Characterization of Chicken-Derived Single Chain Antibody Fragments against Venom of Naja Naja Atra
Abstract
:1. Introduction
2. Results
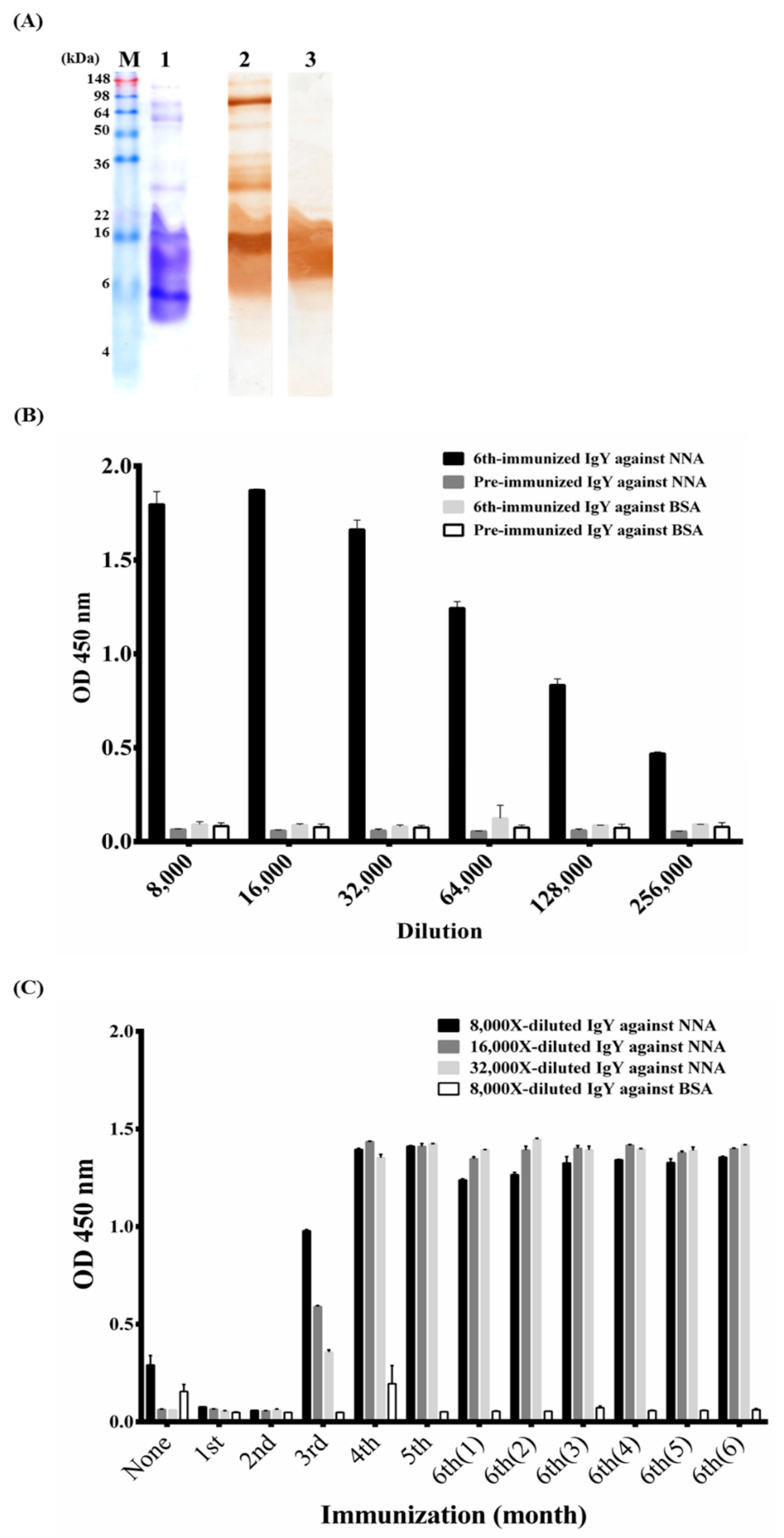
2.1. Characterization of Polyclonal Anti-NNA IgY Antibodies
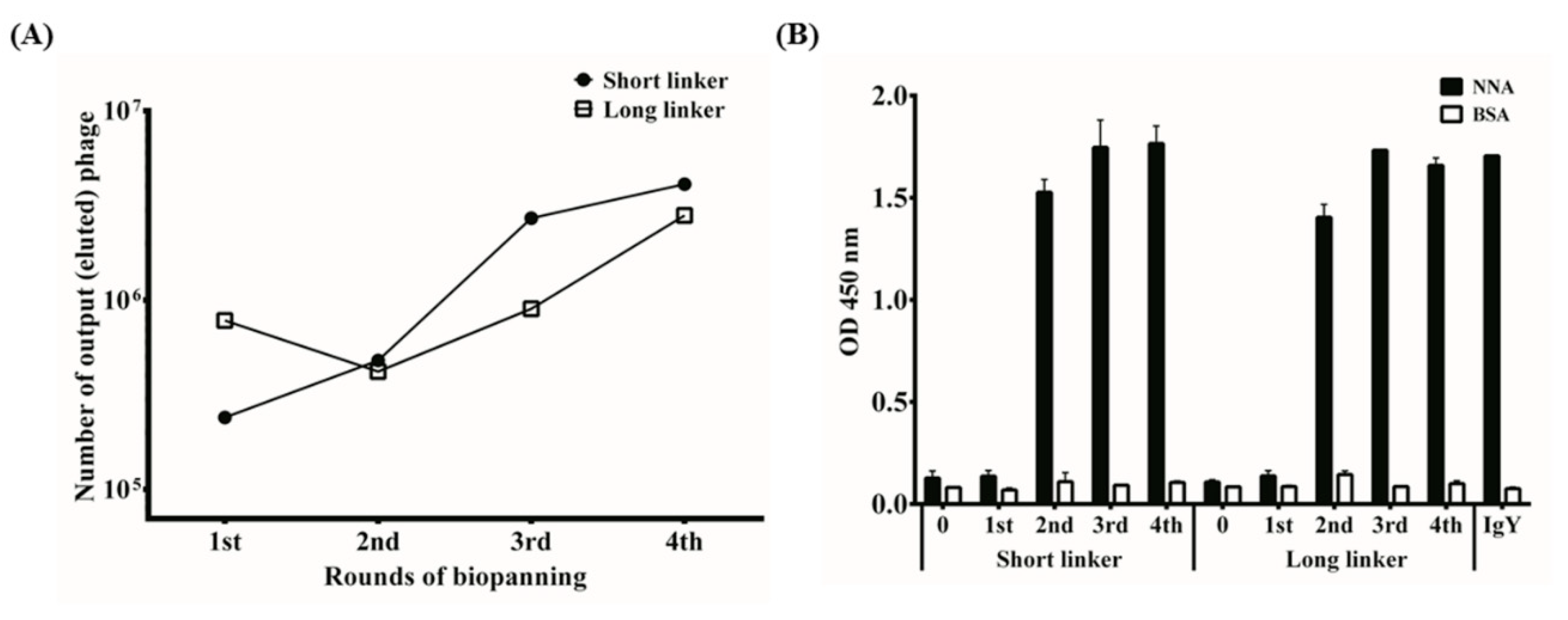
2.2. Antibody Library Construction
2.3. Selection of Monoclonal Anti-NNA scFv Antibodies
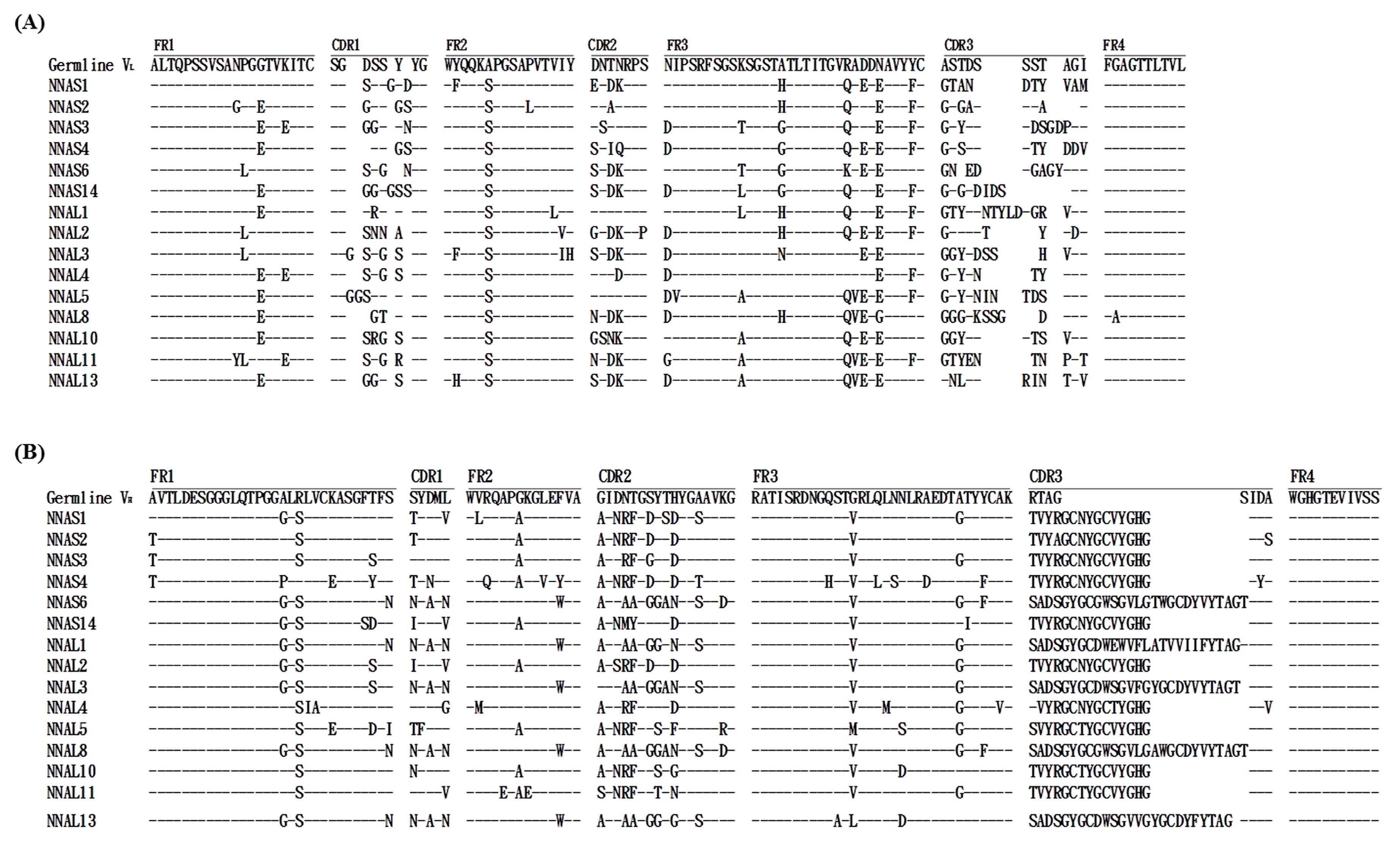
2.4. Sequencing Analysis of Anti-NNA scFv Antibodies
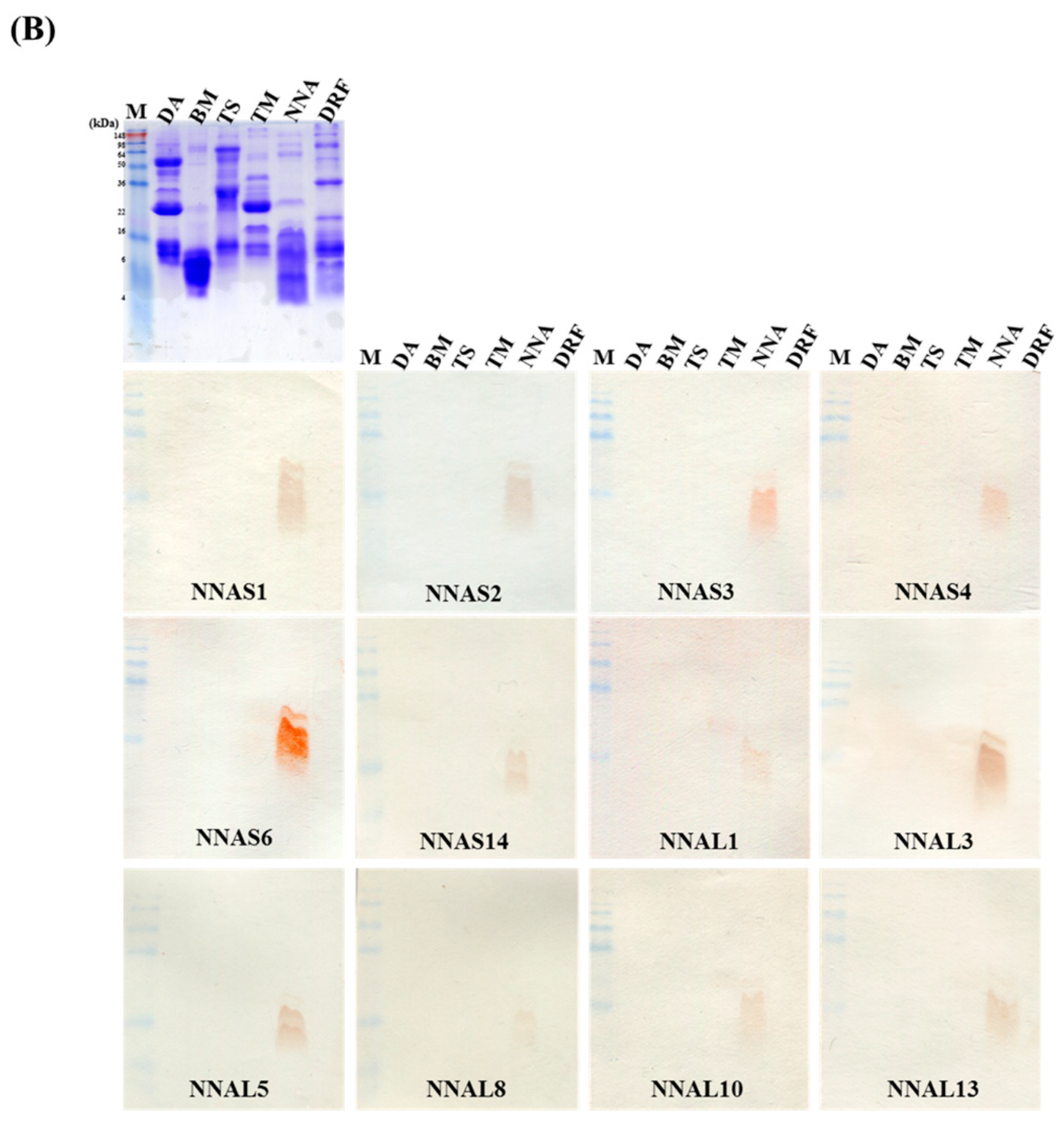
2.5. Expression, Purification and Characterization of Selected scFv Antibodies
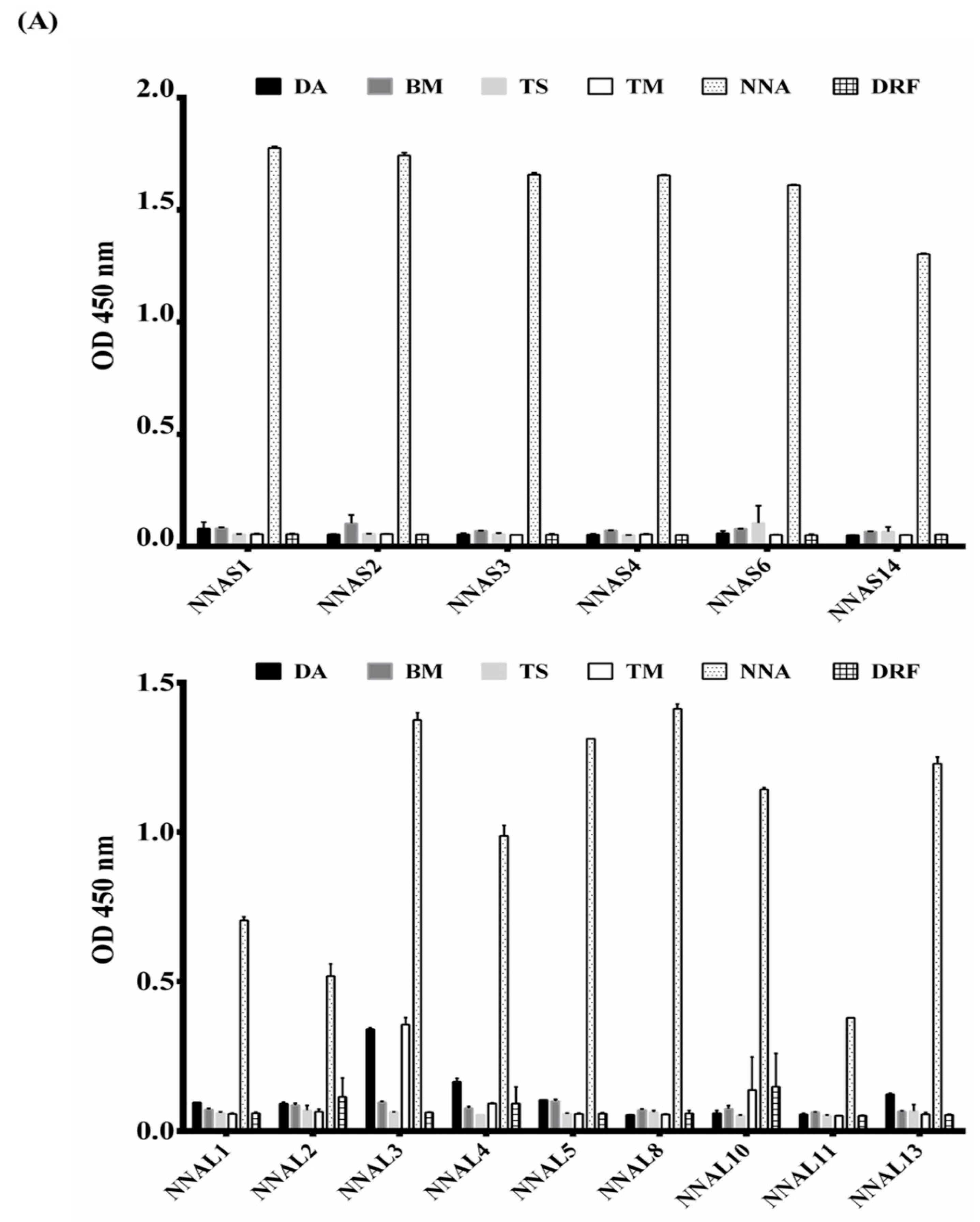
2.6. Specific Binding of Anti-NNA scFv Clones
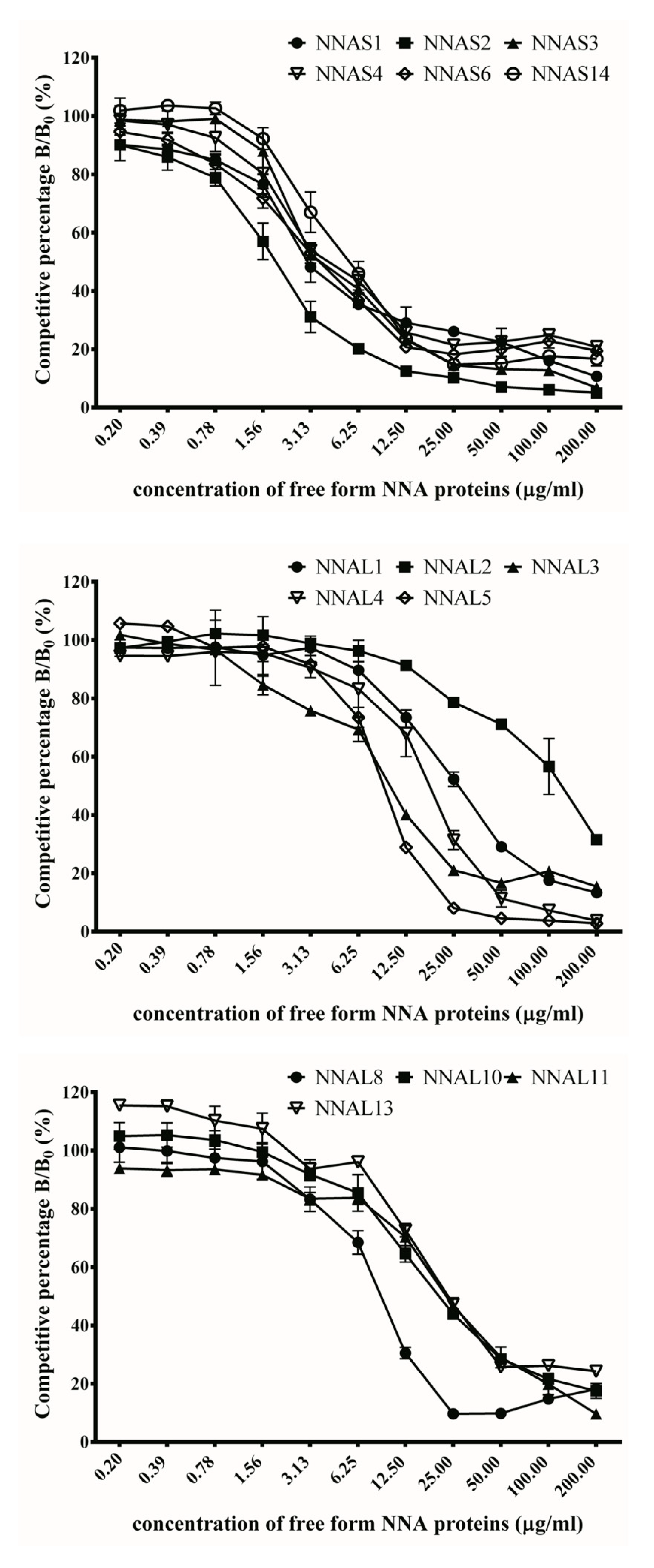
2.7. Inhibition Assay by Competitive ELISA
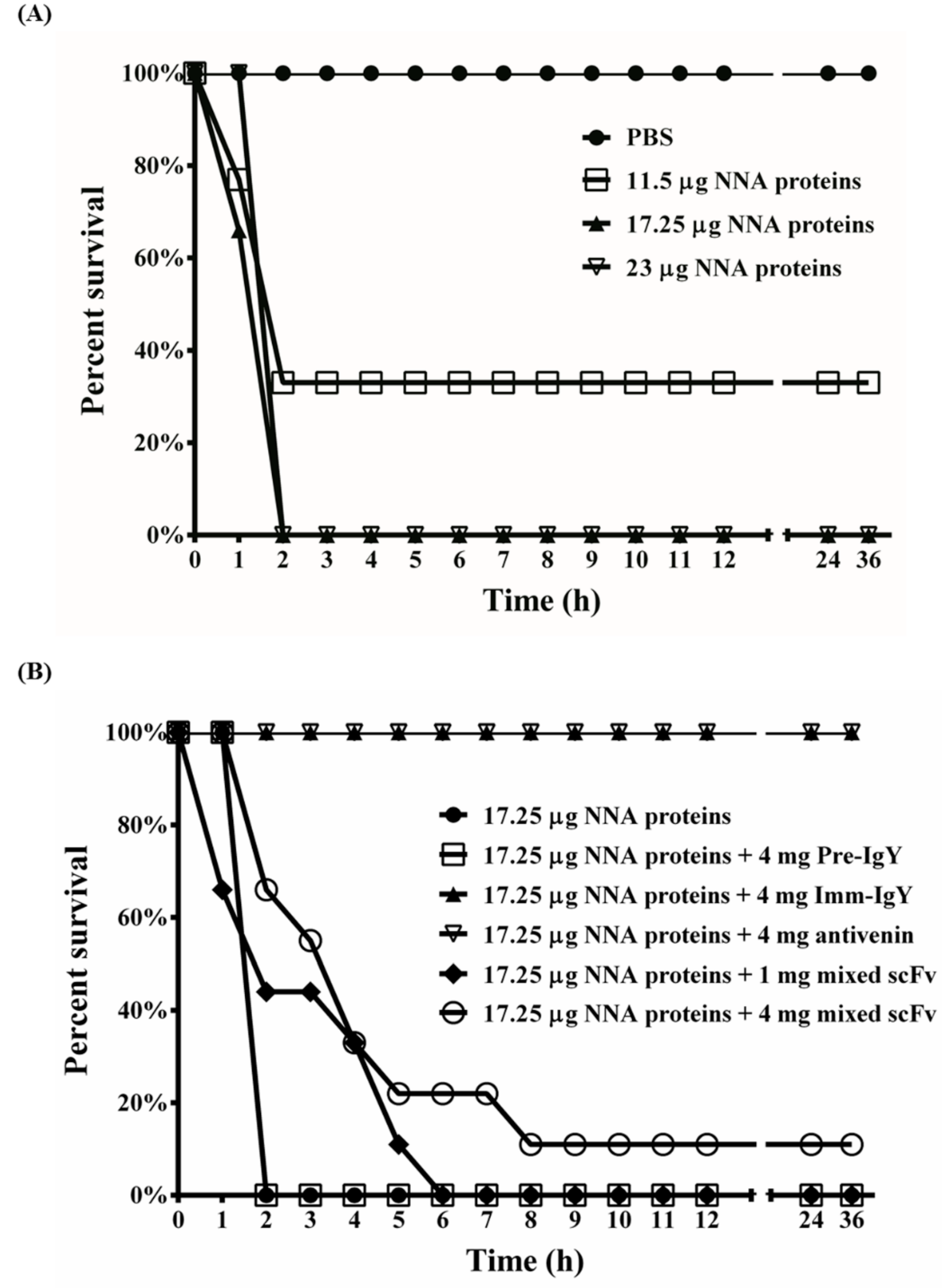
2.8. Neutralization Assay of Anti-NNA IgY and scFv Antibodies
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chicken Immunization and IgY Purification
4.3. Library Construction
4.4. Biopanning
4.5. Expression and Purification of scFv Antibodies
4.6. Sequencing
4.7. Western Blotting
4.8. Enzyme-Linked Immunosorbent Assay (ELISA) and Competitive ELISA
4.9. Neutralization Activity of scFv Antibodies
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Warrell, D.A. Snake bite. Lancet 2010, 375, 77–88. [Google Scholar] [CrossRef]
- Searo, W. Guidelines on Management of Snake-Bites; Who Regional Office for South-East Asia: New Delhi, India, 2010. [Google Scholar]
- Chippaux, J.P. Snake-bites: Appraisal of the global situation. Bull. WHO 1998, 76, 515–524. [Google Scholar] [PubMed]
- Aird, S.D. Ophidian envenomation strategies and the role of purines. Toxicon 2002, 40, 335–393. [Google Scholar] [CrossRef]
- Huang, H.-W.; Liu, B.-S.; Chien, K.-Y.; Chiang, L.-C.; Huang, S.-Y.; Sung, W.-C.; Wu, W.-G. Cobra venom proteome and glycome determined from individual snakes of naja atra reveal medically important dynamic range and systematic geographic variation. J. Proteom. 2015, 128, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Doley, R.; Kini, R.M. Protein complexes in snake venom. Cell. Mol. Life Sci. 2009, 66, 2851–2871. [Google Scholar] [CrossRef] [PubMed]
- Weiner, L.M.; Surana, R.; Wang, S. Monoclonal antibodies: Versatile platforms for cancer immunotherapy. Nat. Rev. Immunol. 2010, 10, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Gold, B.S.; Dart, R.C.; Barish, R.A. Bites of venomous snakes. N. Engl. J. Med. 2002, 347, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Warr, G.W.; Magor, K.E.; Higgins, D.A. Igy: Clues to the origins of modern antibodies. Immunol. Today 1995, 16, 392–398. [Google Scholar] [CrossRef]
- Gassmann, M.; Thommes, P.; Weiser, T.; Hubscher, U. Efficient production of chicken egg yolk antibodies against a conserved mammalian protein. FASEB J. 1990, 4, 2528–2532. [Google Scholar] [CrossRef] [PubMed]
- Carlander, D. Avian IgY Antibody: In Vitro and In Vivo; Acta Universitatis Upsaliensis: Wu oprah, Sweden, 2002. [Google Scholar]
- Nilsson, E.; Larsson, A.; Olesen, H.V.; Wejåker, P.-E.; Kollberg, H. Good effect of igy against pseudomonas aeruginosa infections in cystic fibrosis patients. Pediatr. Pulmonol. 2008, 43, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Sugano, N.; Shimada, T.; Shofiqur, R.A.K.M.; Ibrahim el, E.-S.M.; Isoda, R.; Umeda, K.; Sa, N.V.; Kodama, Y.; Ito, K. Effects of egg yolk antibody against porphyromonas gingivalis gingipains in periodontitis patients. J. Oral Sci. 2007, 49, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Kovacs-Nolan, J.; Mine, Y. Egg yolk antibodies for passive immunity. Annu. Rev. Food Sci. Technol. 2012, 3, 163–182. [Google Scholar] [CrossRef] [PubMed]
- McCormack, W.T.; Tjoelker, L.W.; Thompson, C.B. Immunoglobulin gene diversification by gene conversion. Acad. Press 1993, 45, 27–45. [Google Scholar]
- Rucavado, A.; Escalante, T.; Shannon, J.; Gutierrez, J.M.; Fox, J.W. Proteomics of wound exudate in snake venom-induced pathology: Search for biomarkers to assess tissue damage and therapeutic success. J. Proteome Res. 2011, 10, 1987–2005. [Google Scholar] [CrossRef] [PubMed]
- Rucavado, A.; Escalante, T.; Shannon, J.D.; Ayala-Castro, C.N.; Villalta, M.; Gutiérrez, J.M.; Fox, J.W. Efficacy of igg and f(ab’)2 antivenoms to neutralize snake venom-induced local tissue damage as assessed by the proteomic analysis of wound exudate. J. Proteome Res. 2012, 11, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Huse, W.D.; Sastry, L.; Iverson, S.A.; Kang, A.S.; Alting-Mees, M.; Burton, D.R.; Benkovic, S.J.; Lerner, R.A. Generation of a large combinatorial library of the immunoglobulin repertoire in phage lambda. Science 1989, 246, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Andris-Widhopf, J.; Rader, C.; Steinberger, P.; Fuller, R.; Barbas, C.F., 3rd. Methods for the generation of chicken monoclonal antibody fragments by phage display. J. Immunol. Met. 2000, 242, 159–181. [Google Scholar] [CrossRef]
- Holliger, P.; Hudson, P.J. Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 2005, 23, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Kulkeaw, K.; Sakolvaree, Y.; Srimanote, P.; Tongtawe, P.; Maneewatch, S.; Sookrung, N.; Tungtrongchitr, A.; Tapchaisri, P.; Kurazono, H.; Chaicumpa, W. Human monoclonal scfv neutralize lethal Thai cobra, Naja kaouthia, neurotoxin. J. Proteom. 2009, 72, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Theakston, R.; Warrell, D.; Griffiths, E. Erratum to report of who workshop on the standardization and control on antivenoms. Toxicon 2003, 42, 223. [Google Scholar] [CrossRef]
- Schade, R.; Bürger, W.; Schöneberg, T.; Schniering, A.; Schwarzkopf, C.; Hlinak, A.; Kobilke, H. Avian egg yolk antibodies. The egg laying capacity of hens following immunisation with antigens of different kind and origin and the efficiency of egg yolk antibodies in comparison to mammalian antibodies. Altex 1994, 11, 75–84. [Google Scholar] [PubMed]
- Hatta, H.; Tsuda, K.; Akachi, S.; Kim, M.; Yamamoto, T. Productivity and some properties of egg yolk antibody (igy) against human rotavirus compared with rabbit igg. Biosci. Biotechnol. Biochem. 1993, 57, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Chacana, P.A.; Terzolo, H.R.; Calzado, E.G.; Schade, R. Tecnología igy o aplicaciones de los anticuerpos de yema de huevo de gallina. Rev. Med. Vet. 2004, 85, 179–189. [Google Scholar]
- Davalos-Pantoja, L.; Ortega-Vinuesa, J.L.; Bastos-Gonzalez, D.; Hidalgo-Alvarez, R. A comparative study between the adsorption of igy and igg on latex particles. J. Biomater. Sci. 2000, 11, 657–673. [Google Scholar] [CrossRef]
- Carlander, D.; Larsson, A. Avian antibodies can eliminate interference due to complement activation in elisa. Upsal. J. Med. Sci. 2001, 106, 189–195. [Google Scholar] [CrossRef]
- Schade, R.; Calzado, E.G.; Sarmiento, R.; Chacana, P.A.; Porankiewicz-Asplund, J.; Terzolo, H.R. Chicken egg yolk antibodies (igy-technology): A review of progress in production and use in research and human and veterinary medicine. ATLA 2005, 33, 129–154. [Google Scholar] [PubMed]
- Warrell, D.A. WHO/SEARO Guidelines for the clinical management of snake bites in the southeast asian region. Southeast Asian J. Trop. Med. Public Health 1999, 30, 1–85. [Google Scholar]
- Ming-Yi, L.; Ruey-Jen, H. Toxoids and antivenoms of venomous snakes in taiwan. Toxin Rev. 1997, 16, 163–175. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, Y.C.; Liang, M.H.; Leu, S.J.; Lin, L.T.; Chiang, J.R.; Yang, Y.-Y. Antibodies against venom of the snake deinagkistrodon acutus. Appl. Environ. Microbiol. 2016, 82, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lee, Y.C.; Leu, S.J.; Lin, L.T.; Chiang, J.R.; Hsu, W.J.; Yang, Y.-Y. Production and characterization of neutralizing antibodies against bungarus multicinctus snake venom. Appl. Environ. Microbiol. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lee, Y.C.; Lee, Y.L.; Leu, S.J.; Lin, L.T.; Chen, C.C.; Chiang, J.R.; Mwale, P.F.; Tsai, B.Y.; Hung, C.S.; et al. Single chain antibody fragment against venom from the snake daboia russelii formosensis. Toxins 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Darvish, M.; Behdani, M.; Shokrgozar, M.A.; Pooshang-Bagheri, K.; Shahbazzadeh, D. Development of protective agent against hottentotta saulcyi venom using camelid single-domain antibody. Mol. Immunol. 2015, 68, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Yardehnavi, N.; Behdani, M.; Bagheri, K.P.; Mahmoodzadeh, A.; Khanahmad, H.; Shahbazzadeh, D.; Habibi-Anbouhi, M.; Hassanzadeh Ghassabeh, G.; Muyldermans, S. A camelid antibody candidate for development of a therapeutic agent against hemiscorpius lepturus envenomation. FASEB J. 2014, 28, 4004–4014. [Google Scholar] [CrossRef] [PubMed]
- Hammers, C.M.; Stanley, J.R. Antibody phage display: Technique and applications. J. Investig. Dermatol. 2014, 134. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S. Hybridoma technology for production of monoclonal antibodies. Hybridoma 2010, 1, 88–94. [Google Scholar]
- Xu, J.L.; Davis, M.M. Diversity in the cdr3 region of v(h) is sufficient for most antibody specificities. Immunity 2000, 13, 37–45. [Google Scholar] [CrossRef]
- Wu, L.; Oficjalska, K.; Lambert, M.; Fennell, B.J.; Darmanin-Sheehan, A.; Ní Shúilleabháin, D.; Autin, B.; Cummins, E.; Tchistiakova, L.; Bloom, L.; et al. Fundamental characteristics of the immunoglobulin vh repertoire of chickens in comparison with those of humans, mice, and camelids. J. Immunol. 2012, 188, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Leu, S.-J.; Lee, Y.-C.; Shih, N.-Y.; Huang, I.-J.; Liu, K.-J.; Lu, H.-F.; Huang, S.-Y.; Yang, Y.-Y. Generation and characterization of anti-alpha-enolase single-chain antibodies in chicken. Appl. Environ. Microbiol. 2010, 137, 251–260. [Google Scholar]
- Kumar, T.K.S.; Jayaraman, G.; Lee, C.S.; Arunkumar, A.I.; Sivaraman, T.; Samuel, D.; Yu, C. Snake venom cardiotoxins—structure, dynamics, function and folding. J. Biomol. Struct. Dyn. 1997, 15, 431–463. [Google Scholar] [CrossRef] [PubMed]
- Kulkeaw, K.; Chaicumpa, W.; Sakolvaree, Y.; Tongtawe, P.; Tapchaisri, P. Proteome and immunome of the venom of the thai cobra, naja kaouthia. Toxicon 2007, 49, 1026–1041. [Google Scholar] [CrossRef] [PubMed]
- Standker, L.; Harvey, A.L.; Furst, S.; Mathes, I.; Forssmann, W.G.; Escalona de Motta, G.; Béress, L. Improved method for the isolation, characterization and examination of neuromuscular and toxic properties of selected polypeptide fractions from the crude venom of the taiwan cobra naja naja atra. Toxicon 2012, 60, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Asad, M.H.; Durr, E.S.; Yaqab, T.; Murtaza, G.; Hussain, M.S.; Nasir, M.T.; Azhar, S.; Khan, S.A.; Hussain, I. Phospholipases a2: Enzymatic assay for snake venom (naja naja karachiensis) with their neutralization by medicinal plants of pakistan. Acta Pharm. 2014, 71, 625–630. [Google Scholar]
- Debnath, A.; Saha, A.; Gomes, A.; Biswas, S.; Chakrabarti, P.; Giri, B.; Biswas, A.K.; Gupta, S.D. A lethal cardiotoxic-cytotoxic protein from the indian monocellate cobra (naja kaouthia) venom. Toxicon 2010, 56, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Shashidharamurthy, R.; Kemparaju, K. A neurotoxic phospholipase a2 variant: Isolation and characterization from eastern regional indian cobra (naja naja) venom. Toxicon 2006, 47, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, G.; Kumar, T.K.; Tsai, C.C.; Srisailam, S.; Chou, S.H.; Ho, C.L.; Yu, C. Elucidation of the solution structure of cardiotoxin analogue v from the taiwan cobra (naja naja atra)—Identification of structural features important for the lethal action of snake venom cardiotoxins. Protein Sci. 2000, 9, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Cristofori-Armstrong, B.; Rash, L.D.; Hodgson, W.C.; Isbister, G.K. Defining the role of post-synaptic alpha-neurotoxins in paralysis due to snake envenoming in humans. Cell. Mol. Life Sci. 2018, 2018, 1–14. [Google Scholar]
- Maduwage, K.P.; Scorgie, F.E.; Lincz, L.F.; O’Leary, M.A.; Isbister, G.K. Procoagulant snake venoms have differential effects in animal plasmas: Implications for antivenom testing in animal models. Thromb. Res. 2016, 137, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Tsurushita, N.; Park, M.; Pakabunto, K.; Ong, K.; Avdalovic, A.; Fu, H.; Jia, A.; Vasquez, M.; Kumar, S. Humanization of a chicken anti-il-12 monoclonal antibody. J. Immunol. Met. 2004, 295, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Akita, E.M.; Nakai, S. Comparison of four purification methods for the production of immunoglobulins from eggs laid by hens immunized with an enterotoxigenic e. Coli strain. J. Immunol. Methods 1993, 160, 207–214. [Google Scholar] [CrossRef]
- Friguet, B.; Chaffotte, A.F.; Djavadi-Ohaniance, L.; Goldberg, M.E. Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J. Immunol. Met. 1985, 77, 305–319. [Google Scholar] [CrossRef]

| Short Linker | Long Linker | |||||
|---|---|---|---|---|---|---|
| VL | VH | Percentage | VL | VH | Percentage | |
| Group 1 | 1, 7, 15 | 1, 7, 15 | 20% | 1 | 1 | 6.67% |
| Group 2 | 2, 5, 8, 9, 10, 13 | 2, 5, 8, 9, 10, 13 | 40% | 2 | 2 | 6.67% |
| Group 3 | 3, 11, 12 | 3, 11, 12 | 20% | 3, 15 | 3, 15 | 13.33% |
| Group 4 | 4 | 4 | 6.67% | 4, 6, 9, 14 | 4, 6, 9, 14 | 26.67% |
| Group 5 | 6 | 6 | 6.67% | 5, 7 | 5, 7 | 13.33% |
| Group 6 | 14 | 14 | 6.67% | 8, 12 | 8, 12 | 13.33% |
| Group 7 | 10 | 10 | 6.67% | |||
| Group 8 | 11 | 11 | 6.67% | |||
| Group 9 | 13 | 13 | 6.67% | |||
| Region | CDR1 | CDR2 | CDR3 | Total CDRs | FR1 | FR2 | FR3 | FR4 | Total FRs |
|---|---|---|---|---|---|---|---|---|---|
| VL | 13~50% | 0~57% | 40~100% | 32~60% | 0~15% | 6~29% | 9~25% | 0~10% | 8~14% |
| VH | 0~60% | 24~53% | 83~90% | 50~75% | 3~13% | 7~29% | 6~19% | 0% | 5~16% |
| Clone | Inhibition of 50% Binding of Total Protein (μg/mL) | Kd Values (M) |
|---|---|---|
| NNAS1 | 4.07 ± 0.127 | 3.1 ± 0.009 × 10−7 |
| NNAS2 | 1.73 ± 0.255 | 1.3 ± 0.191 × 10−7 |
| NNAS3 | 4.77 ± 0.373 | 3.6 ± 0.283 × 10−7 |
| NNAS4 | 4.93 ± 0.067 | 3.7 ± 0.050 × 10−7 |
| NNAS6 | 4.19 ± 0.329 | 3.1 ± 0.248 × 10−7 |
| NNAS14 | 6.29 ± 1.233 | 4.7 ± 0.919 × 10−7 |
| NNAL1 | 30.58 ± 0.840 | 23 ± 0.645 × 10−7 |
| NNAL2 | 128.59 ± 9.549 | * |
| NNAL3 | 11.89 ± 0.929 | 8.9 ± 0.700 × 10−7 |
| NNAL4 | 18.48 ± 4.040 | * |
| NNAL5 | 10.50 ± 0.643 | 7.9 ± 0.481 × 10−7 |
| NNAL8 | 9.87 ± 0.508 | 7.4 ± 0.382 × 10−7 |
| NNAL10 | 21.42 ± 1.012 | 16 ± 0.778 × 10−7 |
| NNAL11 | 23.18 ± 5.571 | * |
| NNAL13 | 27.97 ± 0.56 | 21 ± 0.424 × 10−7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-H.; Leu, S.-J.; Lee, Y.-C.; Liu, C.-I.; Lin, L.-T.; Mwale, P.F.; Chiang, J.-R.; Tsai, B.-Y.; Chen, C.-C.; Hung, C.-S.; et al. Characterization of Chicken-Derived Single Chain Antibody Fragments against Venom of Naja Naja Atra. Toxins 2018, 10, 383. https://doi.org/10.3390/toxins10100383
Lee C-H, Leu S-J, Lee Y-C, Liu C-I, Lin L-T, Mwale PF, Chiang J-R, Tsai B-Y, Chen C-C, Hung C-S, et al. Characterization of Chicken-Derived Single Chain Antibody Fragments against Venom of Naja Naja Atra. Toxins. 2018; 10(10):383. https://doi.org/10.3390/toxins10100383
Chicago/Turabian StyleLee, Chi-Hsin, Sy-Jye Leu, Yu-Ching Lee, Chia-I Liu, Liang-Tzung Lin, Pharaoh Fellow Mwale, Jen-Ron Chiang, Bor-Yu Tsai, Chi-Ching Chen, Ching-Sheng Hung, and et al. 2018. "Characterization of Chicken-Derived Single Chain Antibody Fragments against Venom of Naja Naja Atra" Toxins 10, no. 10: 383. https://doi.org/10.3390/toxins10100383
APA StyleLee, C.-H., Leu, S.-J., Lee, Y.-C., Liu, C.-I., Lin, L.-T., Mwale, P. F., Chiang, J.-R., Tsai, B.-Y., Chen, C.-C., Hung, C.-S., & Yang, Y.-Y. (2018). Characterization of Chicken-Derived Single Chain Antibody Fragments against Venom of Naja Naja Atra. Toxins, 10(10), 383. https://doi.org/10.3390/toxins10100383






