A Cross-Sectional Study of Dietary and Genetic Predictors of Blood Folate Levels in Healthy Young Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Procedures
2.3. Statistical Analysis
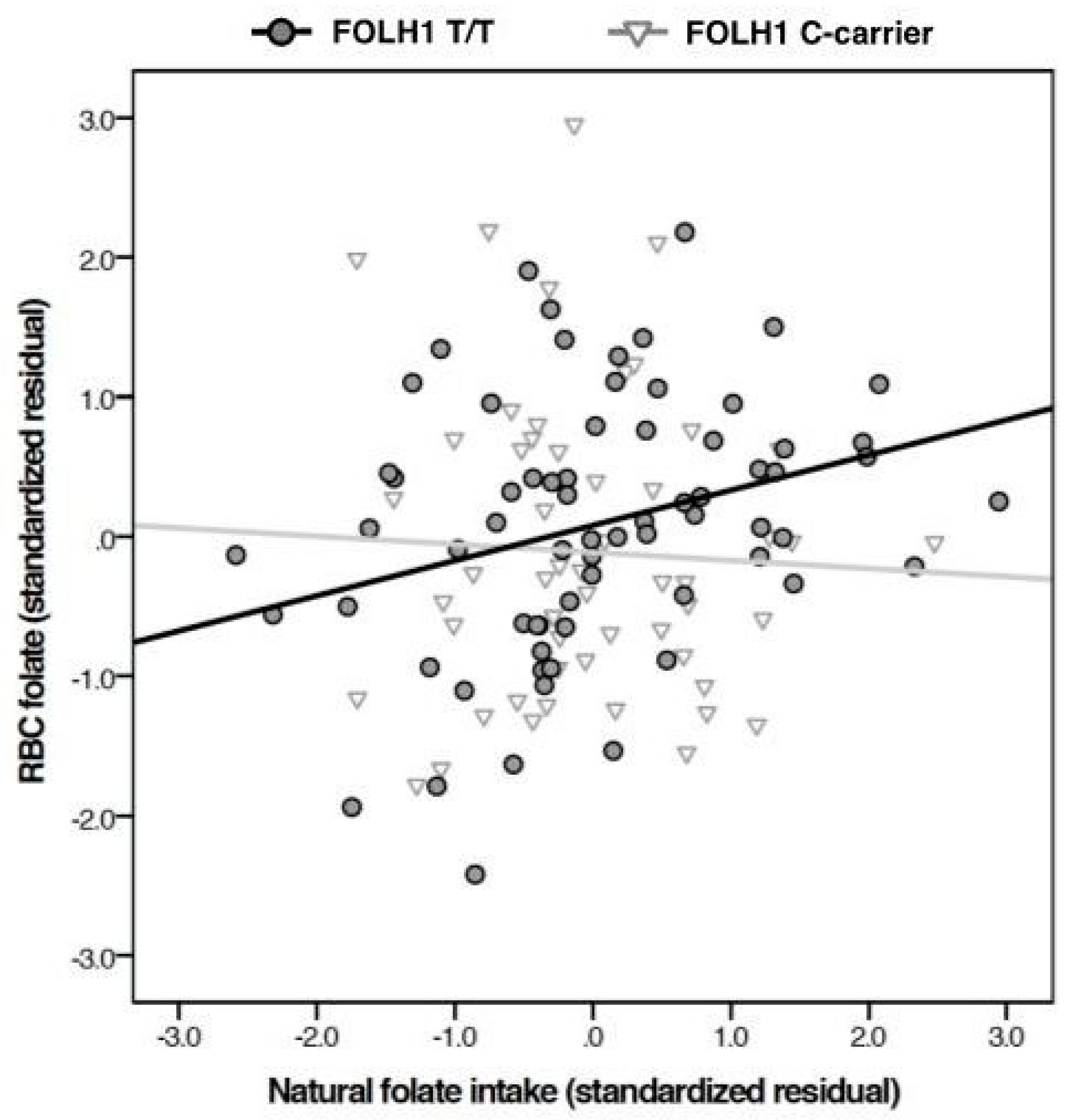
3. Results
3.1. Demographics
3.2. Folate Intake
3.3. RBC Folate Concentration
3.4. Predictors of RBC Folate Concentration
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Caudill, M.A. Folate bioavailability: Implications for establishing dietary recommendations and optimizing status. Am. J. Clin. Nutr. 2010. [Google Scholar] [CrossRef] [PubMed]
- Crider, K.S.; Bailey, L.B.; Berry, R.J. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients 2011, 3, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Roffman, J.L.; Brohawn, D.G.; Nitenson, A.Z.; Macklin, E.A.; Smoller, J.W.; Goff, D.C. Genetic variation throughout the folate metabolic pathway influences negative symptom severity in schizophrenia. Schizophr. Bull. 2013, 39, 330–338. [Google Scholar] [CrossRef] [PubMed]
- DeVos, L.; Chanson, A.; Liu, Z.; Ciappio, E.D.; Parnell, L.D.; Mason, J.B.; Tucker, K.L.; Crott, J.W. Associations between single nucleotide polymorphisms in folate uptake and metabolizing genes with blood folate, homocysteine, and DNA uracil concentrations. Am. J. Clin. Nutr. 2008, 88, 1149–1158. [Google Scholar] [PubMed]
- Halsted, C.H.; Ling, E.; Luthi-Carter, R.; Villanueva, J.A.; Gardner, J.M.; Coyle, J.T. Folypoly-γ-glutamate carboxypeptidase from pig jejunum molecular characterization and relation to glutamate carboxypeptidase II. J. Biol. Chem. 1998, 273, 20417–20424. [Google Scholar] [CrossRef] [PubMed]
- Salbaum, J.M.; Kappen, C. Genetic and epigenomic footprints of folate. Prog. Mol. Biol. Transl. Sci. 2012, 108, 129–158. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.J. The importance of folic acid. J. Gend. Specif. Med. 1999, 2, 24–28. [Google Scholar] [PubMed]
- Ohrvik, V.E.; Witthoft, C.M. Human folate bioavailability. Nutrients 2011, 3, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.; Magnus, P.; Schjølberg, S.; Stoltenberg, C.; Surén, P.; McKeague, I.W.; Davey Smith, G.; Reichborn-Kjennerud, T.; Susser, E. Folic acid supplements in pregnancy and severe language delay in children. JAMA 2011, 306, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Surén, P.; Roth, C.; Bresnahan, M.; Haugen, M.; Hornig, M.; Hirtz, D.; Lie, K.K.; Lipkin, W.I.; Magnus, P.; Reichborn-Kjennerud, T.; et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA 2013, 309, 570–577. [Google Scholar] [CrossRef] [PubMed]
- MRC Vitamin Study Research Group. Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet 1991, 338, 131–137. [Google Scholar] [CrossRef]
- Bower, C.; Stanley, F.J. Dietary folate as a risk factor for neural-tube defects: Evidence from a case-control study in Western Australia. Med. J. Aust. 1989, 150, 613–619. [Google Scholar] [PubMed]
- Centers for Disease Control. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR Recomm. Rep. 1992, 41, 1–7. [Google Scholar]
- Maberly, G.; Grummer-Strawn, L.; Jefferds, M.E.; Peña-Rosas, J.P.; Serdula, M.K.; Tyler, V.Q.; Berry, R.J.; Mulinare, J.; Parvanta, I.; Aburto, N.J. Trends in wheat-flour fortification with folic acid and iron—Worldwide, 2004 and 2007. MMWR 2008, 57, 8–10. [Google Scholar]
- Bailey, LB. Dietary reference intakes for folate: The debut of dietary folate equivalents. Nutr. Rev. 1998, 56, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.L.; Hung, J.; Caudill, M.A.; Urrutia, T.F.; Alamilla, A.; Perry, C.A.; Li, R.; Hata, H.; Cogger, E.A. A long-term controlled folate feeding study in young women supports the validity of the 1.7 multiplier in the dietary folate equivalency equation. J. Nutr. 2005, 135, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. 8, Folate. In Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press: Washington, DC, USA, 1998; pp. 196–305. [Google Scholar]
- Fulgoni, V.L., 3rd; Keast, D.R.; Bailey, R.L.; Dwyer, J. Foods, fortificants, and supplements: Where do Americans get their nutrients? J. Nutr. 2011, 141, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Ganji, V.; Kafai, M.R. Trends in serum folate, RBC folate, and circulating total homocysteine concentrations in the United States: Analysis of data from National Health and Nutrition Examination Surveys, 1988–1994, 1999–2000, and 2001–2002. J. Nutr. 2006, 136, 153–158. [Google Scholar] [PubMed]
- Choumenkovitch, S.F.; Selhub, J.; Wilson, P.W.; Rader, J.I.; Rosenberg, I.H.; Jacques, P.F. Folic acid intake from fortification in United States exceeds predictions. J. Nutr. 2002, 132, 2792–2798. [Google Scholar] [PubMed]
- Pfeiffer, C.M.; Hughes, J.P.; Lacher, D.A.; Bailey, R.L.; Berry, R.J.; Zhang, M.; Yetley, E.A.; Rader, J.I.; Sempos, C.T.; Johnson, C.L. Estimation of trends in serum and RBC folate in the U.S. population from pre- to postfortification using assay-adjusted data from the NHANES 1988–2010. J. Nutr. 2012, 142, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; Dodd, K.W.; Gahche, J.J.; Dwyer, J.T.; McDowell, M.A.; Yetley, E.A.; Sempos, C.A.; Burt, V.L.; Radimer, K.L.; Picciano, M.F. Total folate and folic acid intake from foods and dietary supplements in the United States: 2003–2006. Am. J. Clin. Nutr. 2010, 91, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Durga, J.; van Boxtel, M.P.; Schouten, E.G.; Kok, F.J.; Jolles, J.; Katan, M.B.; Verhoef, P. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: A randomised, double blind, controlled trial. Lancet 2007, 369, 208–216. [Google Scholar] [CrossRef]
- Van Oort, F.V.; Melse-Boonstra, A.; Brouwer, I.A.; Clarke, R.; West, C.E.; Katan, M.B.; Verhoef, P. Folic acid and reduction of plasma homocysteine concentrations in older adults: A dose-response study. Am. J. Clin. Nutr. 2003, 77, 1318–1323. [Google Scholar] [PubMed]
- Winkels, R.M.; Brouwer, I.A.; Verhoef, P.; van Oort, F.V.; Durga, J.; Katan, M.B. Gender and body size affect the response of erythrocyte folate to folic acid treatment. J. Nutr. 2008, 138, 1456–1461. [Google Scholar] [PubMed]
- Roffman, J.L.; Lamberti, J.S.; Achtyes, E.; Macklin, E.A.; Galendez, G.C.; Raeke, L.H.; Silverstein, N.J.; Smoller, J.W.; Hill, M.; Goff, D.C. Randomized multicenter investigation of folate plus vitamin B12 supplementation in schizophrenia. JAMA Psychiatry 2013, 70, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Honein, M.A.; Paulozzi, L.J.; Mathews, T.J.; Erickson, J.D.; Wong, L.Y. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA 2001, 285, 2981–2986. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.L.; Signore, C. Neural tube defect rates before and after food fortification with folic acid. Birth Defects Res. A Clin. Mol. Teratol. 2004, 70, 844–845. [Google Scholar] [CrossRef] [PubMed]
- Boulet, S.L.; Yang, Q.; Mai, C.; Mulinare, J.; Pfeiffer, C.M. Folate status in women of childbearing age, by race/ethnicity—United States, 1999–2000, 2001–2002, and 2003–2004. MMWR Morb. Mortal. Wkly. Rep. 2007, 55, 1377–1380. [Google Scholar]
- U.S. Centers for Disease Control and Prevention. Second National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population 2012; National Center for Environmental Health: Atlanta, GA, USA, 2012; pp. 13–71.
- Pfeiffer, C.M.; Sternberg, M.R.; Hamner, H.C.; Crider, K.S.; Lacher, D.A.; Rogers, L.M.; Bailey, R.L.; Yetley, E.A. Applying inappropriate cutoffs leads to misinterpretation of folate status in the US populations. Am. J. Clin. Nutr. 2016, 104, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Tinker, S.C.; Hamner, H.C.; Qi, Y.P.; Crider, K.S. U.S. women of childbearing age who are at possible increased risk of a neural tube defect-affected pregnancy due to suboptimal red blood cell concentrations, National Health and Nutrition Examination Survey 2007 to 2012. Birth Defects Res. A Clin. Mol. Teratol. 2015, 103, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Herbert, V. Making sense of laboratory tests of folate status: Folate requirements to sustain normality. Am. J. Hematol. 1987, 26, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L. The rise and fall of blood folate in the United States emphasizes the need to identify all sources of folic acid. Am. J. Clin. Nutr. 2007, 86, 528–530. [Google Scholar] [PubMed]
- Rader, J.I.; Weaver, C.M.; Angyal, G. Total folate in enriched cereal-grain products in the United States following fortification. Food Chem. 2000, 70, 275–289. [Google Scholar] [CrossRef]
- Johnston, K.E.; Tamura, T. Folate content in commercial white and whole wheat sandwich breads. J. Agric. Food Chem. 2004, 62, 6338–6340. [Google Scholar] [CrossRef] [PubMed]
- Póo-Prieto, R.; Haytowitz, D.B.; Holden, J.M.; Rogers, G.; Choumenkovitch, S.F.; Jacques, P.F.; Selhub, J. Use of the affinity/HPLC method for quantitative estimation of folic acid in enriched cereal-grain products. J. Nutr. 2006, 136, 3079–3083. [Google Scholar] [PubMed]
- Subar, A.F.; Midthune, D.; Kulldorff, M.; Brown, C.C.; Thompson, F.E.; Kipnis, V.; Schatzkin, A. Evaluation of alternative approaches to assign nutrient values to food groups in food frequency questionnaires. Am. J. Epidemiol. 2000, 152, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Revelle, W. Psych: Procedures for Personality and Psychological Research. Northwestern University: Evanston, IL, USA, 2015. Version = 1.5.8. Available online: http://CRAN.R-project.org/package=psych (accessed on 16 May 2017).
- Yang, Q.; Botto, L.D.; Gallagher, M.; Friedman, J.M.; Sanders, C.L.; Koontz, D.; Nikolova, S.; Erickson, J.D.; Steinberg, K. Prevalence and effects of gene-gene and gene-nutrient interactions on serum folate and serum total homocysteine concentrations in the United States: Findings from the third National health and Nutrition Examination Survey DNA Bank. Am. J. Clin. Nutr. 2008, 88, 232–246. [Google Scholar] [PubMed]
- Botto, L.D.; Moore, C.A.; Khoury, M.J.; Erickson, J.D. Neural-tube defects. NEJM 1999, 341, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Wilcken, B.; Bamforth, F.; Li, Z.; Zhu, H.; Ritvanen, A.; Redlund, M.; Stoll, C.; Alembik, Y.; Dott, B.; Czeizel, A.; et al. Geographical and ethnic variation of the 677C>T allele of 5,10 methylenetetrahydrofolate reductase (MTHFR): Findings from over 7000 newborns from 16 areas worldwide. J. Med. Genet. 2003, 40, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Guéant-Rodriguez, R.; Guéant, J.; Debard, R.; Thirion, S.; Hong, L.X.; Bronowicki, J.P.; Namour, F.; Chabi, N.W.; Sanni, A.; Anello, G.; et al. Prevalence of methylenetetrahydrofolate reductase 677T and 1298C alleles and folate status: A comparative study in Mexican, West African, and European populations. Am. J. Clin. Nutr. 2006, 83, 701–707. [Google Scholar] [PubMed]
- Bagley, P.J.; Selhub, J. A common mutation in the methylenetetrahydrofolate reductase gene is associated with an accumulation of formylated tetrahydrofolates in red blood cells. Proc. Natl. Acad. Sci. USA 1998, 95, 13217–13220. [Google Scholar] [CrossRef] [PubMed]
- Fazili, Z.; Pfeiffer, C.M.; Zhang, M.; Jain, R.B.; Koontz, D. Influence of 5,10-methylenetetrahydrofolate reductase polymorphism on whole-blood folate concentrations measured by LC-MS/MS, microbiologic assay, and bio-rad radioassay. Clin. Chem. 2008, 54, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Farrell, C.; Kirsch, S.; Herrmann, M. Red cell or serum folate: What to do in clinical practice? Clin. Chem. Lab. Med. 2013, 51, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.C.; Witte, J.S. Point: Population stratification: A problem for case-control studies of candidate gene associations? Cancer Epidemiol. Biomark. Prev. 2002, 11, 505–512. [Google Scholar]
- Thompson, F.E.; Subar, A.D. Dietary Assessment Methodology. In Nutrition in the Prevention of Disease, 2nd ed.; Academic Press: Burlington, MA, USA, 2008; pp. 2–39. [Google Scholar]
- Yetley, E.A.; Pfeiffer, C.M.; Phinney, K.W.; Fazili, Z.; Lacher, D.A.; Bailey, R.L.; Blackmore, S.; Bock, J.L.; Brody, L.C.; Carmel, R.; et al. Biomarkers of folate status in NHANES: A roundtable summary. Am. J. Clin. Nutr. 2011. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.R.; Tyagi, S.C. Homocysteine and reactive oxygen species in metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: The pleiotropic effects of folate supplementation. Nutr. J. 2004, 3, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, W.E.; Roberts, W.L. Comparison of five automated serum and whole blood folate assays. Am. J. Clin. Pathol. 2003, 120, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Guo, J.; Wang, J.; Wang, F.; Zhao, H.; Liu, C.; Wang, L.; Lu, X.; Wu, L.; Bao, Y.; et al. Glutamate carboxypeptidase II gene polymorphisms and neural tube defects in a high-risk Chinese population. Metab. Brain Dis. 2012, 27, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Shelnutt, K.P.; Gail, P.A.; Kauwell, C.M.; Chapman, J.F., III; Maneval, D.R.; Browdy, A.A.; Theriaque, D.W.; Bailey, L.B. Folate status response to controlled folate intake is affected in methylenetetrahydrofolate reductase 677C→T polymorphism in young women. J. Nutr. 2003, 133, 4107–4111. [Google Scholar] [PubMed]
- Melse-Boonstra, A.; Lievers, K.J.A.; Blom, H.J.; Verhoef, P. Bioavailability of polyglutamyl folic acid relative to that of monoglutamyl folic acid in subjects with different genotypes of the glutamate carboxypeptidase II gene. Am. J. Clin. Nutr. 2004, 80, 700–704. [Google Scholar] [PubMed]
- Lievers, K.J.A.; Kluijtmans, L.A.; Boers, G.H.; Verhoef, P.; den Heijer, M.; Trijbels, F.J.M.; Blom, H.J. Influence of a glutamate carboxypeptidase II (GCPII) polymorphism (1561C→T) on plasma homocysteine, folate and vitamin B12 levels and its relationship to cardiovascular disease risk. Atherosclerosis 2002, 164, 269–273. [Google Scholar] [CrossRef]
- Vargas-Martinez, C.; Ordovas, J.M.; Wilson, P.W.; Selhub, J. The glutamate carboxypeptidase gene II (C>T) polymorphism does not affect folate status in the Framingham Offspring cohort. J. Nutr. 2002, 132, 1176–1179. [Google Scholar] [PubMed]
- Devlin, A.M.; Ling, E.; Peerson, J.; Fernando, S.; Clarke, R.; Smith, A.D.; Halsted, C.H. Glutamate carboxypeptidase II: A polymorphism associated with lower levels of serum folate and hyperhomocystenemia. Hum. Mol. Genet. 2000, 9, 2837–2844. [Google Scholar] [CrossRef] [PubMed]
- Halsted, C.H.; Wong, D.H.; Peerson, J.M.; Warden, C.H.; Refsum, H.; Smith, A.D.; Nygärd, O.K.; Ueland, P.M.; Vollset, S.E.; Tell, G.S. Relations of glutamate carboxypeptidase II (GCPII) polymorphisms to folate and homocysteine concentrations and to scores of cognition, anxiety, and depression in a homogenous Norwegian population: The Hordaland Homocysteine Study. Am. J. Clin. Nutr. 2007, 86, 514–521. [Google Scholar] [PubMed]
- Eklöf, V.; Van Guelpen, B.; Hultdin, J.; Johansson, I.; Hallmans, G.; Palmqvist, R. The reduced folate carrier (RFC1) 80G>A and folate hydrolase 1 (FOLH1) 1561C>T polymorphisms and the risk of colorectal cancer: A nested case-referent study. Scand. J. Clin. Lab. Investig. 2009, 68, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Devlin, A.M.; Clarke, R.; Birks, J.; Grimely Evans, J.; Halsted, C.H. Interactions among polymorphism in folate-metabolizing genes and serum in total homocysteine concentrations in a healthy elderly population. Am. J. Clin. Nutr. 2006, 83, 708–713. [Google Scholar] [PubMed]
- Morin, J.; Devlin, A.M.; Leclerc, D.; Sabbaghian, N.; Halsted, C.H.; Ninnel, R.; Rozen, R. Evaluation of genetic variants in the reduced folate carrier and in glutamate carboxypeptidase II for spina bifida risk. Mol. Genet. Metab. 2003, 79, 197–200. [Google Scholar] [CrossRef]
- Freedman, L.S.; Commins, J.M.; Moler, J.E.; Willett, W.; Tinker, L.F.; Subar, A.F.; Spiegelman, D.; Rhodes, D.; Potischman, N.; Neuhouser, M.L.; et al. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for potassium and sodium intake. Am. J. Epidemiol. 2015, 181, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Mensink, G.B.M.; Fletcher, R.; Gurinovic, M.; Huybrechts, I.; Lafay, L.; Serra-Majem, L.; Szponar, L.; Tetens, I.; Verkaik-Kloosterman, J.; Baka, A.; et al. Mapping low micronutrients across Europe. Br. J. Nutr. 2013, 110, 755–773. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Hattori, M.; Wada, S.; Iwase, H.; Kadono, M.; Tatsumi, H.; Kuwahata, M.; Fukui, M.; Hasegawa, G.; Nakamura, N.; et al. Assessment of daily food and nutrient intake in Japanese type 2 diabetes mellitus patients using dietary reference intakes. Nutrients 2013, 5, 2276–2288. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Tachiki, A.; Horiuchi, H.; Mukaeda, K.; Morioka, M.; Fukuwatari, T.; Sasaki, S.; Jinno, Y. More than 50% of Japanese women with an intake of 150 µg dietary folate per 1000 kcal can maintain values above the cut-off. J. Nutr. Sci. Vitaminol. 2014, 60, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; Fulgoni, V.L.; Taylor, C.L.; Pfeiffer, C.M.; Thuppal, S.V.; McCabe, G.P.; Yetley, E.A. Correspondence of folate dietary intake and biomarker data. Am. J. Clin. Nutr. 2017, 105, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Block, G.; Subar, A.F. Estimates of nutrient intake from a food frequency questionnaire: The 1987 National Health Interview Survey. J. Am. Diet. Assoc. 1992, 92, 969–977. [Google Scholar] [PubMed]
- Lassale, C.; Guilbert, C.; Keogh, J.; Syrette, J.; Lange, K.; Cox, D.N. Estimating food intakes in Australia: Validation of Commonwealth Scientific and Industrial Research Organization (CSIRO) food frequency questionnaire against weighted dietary intakes. J. Hum. Nutr. Diet. 2009, 22, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Haftenberger, M.; Heuer, T.; Heidemann, C.; Kube, F.; Krems, C.; Mensink, G.B. Relative validation of a food frequency questionnaire for national health and nutrition monitoring. Nutr. J. 2010, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Yanagibori, R.; Amano, K. Self-administered diet history questionnaire developed for health education: A relative validation of the test-version by comparison with 3-day diet record in women. J. Epidemiol. 1998, 8, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Subar, A.; Thompson, F.E.; Kipnis, V.; Midthune, D.; Hurwitz, P.; McNutt, S.; McIntosh, A.; Rosenfeld, S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: The Eating at America’s Table Study. Am. J. Epidemiol. 2001, 154, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Zekovic, M.; Djekic-Ivankovic, M.; Nikolic, M.; Gurinovic, M.; Krajnovic, D.; Glibetic, M. Validity of the food frequency questionnaire assessing the folate intake in women of reproductive age living in a country without food fortification: Application of the method of triads. Nutrients 2017, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Thompson, F.E.; Kipnis, V.; Midthune, D.; Freedman, L.S.; Carroll, R.J.; Subar, A.F.; Brown, C.C.; Butcher, M.S.; Mouw, T.; Leitzmann, M.; et al. Performance of a food frequency questionnaire in the U.S. NIH-AARP (National Institutes of Healthy-American Association of Retired Persons) diet and health study. Public Health Nutr. 2008, 11, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Pavlíková, M.; Sokolová, J.; Janošiková, B.; Melenovská, P.; Krupková, L.; Zvárová, J.; Kožich, V. Rare allelic variants determine folate status in an unsupplemented European population. J. Nutr. 2012, 142, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Xie, H.; Wang, J.; Zhao, H.; Wang, F.; Liu, C.; Wang, L.; Lu, X.; Bao, Y.; Zou, J.; et al. The maternal folate hydrolase gene polymorphism is associated with neural tube defects in a high-risk Chinese population. Genes Nutr. 2013, 8, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Said, H.M.; Chatterjee, N.; Ul Haq, R.; Subramanian, V.S.; Ortiz, A.; Matherly, L.H.; Sirotnak, F.M.; Halsted, C.; Rubin, S.A. Adaptive regulation of intestinal folate uptake: Effect of dietary folate deficiency. Am. J. Cell Physiol. 2000, 279, C1889–C1895. [Google Scholar]
- Bailey, L.B.; Stover, P.J.; McNulty, H.; Fenech, M.F.; Gregory, J.F., III; Mills, J.L.; Pfeiffer, C.M.; Fazili, Z.; Zhang, M.; Ueland, P.M.; et al. Biomarkers of nutrition for development—Folate review. J. Nutr. 2015, 145, 1636S–1680S. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Chanarin, I.; Slavin, G.; Levi, A.J. Folate deficiency in the alcoholic—Its relation to clinical and haematological abnormalities, liver disease, and folate stores. Br. J. Haematol. 1975, 29, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Crider, K.S.; Devine, O.; Hao, I.; Dowling, N.F.; Li, S.; Molloy, A.M.; Li, Z.; Zhu, J.; Berry, R.J. Population red blood cell folate concentrations for prevention of neural tube defects: Bayesian model. BMJ 2014, 349, g4554. [Google Scholar] [CrossRef] [PubMed]
- Ayoola, A.B.; Stommel, M.; Nettleman, M.D. Late recognition of pregnancy as a predictor of adverse birth outcomes. Am. J. Obstet. Gynecol. 2009, 201, 156.e1–156.e6. [Google Scholar] [CrossRef] [PubMed]
- Finer, L.B.; Henshaw, S.K. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspect. Sex. Reprod. Health 2006, 38, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Orozco, A.M.; Yeung, L.F.; Guo, J.; Carriquiry, A.; Berry, R.J. Characteristics of U.S. adults with usual daily folic acid intake above the tolerable upper intake level: National health and Nutrition Examination Survey, 2003–2010. Nutrients 2016, 8, 195. [Google Scholar] [CrossRef] [PubMed]
| Total Sample (n = 265) | Males (n = 118) | Females (n = 147) | p-Value | |
|---|---|---|---|---|
| Age (years) ± SD | 23.6 ± 4.4 | 24.4 ± 4.5 | 22.9 ± 4.1 | 0.005 |
| Self-reported race (White/non-White) | 176/89 | 86/32 | 90/57 | 0.062 |
| Education (years) ± SD | 15.3 ± 2.5 | 15.4 ± 2.4 | 15.2 ± 2.6 | 0.46 |
| FOLH1 genotype (TT/C-carrier) | 131/115 | 63/50 | 68/65 | 0.55 |
| Men | Women | p-Value | |
|---|---|---|---|
| Variable | Mean ± SD | Mean ± SD | |
| Naturally occurring food folate (mcg) | 361 ± 197 | 309 ± 211 | 0.0059 |
| Folic acid from fortified foods (mcg DFE) | 432 ± 285 | 266 ± 177 | <0.0001 |
| Supplemental folic acid (mcg DFE) | 112 ± 221 | 136 ± 247 | 0.15 |
| Folate from all sources (mcg DFE) | 905 ± 482 | 712 ± 433 | 0.0002 |
| Percent of Men (n = 117) below EAR | Percent of Women (n = 147) below EAR | p-Value | |
|---|---|---|---|
| Naturally occurring food folate | 53.0 | 64.6 | 0.074 |
| Food folate | 6.0 | 20.4 | 0.0015 |
| Folate from all sources | 6.0 | 13.6 | 0.068 |
| Model Number | Variables | Beta | p-Value | Adjusted R2 | R2 Change | Significance of R2 Change |
|---|---|---|---|---|---|---|
| 1 | Log of naturally occurring food folate | 0.07 | 0.37 | −0.001 | 0. | 0.37 |
| 2 | Log of naturally occurring food folate | −0.09 | 0.26 | 0.0725 | 0.0736 | <0.001 |
| Log of total synthetic folic acid | 0.32 | <0.001 | ||||
| 3 | Log of naturally occurring food folate | −0.08 | 0.31 | 0.0836 | 0.0111 | 0.078 |
| Log of total synthetic folic acid | 0.32 | <0.001 | ||||
| Genotype | 0.13 | 0.078 | ||||
| 4 | Log of naturally occurring food folate | −0.13 | 0.27 | 0.0801 | −0.0036 | 0.57 |
| Log of total synthetic folic acid | 0.32 | <0.001 | ||||
| Genotype | −0.27 | 0.70 | ||||
| Genotype x log of naturally occurring food folate interaction | 0.40 | 0.57 |
| Model Number | Variables | Beta | p-Value | Adjusted R2 | R2 Change | Significance of R2 Change |
|---|---|---|---|---|---|---|
| 1 | Log of naturally occurring food folate | 0.03 | 0.77 | −0.008 | 0. | 0.77 |
| 2 | Log of naturally occurring food folate | −0.17 | 0.082 | 0.114 | 0.121 | <0.001 |
| Log of total synthetic folic acid | 0.41 | <0.001 | ||||
| 3 | Log of naturally occurring food folate | −0.17 | 0.079 | 0.115 | 0.001 | 0.27 |
| Log of total synthetic folic acid | 0.41 | <0.001 | ||||
| Genotype | 0.09 | 0.2773 | ||||
| 4 | Log of naturally occurring food folate | −0.42 | 0.008 | 0.138 | 0.023 | 0.043 |
| Log of total synthetic folic acid | 0.43 | <0.001 | ||||
| Genotype | −10.87 | 0.054 | ||||
| Genotype x log of naturally occurring food folate interaction | 10.99 | 0.043 |
| Model Number | Variables | Beta | p-Value | Adjusted R2 | R2 Change | Significance of R2 Change |
|---|---|---|---|---|---|---|
| 1 | Log of naturally occurring food folate | −0.15 (0.18) | 0.27 (0.14) | 0.004 (0.018) | 0. | 0.27 (0.14) |
| 2 | Log of naturally occurring food folate | −0.32 (−0.003) | 0.014 (0.98) | 0.191 (0.086) | 0.187 (0.068) | <0.001 (0.021) |
| Log of total synthetic folic acid | 0.48 (0.34) | <0.001 (0.021) | ||||
| 3 | Log of naturally occurring food folate | −0.32 (−0.01) | 0.016 (0.94) | 0.178 (0.091) | −0.0139 (0.005) | 0.79 (0.25) |
| Log of total synthetic folic acid | 0.48 (0.35) | <0.001 (0.018) | ||||
| Genotype | 0.03 (0.14) | 0.79 (0.25) | ||||
| 4 | Log of naturally occurring food folate | −0.88 (−0.07) | <0.001 (0.72) | 0.29 (0.071) | 0.112 (−0.02) | 0.003 (0.67) |
| Log of total synthetic folic acid | 0.54 (0.35) | <0.001 (0.019) | ||||
| Genotype | −40.17 (−0.44) | 0.0036 (0.75) | ||||
| Genotype x log of naturally occurring food folate interaction | 40.25 (0.59) | 0.0033 (0.67) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cummings, D.; Dowling, K.F.; Silverstein, N.J.; Tanner, A.S.; Eryilmaz, H.; Smoller, J.W.; Roffman, J.L. A Cross-Sectional Study of Dietary and Genetic Predictors of Blood Folate Levels in Healthy Young Adults. Nutrients 2017, 9, 994. https://doi.org/10.3390/nu9090994
Cummings D, Dowling KF, Silverstein NJ, Tanner AS, Eryilmaz H, Smoller JW, Roffman JL. A Cross-Sectional Study of Dietary and Genetic Predictors of Blood Folate Levels in Healthy Young Adults. Nutrients. 2017; 9(9):994. https://doi.org/10.3390/nu9090994
Chicago/Turabian StyleCummings, Daniel, Kevin F. Dowling, Noah J. Silverstein, Alexandra S. Tanner, Hamdi Eryilmaz, Jordan W. Smoller, and Joshua L. Roffman. 2017. "A Cross-Sectional Study of Dietary and Genetic Predictors of Blood Folate Levels in Healthy Young Adults" Nutrients 9, no. 9: 994. https://doi.org/10.3390/nu9090994
APA StyleCummings, D., Dowling, K. F., Silverstein, N. J., Tanner, A. S., Eryilmaz, H., Smoller, J. W., & Roffman, J. L. (2017). A Cross-Sectional Study of Dietary and Genetic Predictors of Blood Folate Levels in Healthy Young Adults. Nutrients, 9(9), 994. https://doi.org/10.3390/nu9090994





