K. Contribution of intracellular calcium stores to an increase in cytosolic calcium concentration induced by mannheimia haemolytica leukotoxin. FEMS Microbiol. Lett. 2003, 225, 23–27. [Google Scholar] [CrossRef]
- Gekara, N.O.; Groebe, L.; Viegas, N.; Weiss, S. Listeria monocytogenes desensitizes immune cells to subsequent Ca2+ signaling via listeriolysin o-induced depletion of intracellular Ca2+ stores. Infect. Immun. 2008, 76, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Gekara, N.O.; Westphal, K.; Ma, B.; Rohde, M.; Groebe, L.; Weiss, S. The multiple mechanisms of Ca2+ signalling by listeriolysin o, the cholesterol-dependent cytolysin of listeria monocytogenes. Cell. Microbiol. 2007, 9, 2008–2021. [Google Scholar] [CrossRef] [PubMed]
- Hsuan, S.L.; Kannan, M.S.; Jeyaseelan, S.; Prakash, Y.S.; Sieck, G.C.; Maheswaran, S.K. Pasteurella haemolytica a1-derived leukotoxin and endotoxin induce intracellular calcium elevation in bovine alveolar macrophages by different signaling pathways. Infect. Immun. 1998, 66, 2836–2844. [Google Scholar] [PubMed]
- Jover, E.; Tawk, M.Y.; Laventie, B.J.; Poulain, B.; Prevost, G. Staphylococcal leukotoxins trigger free intracellular Ca2+ rise in neurones, signalling through acidic stores and activation of store-operated channels. Cell. Microbiol. 2013, 15, 742–758. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.H.; Fivaz, M.; Monod, A.; van der Goot, F.G. Aerolysin induces g-protein activation and Ca2+ release from intracellular stores in human granulocytes. J. Biol. Chem. 1998, 273, 18122–18129. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Gomez-Bilbao, G.; Ostolaza, H. Bordetella adenylate cyclase toxin promotes calcium entry into both cd11b+ and cd11b- cells through camp-dependent l-type-like calcium channels. J. Biol. Chem. 2010, 285, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Staali, L.; Monteil, H.; Colin, D.A. The staphylococcal pore-forming leukotoxins open Ca2+ channels in the membrane of human polymorphonuclear neutrophils. J. Membr. Biol. 1998, 162, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Tanoue, N.; Nakano, M.; Hamamoto, A.; Okamoto, K.; Fujii, Y.; Harada, N.; Nakaya, Y. A pore-forming toxin produced by aeromonas sobria activates Ca2+ dependent cl- secretion. Microb. Pathog. 2005, 38, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Carafoli, E.; Krebs, J. Why calcium? How calcium became the best communicator. J. Biol. Chem. 2016, 291, 20849–20857. [Google Scholar] [CrossRef] [PubMed]
- Lodish, H.; Matsudaira, P. Molecular Cell Biology; Freeman: New York, NY, USA, 2000. [Google Scholar]
- Von Hoven, G.; Rivas, A.J.; Neukirch, C.; Meyenburg, M.; Qin, Q.; Parekh, S.; Hellmann, N.; Husmann, M. Repair of a bacterial small beta-barrel toxin pore depends on channel width. mBio 2017, 8, e02083-16. [Google Scholar] [CrossRef] [PubMed]
- Aroian, R.; van der Goot, F.G. Pore-forming toxins and cellular non-immune defenses (CNIDs). Curr. Opin. Microbiol. 2007, 10, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Bravo-San Pedro, J.M.; Kroemer, G. Necrosis: Linking the inflammasome to inflammation. Cell Rep. 2015, 11, 1501–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, S.M.; Kanneganti, T.D. Regulation of inflammasome activation. Immunol. Rev. 2015, 265, 6–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, D.; Ahn, D.; Cohen, T.; Prince, A. Innate immune signaling activated by mdr bacteria in the airway. Physiol. Rev. 2016, 96, 19–53. [Google Scholar] [CrossRef] [PubMed]
- Rimessi, A.; Bezzerri, V.; Patergnani, S.; Marchi, S.; Cabrini, G.; Pinton, P. Mitochondrial Ca2+-dependent nlrp3 activation exacerbates the pseudomonas aeruginosa-driven inflammatory response in cystic fibrosis. Nat. Commun. 2015, 6, 6201. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Ockinger, J.; Yu, J.; Byles, V.; McColl, A.; Hofer, A.M.; Horng, T. Critical role for calcium mobilization in activation of the nlrp3 inflammasome. Proc. Natl. Acad. Sci. USA 2012, 109, 11282–11287. [Google Scholar] [CrossRef] [PubMed]
- Cosker, F.; Cheviron, N.; Yamasaki, M.; Menteyne, A.; Lund, F.E.; Moutin, M.J.; Galione, A.; Cancela, J.M. The ecto-enzyme CD38 is a nicotinic acid adenine dinucleotide phosphate (NAADP) synthase that couples receptor activation to Ca2+ mobilization from lysosomes in pancreatic acinar cells. J. Biol. Chem. 2010, 285, 38251–38259. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.X.; Ma, J.; Parrington, J.; Galione, A.; Evans, A.M. Tpcs: Endolysosomal channels for Ca2+ mobilization from acidic organelles triggered by NAADP. FEBS Lett. 2010, 584, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Iwase, M.; Korchak, H.M.; Lally, E.T.; Berthold, P.; Taichman, N.S. Lytic effects of actinobacillus actinomycetemcomitans leukotoxin on human neutrophil cytoplasts. J. Leukoc. Biol. 1992, 52, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Bucker, R.; Krug, S.M.; Rosenthal, R.; Gunzel, D.; Fromm, A.; Zeitz, M.; Chakraborty, T.; Fromm, M.; Epple, H.J.; Schulzke, J.D. Aerolysin from aeromonas hydrophila perturbs tight junction integrity and cell lesion repair in intestinal epithelial ht-29/b6 cells. J. Infect. Dis. 2011, 204, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.L.; Brodsky, R.A.; Buckley, J.T. Channels formed by subnanomolar concentrations of the toxin aerolysin trigger apoptosis of t lymphomas. Cell. Microbiol. 1999, 1, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Tschodrich-Rotter, M.; Kubitscheck, U.; Ugochukwu, G.; Buckley, J.T.; Peters, R. Optical single-channel analysis of the aerolysin pore in erythrocyte membranes. Biophys. J. 1996, 70, 723–732. [Google Scholar] [CrossRef] [Green Version]
- Fiser, R.; Masin, J.; Basler, M.; Krusek, J.; Spulakova, V.; Konopasek, I.; Sebo, P. Third activity of bordetella adenylate cyclase (ac) toxin-hemolysin. Membrane translocation of ac domain polypeptide promotes calcium influx into cd11b+ monocytes independently of the catalytic and hemolytic activities. J. Biol. Chem. 2007, 282, 2808–2820. [Google Scholar] [CrossRef] [PubMed]
- Fiser, R.; Masin, J.; Bumba, L.; Pospisilova, E.; Fayolle, C.; Basler, M.; Sadilkova, L.; Adkins, I.; Kamanova, J.; Cerny, J.; et al. Calcium influx rescues adenylate cyclase-hemolysin from rapid cell membrane removal and enables phagocyte permeabilization by toxin pores. PLoS Pathog. 2012, 8, e1002580. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.; Keyel, M.; Shi, G.; Bhattacharjee, P.; Roth, R.; Heuser, J.E.; Keyel, P.A. Intrinsic repair protects cells from pore-forming toxins by microvesicle shedding. Cell Death Differ. 2017, 24, 798–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakrabarti, G.; McClane, B.A. The importance of calcium influx, calpain and calmodulin for the activation of caco-2 cell death pathways by clostridium perfringens enterotoxin. Cell. Microbiol. 2005, 7, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Chassin, C.; Bens, M.; de Barry, J.; Courjaret, R.; Bossu, J.L.; Cluzeaud, F.; Ben Mkaddem, S.; Gibert, M.; Poulain, B.; Popoff, M.R.; et al. Pore-forming epsilon toxin causes membrane permeabilization and rapid atp depletion-mediated cell death in renal collecting duct cells. Am. J. Physiol. Ren. Physiol. 2007, 293, F927–F937. [Google Scholar] [CrossRef] [PubMed]
- Petit, L.; Maier, E.; Gibert, M.; Popoff, M.R.; Benz, R. Clostridium perfringens epsilon toxin induces a rapid change of cell membrane permeability to ions and forms channels in artificial lipid bilayers. J. Biol. Chem. 2001, 276, 15736–15740. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C.L.; Smith, D.J.; Lyras, D.; Chakravorty, A.; Rood, J.I. Programmed cellular necrosis mediated by the pore-forming alpha-toxin from clostridium septicum. PLoS Pathog. 2009, 5, e1000516. [Google Scholar] [CrossRef] [PubMed]
- Koschinski, A.; Repp, H.; Unver, B.; Dreyer, F.; Brockmeier, D.; Valeva, A.; Bhakdi, S.; Walev, I. Why escherichia coli alpha-hemolysin induces calcium oscillations in mammalian cells—The pore is on its own. FASEB J. 2006, 20, 973–975. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, P.; Laestadius, A.; Jahnukainen, T.; Soderblom, T.; Backhed, F.; Celsi, G.; Brismar, H.; Normark, S.; Aperia, A.; Richter-Dahlfors, A. Alpha-haemolysin of uropathogenic e. Coli induces Ca2+ oscillations in renal epithelial cells. Nature 2000, 405, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Valeva, A.; Walev, I.; Kemmer, H.; Weis, S.; Siegel, I.; Boukhallouk, F.; Wassenaar, T.M.; Chavakis, T.; Bhakdi, S. Binding of escherichia coli hemolysin and activation of the target cells is not receptor-dependent. J. Biol. Chem. 2005, 280, 36657–36663. [Google Scholar] [CrossRef] [PubMed]
- Soderblom, T.; Oxhamre, C.; Wai, S.N.; Uhlen, P.; Aperia, A.; Uhlin, B.E.; Richter-Dahlfors, A. Effects of the escherichia coli toxin cytolysin a on mucosal immunostimulation via epithelial Ca2+ signalling and toll-like receptor 4. Cell. Microbiol. 2005, 7, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Dramsi, S.; Cossart, P. Listeriolysin o-mediated calcium influx potentiates entry of listeria monocytogenes into the human hep-2 epithelial cell line. Infect. Immun. 2003, 71, 3614–3618. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.G.T.; Vadia, S.; Pathak-Sharma, S.; McLaughlin, E.; Zhang, X.; Swanson, J.; Seveau, S. Host cell perforation by listeriolysin o (llo) activates a Ca2+-dependent cpkc/rac1/arp2/3 signaling pathway that promotes listeria monocytogenes internalization independently of membrane resealing. Mol. Biol. Cell 2018, 29, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Repp, H.; Pamukci, Z.; Koschinski, A.; Domann, E.; Darji, A.; Birringer, J.; Brockmeier, D.; Chakraborty, T.; Dreyer, F. Listeriolysin of listeria monocytogenes forms Ca2+-permeable pores leading to intracellular Ca2+ oscillations. Cell. Microbiol. 2002, 4, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Cudd, L.; Clarke, C.; Clinkenbeard, K. Mannheimia haemolytica leukotoxin-induced increase in leukotriene b4 production by bovine neutrophils is mediated by a sustained and excessive increase in intracellular calcium concentration. FEMS Microbiol. Lett. 2003, 224, 85–90. [Google Scholar] [CrossRef]
- Cudd, L.; Clarke, C.; Clinkenbeard, K.; Shelton, M.; Clinkenbeard, P.; Murphy, G. Role of intracellular calcium in pasteurella haemolytica leukotoxin-induced bovine neutrophil leukotriene b4 production and plasma membrane damage. FEMS Microbiol. Lett. 1999, 172, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Carranza, O.; Czuprynski, C.J. Activation of bovine neutrophils by pasteurella haemolytica leukotoxin is calcium dependent. J. Leukoc. Biol. 1992, 52, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Reboud, E.; Bouillot, S.; Patot, S.; Beganton, B.; Attree, I.; Huber, P. Pseudomonas aeruginosa ExlA and serratia marcescens ShlA trigger cadherin cleavage by promoting calcium influx and ADAM10 activation. PLoS Pathog. 2017, 13, e1006579. [Google Scholar] [CrossRef] [PubMed]
- Eichstaedt, S.; Gabler, K.; Below, S.; Muller, C.; Kohler, C.; Engelmann, S.; Hildebrandt, P.; Volker, U.; Hecker, M.; Hildebrandt, J.P. Effects of staphylococcus aureus-hemolysin a on calcium signalling in immortalized human airway epithelial cells. Cell Calcium 2009, 45, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Fink, D.; Contreras, M.L.; Lelkes, P.I.; Lazarovici, P. Staphylococcus aureus alpha-toxin activates phospholipases and induces a Ca2+ influx in PC12 cells. Cell. Signal. 1989, 1, 387–393. [Google Scholar] [CrossRef]
- Inoshima, I.; Inoshima, N.; Wilke, G.A.; Powers, M.E.; Frank, K.M.; Wang, Y.; Bubeck Wardenburg, J. A staphylococcus aureus pore-forming toxin subverts the activity of adam10 to cause lethal infection in mice. Nat. Med. 2011, 17, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Kwak, Y.K.; Vikstrom, E.; Magnusson, K.E.; Vecsey-Semjen, B.; Colque-Navarro, P.; Mollby, R. The staphylococcus aureus alpha-toxin perturbs the barrier function in caco-2 epithelial cell monolayers by altering junctional integrity. Infect. Immun. 2012, 80, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Von Hoven, G.; Rivas, A.J.; Neukirch, C.; Klein, S.; Hamm, C.; Qin, Q.; Meyenburg, M.; Fuser, S.; Saftig, P.; Hellmann, N.; et al. Dissecting the role of adam10 as a mediator of staphylococcus aureus alpha-toxin action. Biochem. J. 2016, 473, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Susilowati, H.; Okamura, H.; Hirota, K.; Shono, M.; Yoshida, K.; Murakami, K.; Tabata, A.; Nagamune, H.; Haneji, T.; Miyake, Y. Intermedilysin induces EGR-1 expression through calcineurin/NFAT pathway in human cholangiocellular carcinoma cells. Biochem. Biophys. Res. Commun. 2011, 404, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.S.; Sublett, J.E.; Freyer, D.; Mitchell, T.J.; Cleveland, J.L.; Tuomanen, E.I.; Weber, J.R. Pneumococcal pneumolysin and H(2)O(2) mediate brain cell apoptosis during meningitis. J. Clin. Investig. 2002, 109, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Fickl, H.; Cockeran, R.; Steel, H.C.; Feldman, C.; Cowan, G.; Mitchell, T.J.; Anderson, R. Pneumolysin-mediated activation of NFkappaB in human neutrophils is antagonized by docosahexaenoic acid. Clin. Exp. Immunol. 2005, 140, 274–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nel, J.G.; Durandt, C.; Mitchell, T.J.; Feldman, C.; Anderson, R.; Tintinger, G.R. Pneumolysin mediates platelet activation in vitro. Lung 2016, 194, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Wolfmeier, H.; Schoenauer, R.; Atanassoff, A.P.; Neill, D.R.; Kadioglu, A.; Draeger, A.; Babiychuk, E.B. Ca(2)(+)-dependent repair of pneumolysin pores: A new paradigm for host cellular defense against bacterial pore-forming toxins. Biochim. Biophys. Acta 2015, 1853, 2045–2054. [Google Scholar] [CrossRef] [PubMed]
- Cywes Bentley, C.; Hakansson, A.; Christianson, J.; Wessels, M.R. Extracellular group a streptococcus induces keratinocyte apoptosis by dysregulating calcium signalling. Cell. Microbiol. 2005, 7, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Idone, V.; Tam, C.; Goss, J.W.; Toomre, D.; Pypaert, M.; Andrews, N.W. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J. Cell Biol. 2008, 180, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Fong, K.P.; Pacheco, C.M.; Otis, L.L.; Baranwal, S.; Kieba, I.R.; Harrison, G.; Hersh, E.V.; Boesze-Battaglia, K.; Lally, E.T. Actinobacillus actinomycetemcomitans leukotoxin requires lipid microdomains for target cell cytotoxicity. Cell. Microbiol. 2006, 8, 1753–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carafoli, E. Calcium pump of the plasma membrane. Physiol. Rev. 1991, 71, 129–153. [Google Scholar] [CrossRef] [PubMed]
- Chemaly, E.R.; Troncone, L.; Lebeche, D. Serca control of cell death and survival. Cell Calcium 2018, 69, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Marchi, S.; Pinton, P. The mitochondrial calcium uniporter complex: Molecular components, structure and physiopathological implications. J. Physiol. 2014, 592, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Babiychuk, E.B.; Draeger, A. Defying death: Cellular survival strategies following plasmalemmal injury by bacterial toxins. Semin. Cell Dev. Biol. 2015, 45, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.T.; McNeil, P.L. Membrane repair: Mechanisms and pathophysiology. Physiol. Rev. 2015, 95, 1205–1240. [Google Scholar] [CrossRef] [PubMed]
- Babiychuk, E.B.; Monastyrskaya, K.; Potez, S.; Draeger, A. Intracellular Ca2+ operates a switch between repair and lysis of streptolysin o-perforated cells. Cell Death Differ. 2009, 16, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Babiychuk, E.B.; Monastyrskaya, K.; Potez, S.; Draeger, A. Blebbing confers resistance against cell lysis. Cell Death Differ. 2011, 18, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Keyel, P.A.; Loultcheva, L.; Roth, R.; Salter, R.D.; Watkins, S.C.; Yokoyama, W.M.; Heuser, J.E. Streptolysin o clearance through sequestration into blebs that bud passively from the plasma membrane. J. Cell Sci. 2011, 124, 2414–2423. [Google Scholar] [CrossRef] [PubMed]
- Wolfmeier, H.; Radecke, J.; Schoenauer, R.; Koeffel, R.; Babiychuk, V.S.; Drucker, P.; Hathaway, L.J.; Mitchell, T.J.; Zuber, B.; Draeger, A.; et al. Active release of pneumolysin prepores and pores by mammalian cells undergoing a streptococcus pneumoniae attack. Biochim. Biophy. Acta 2016, 1860, 2498–2509. [Google Scholar] [CrossRef] [PubMed]
- Draeger, A.; Monastyrskaya, K.; Babiychuk, E.B. Plasma membrane repair and cellular damage control: The annexin survival kit. Biochem. Pharmacol. 2011, 81, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, A.J.; Maiuri, P.; Lafaurie-Janvore, J.; Divoux, S.; Piel, M.; Perez, F. Escrt machinery is required for plasma membrane repair. Science 2014, 343, 1247136. [Google Scholar] [CrossRef] [PubMed]
- Corrotte, M.; Fernandes, M.C.; Tam, C.; Andrews, N.W. Toxin pores endocytosed during plasma membrane repair traffic into the lumen of MVBs for degradation. Traffic 2012, 13, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Los, F.C.; Kao, C.Y.; Smitham, J.; McDonald, K.L.; Ha, C.; Peixoto, C.A.; Aroian, R.V. Rab-5- and rab-11-dependent vesicle-trafficking pathways are required for plasma membrane repair after attack by bacterial pore-forming toxin. Cell Host Microbe 2011, 9, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Husmann, M.; Dersch, K.; Bobkiewicz, W.; Beckmann, E.; Veerachato, G.; Bhakdi, S. Differential role of p38 mitogen activated protein kinase for cellular recovery from attack by pore-forming S. Aureus alpha-toxin or streptolysin O. Biochem. Biophys. Res. Commun. 2006, 344, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.Y.; Los, F.C.; Huffman, D.L.; Wachi, S.; Kloft, N.; Husmann, M.; Karabrahimi, V.; Schwartz, J.L.; Bellier, A.; Ha, C.; et al. Global functional analyses of cellular responses to pore-forming toxins. PLoS Pathog. 2011, 7, e1001314. [Google Scholar] [CrossRef] [PubMed]
- Bischof, L.J.; Kao, C.Y.; Los, F.C.; Gonzalez, M.R.; Shen, Z.; Briggs, S.P.; van der Goot, F.G.; Aroian, R.V. Activation of the unfolded protein response is required for defenses against bacterial pore-forming toxin in vivo. PLoS Pathog. 2008, 4, e1000176. [Google Scholar] [CrossRef] [PubMed]
- Hail, N., Jr.; Carter, B.Z.; Konopleva, M.; Andreeff, M. Apoptosis effector mechanisms: A requiem performed in different keys. Apoptosis 2006, 11, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Braun, P.; de Groot, A.; Bitter, W.; Tommassen, J. Secretion of elastinolytic enzymes and their propeptides by pseudomonas aeruginosa. J. Bacteriol. 1998, 180, 3467–3469. [Google Scholar] [PubMed]
- Joza, N.; Kroemer, G.; Penninger, J.M. Genetic analysis of the mammalian cell death machinery. Trends Genet. 2002, 18, 142–149. [Google Scholar] [CrossRef]
- McCall, K. Genetic control of necrosis—Another type of programmed cell death. Curr. Opin. Cell Biol. 2010, 22, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Fancy, R.M.; Wang, L.; Schmid, T.; Zeng, Q.; Wang, H.; Zhou, T.; Buchsbaum, D.J.; Song, Y. Characterization of the interactions between calmodulin and death receptor 5 in triple-negative and estrogen receptor-positive breast cancer cells: An integrated experimental and computational study. J. Biol. Chem. 2016, 291, 12862–12870. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Juarbe, N.; Bradley, K.M.; Shenoy, A.T.; Gilley, R.P.; Reyes, L.F.; Hinojosa, C.A.; Restrepo, M.I.; Dube, P.H.; Bergman, M.A.; Orihuela, C.J. Pore-forming toxin-mediated ion dysregulation leads to death receptor-independent necroptosis of lung epithelial cells during bacterial pneumonia. Cell Death Differ. 2017, 24, 917–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galluzzi, L.; Kepp, O.; Krautwald, S.; Kroemer, G.; Linkermann, A. Molecular mechanisms of regulated necrosis. Semin. Cell Dev. Biol. 2014, 35, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.E.; Kim, H.K.; Wang, Y.; Bubeck Wardenburg, J. Adam10 mediates vascular injury induced by staphylococcus aureus alpha-hemolysin. J. Infect. Dis. 2012, 206, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Wilke, G.A.; Bubeck Wardenburg, J. Role of a disintegrin and metalloprotease 10 in staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc. Natl. Acad. Sci. USA 2010, 107, 13473–13478. [Google Scholar] [CrossRef] [PubMed]
- Nagano, O.; Murakami, D.; Hartmann, D.; De Strooper, B.; Saftig, P.; Iwatsubo, T.; Nakajima, M.; Shinohara, M.; Saya, H. Cell-matrix interaction via cd44 is independently regulated by different metalloproteinases activated in response to extracellular Ca2+ influx and pkc activation. J. Cell Biol. 2004, 165, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Ponnuchamy, B.; Khalil, R.A. Role of ADAMs in endothelial cell permeability: Cadherin shedding and leukocyte rolling. Circ. Res. 2008, 102, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Dreymueller, D.; Pruessmeyer, J.; Groth, E.; Ludwig, A. The role of ADAM-mediated shedding in vascular biology. Eur. J. Cell Biol. 2012, 91, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Pruessmeyer, J.; Ludwig, A. The good, the bad and the ugly substrates for ADAM10 and ADAM17 in brain pathology, inflammation and cancer. Semin. Cell Dev. Biol. 2009, 20, 164–174. [Google Scholar] [CrossRef] [PubMed]
| Species | Toxin Name 1 | Pore Size 2 | Ca2+ Origin 3 | Ca2+ Kinetics | Reported Effects of PFT-Induced Ca2+ Influx | Refs |
|---|---|---|---|---|---|---|
| Actinobacillus actinomycetemcomitans | Ltx | n. d. | EC | Monophasic | ∙ Neutrophil lysis | [27] |
| Aeromonas hydrophila | Aerolysin | Small | EC + IC | Multiphasic | ∙ Granulocyte chemotaxis ∙ T cell apoptosis ∙ Actomyosin contraction and tight junction disruption | [12,28,29,30] |
| Aeromonas sobria | ASH | Small | EC + IC | Biphasic | [15] | |
| Bordetella pertussis | ACT = CyaA | Small | EC | Multiphasic via non-voltage dependent channels with L-type properties | ∙ Prevents ACT endocytosis and degradation | [31,32] |
| Clostridium perfringens | PFO | Large | EC | Unknown | ∙ Activates/enhances repair mechanism | [33] |
| CPE | Small | Unknown | Biphasic | ∙ Apoptosis and necrosis through calpain and calmodulin-dependent processes | [34] | |
| ET | Small | EC | Monophasic | [35,36] | ||
| Clostridium septicum | α-toxin | Small | EC | Biphasic | ∙ Necrosis induced by multiple pathways | [37] |
| Escherichia coli | HlyA | Small | EC | Oscillations due to Ca2+ channel activation or to rapid formation/closure of the pore | ∙ ROS production by granulocytes ∙ IL-6 and IL-8 production by epithelal cells | [38,39,40] |
| ClyA = HlyE | Small | IC | Oscillations | [41] | ||
| Listeria monocytogenes | LLO | Large | EC IC via G-protein activation-IP3 production | Oscillation due to rapid formation/closure of the pore and release from IC stores | ∙ Bacterial internalization ∙ Mast cell degranulation and cytokine synthesis ∙ Immune cell desensitization | [8,9,42,43,44] |
| Pasteurella hemolytica | LKT | n. d. | EC through voltage-gated Ca2+ channels | Monophasic | ∙ ROS and leukotriene production by neutrophils ∙ Cytokine release from macrophages | [7,10,45,46,47] |
| Pseudomonas aeruginosa | ExlA | Small | EC | Biphasic | ∙ Cadherin cleavage via ADAM10 activation ∙ Necrosis | [48] |
| Photobacterium damselae | PhlyP | Small | Monophasic | ∙ Lysosomal exocytosis | [18] | |
| Serratia marcescens | ShlA | Small | EC | Monophasic | ∙ Cadherin cleavage via ADAM10 activation ∙ Necrosis | [48] |
| Staphylococcus aureus | Hla = α-toxin | Small | EC | Monophasic | ∙ PLA2 activation ∙ Cadherin cleavage through ADAM10 activation | [49,50,51,52,53] |
| Hlg | Small | IC from lysosomes and endoplasmic reticulum EC from store-operated channels | Mono/biphasic | [11,14] | ||
| PVL | Small | As for Hlg | Mono/biphasic | [11,14] | ||
| Streptococcus intermedius | ILY | Large | Unknown | Unknown | ∙ NFAT activation and EGR-1 expression via Ca2+/calcineurin pathway ∙ Activation/enhancement of repair mechanism | [33,54] |
| Streptococcus pneumoniae | PLY | Large | EC | Multiphasic | ∙ Apoptosis ∙ IL-8 production via NFκB activation ∙ Cadherin cleavage through ADAM10 activation ∙ Activation/enhancement of repair mechanism ∙ Platelet activation ∙ NFκB-dependent IL-8 synthesis | [51,55,56,57,58] |
| Streptococcus pyogenes | SLO | Large | EC + IC | Monophasic | ∙ Granulocyte chemotaxis ∙ Keratinocyte apoptosis and ER vacuolation ∙ Membrane repair | [12,33,59,60] |
| Pore-Forming Toxins 1 (Species) | Apoptosis | Necrosis | Necroptosis | Ref. |
|---|---|---|---|---|
| Ltx (A. actinomycetemcomitans) | In T cells. Possibly calpain-dependent | [61] | ||
| Aerolysin (A. hydophila) | At low dose in T cells | [29] | ||
| CPE (C. perfringens) | At low dose in enterocytes | At high dose in enterocytes | [34] | |
| ET (C. perfringens) | In renal collecting duct cells | [35] | ||
| α-toxin (C. septicum) | In myoblasts | [37] | ||
| PLY (S. pneumoniae) | In microglial cells | In pneumocytes | [55,83] | |
| SLO (S. pyogenes) | At low dose in keratinocytes | At high dose in keratinocytes | [59] | |
| ShlA (S. marcescens) | In pneumocytes | [83] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouillot, S.; Reboud, E.; Huber, P. Functional Consequences of Calcium Influx Promoted by Bacterial Pore-Forming Toxins. Toxins 2018, 10, 387. https://doi.org/10.3390/toxins10100387
Bouillot S, Reboud E, Huber P. Functional Consequences of Calcium Influx Promoted by Bacterial Pore-Forming Toxins. Toxins. 2018; 10(10):387. https://doi.org/10.3390/toxins10100387
Chicago/Turabian StyleBouillot, Stéphanie, Emeline Reboud, and Philippe Huber. 2018. "Functional Consequences of Calcium Influx Promoted by Bacterial Pore-Forming Toxins" Toxins 10, no. 10: 387. https://doi.org/10.3390/toxins10100387
APA StyleBouillot, S., Reboud, E., & Huber, P. (2018). Functional Consequences of Calcium Influx Promoted by Bacterial Pore-Forming Toxins. Toxins, 10(10), 387. https://doi.org/10.3390/toxins10100387




