Flavonoid-Rich Extract of Paulownia fortunei Flowers Attenuates Diet-Induced Hyperlipidemia, Hepatic Steatosis and Insulin Resistance in Obesity Mice by AMPK Pathway
Abstract
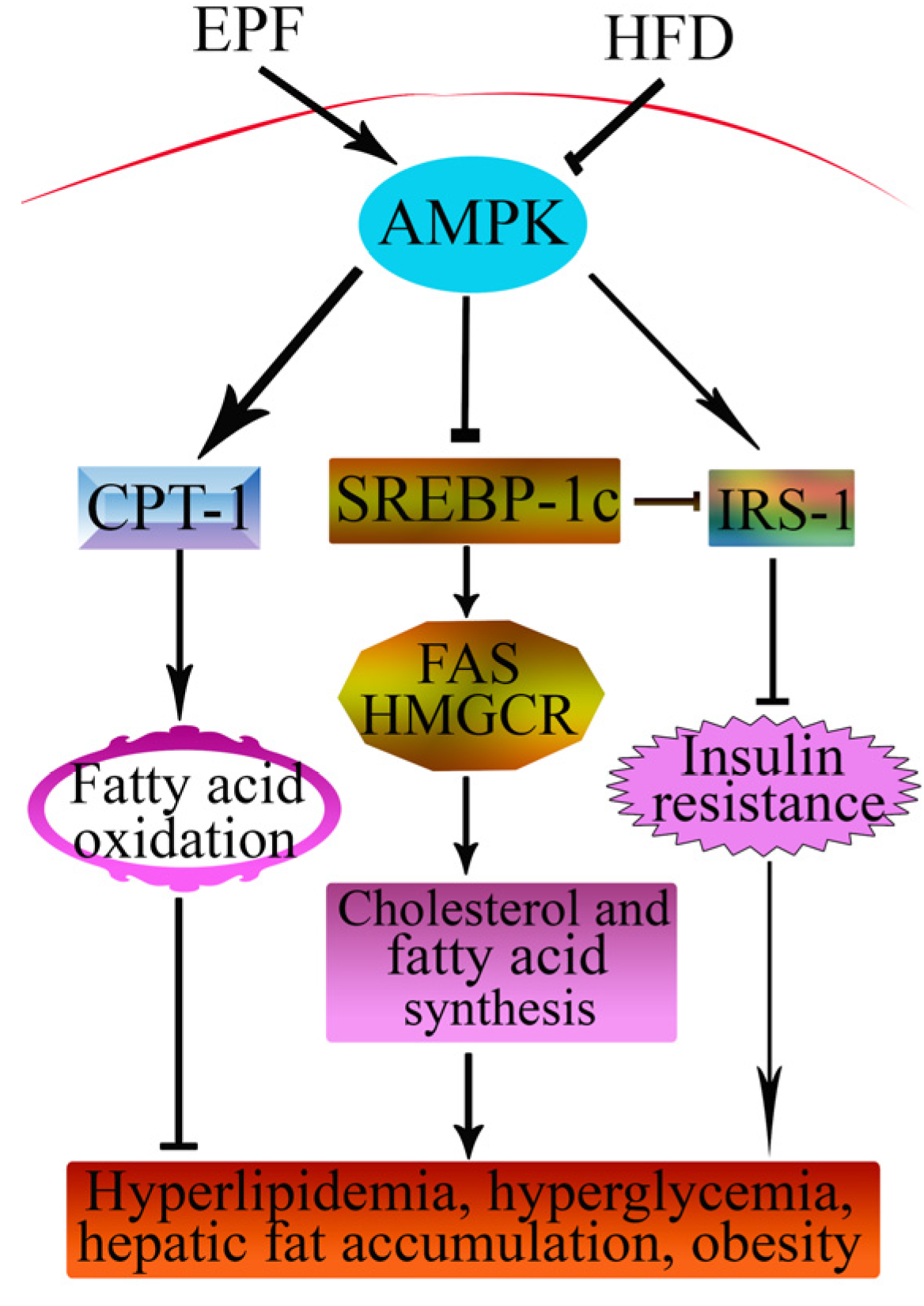
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Sample Preparation
2.3. Cell Culture and Treatments
2.4. Animals and Experimental Procedure
2.5. Biochemical Analysis
2.6. Oral Glucose Tolerance
2.7. Oil Red O Staining
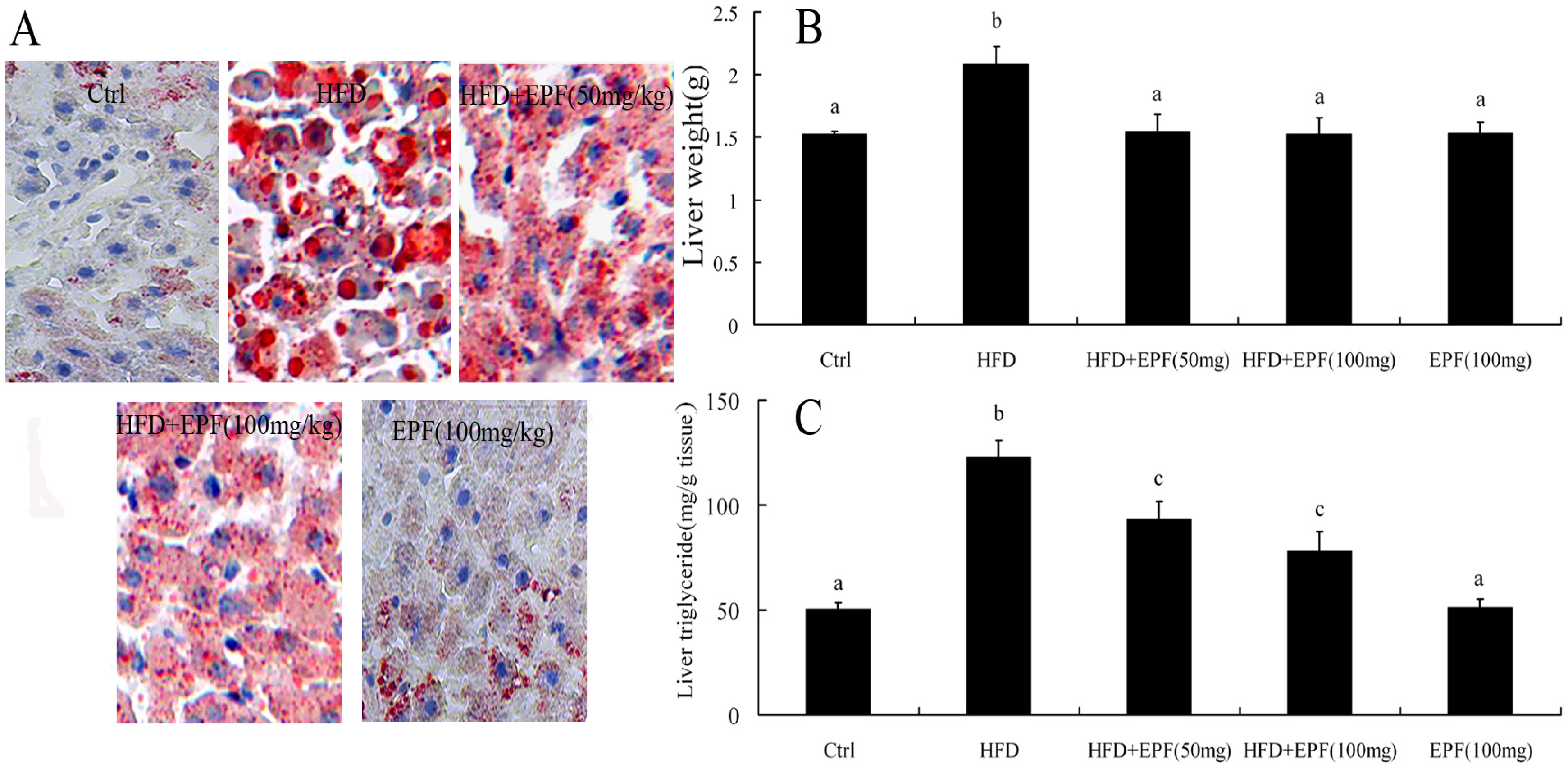
2.8. Measurement of Liver Triglyceride Content
2.9. Western Blot Analyses
2.10. Statistical Analysis
3. Results
3.1. Identification of Purified EPF
3.2. General Characteristics
3.3. Liver Damage Parameters
3.4. Plasma Glucose and Insulin Concentrations
3.5. Serum Lipid Profiles
3.6. Hepatic Lipid Accumulation
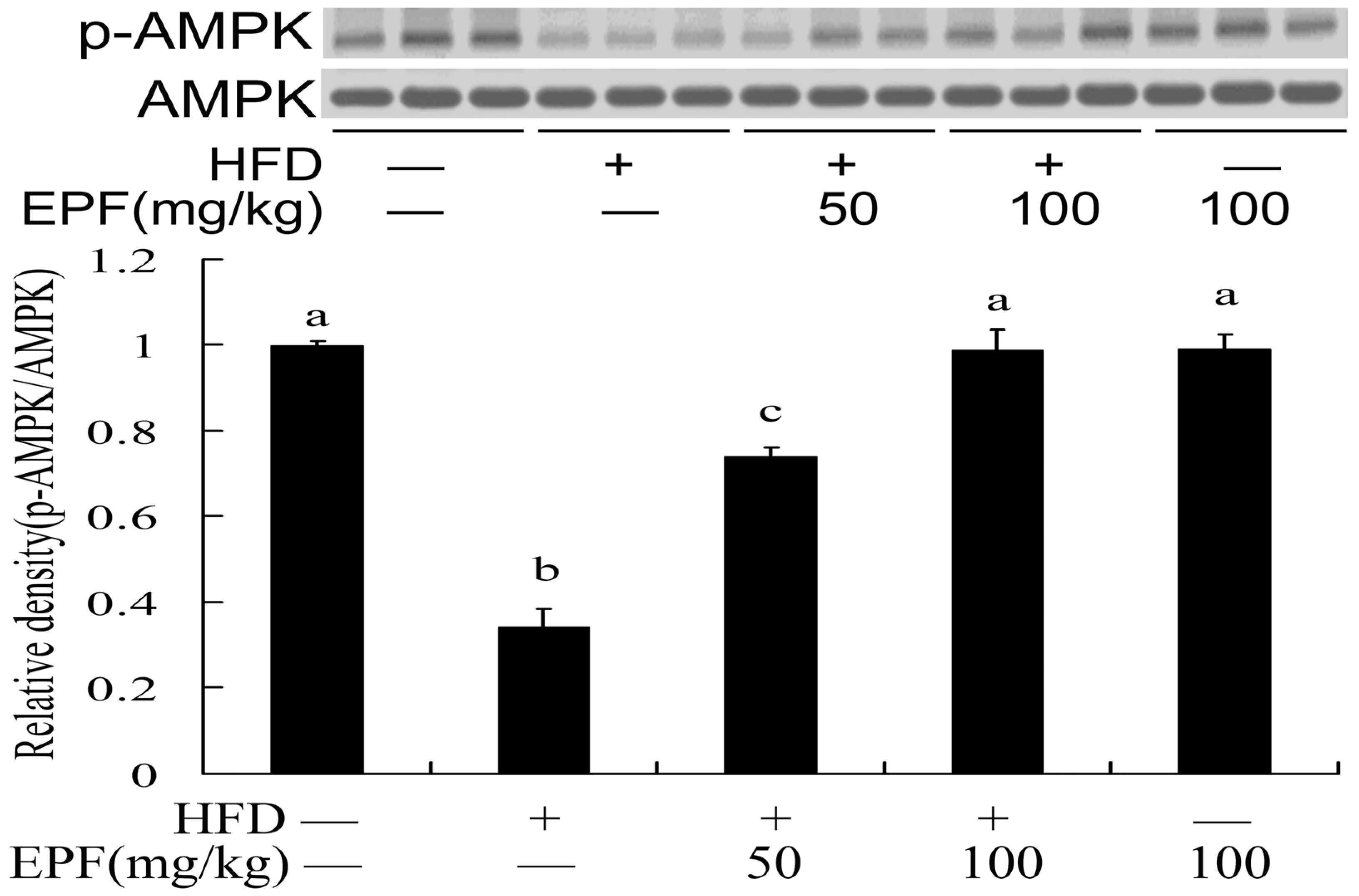
3.7. Hepatic AMPK Activation
3.8. AMPK Activation in HepG2 Cells
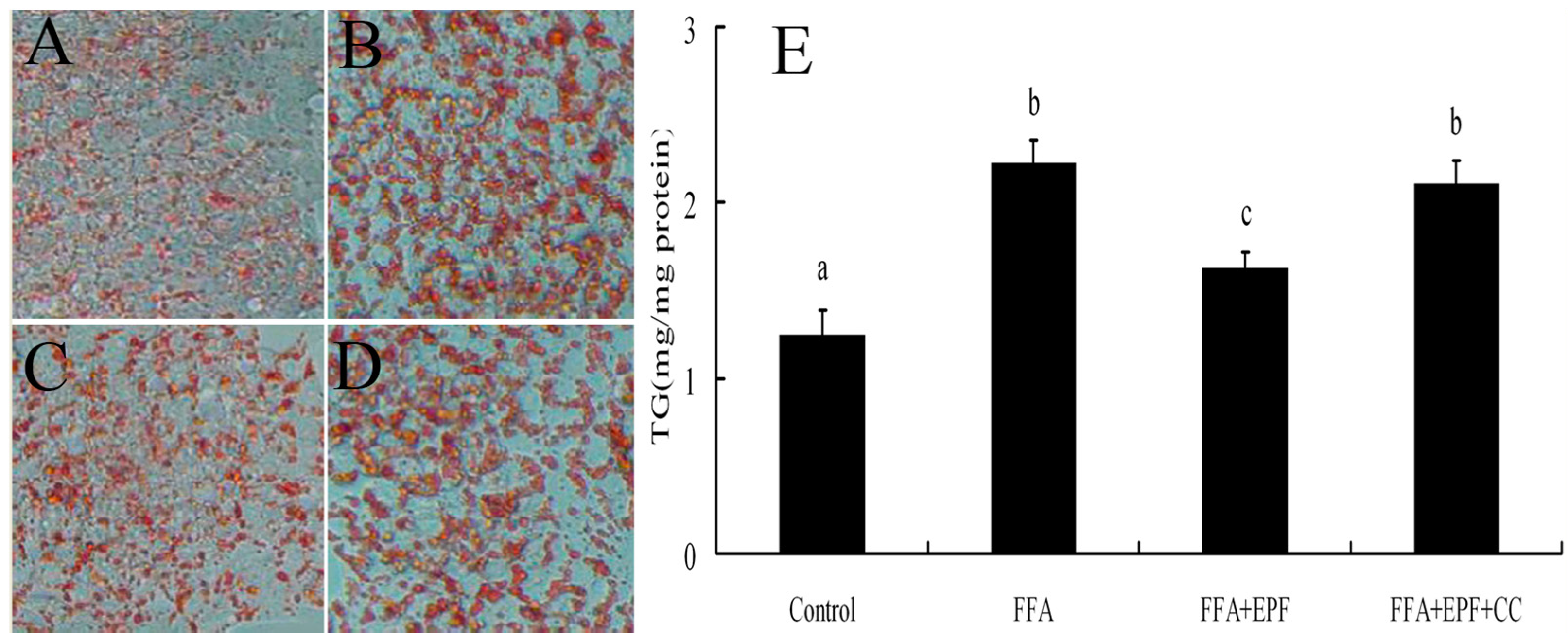
3.9. AMPK Inhibition Reduces the Effect of EPF on Lipid Accumulation in FFA-Exposed Hepatocytes
3.10. Hepatic Expressions of Proteins Associated with Lipid Metabolism in Livers
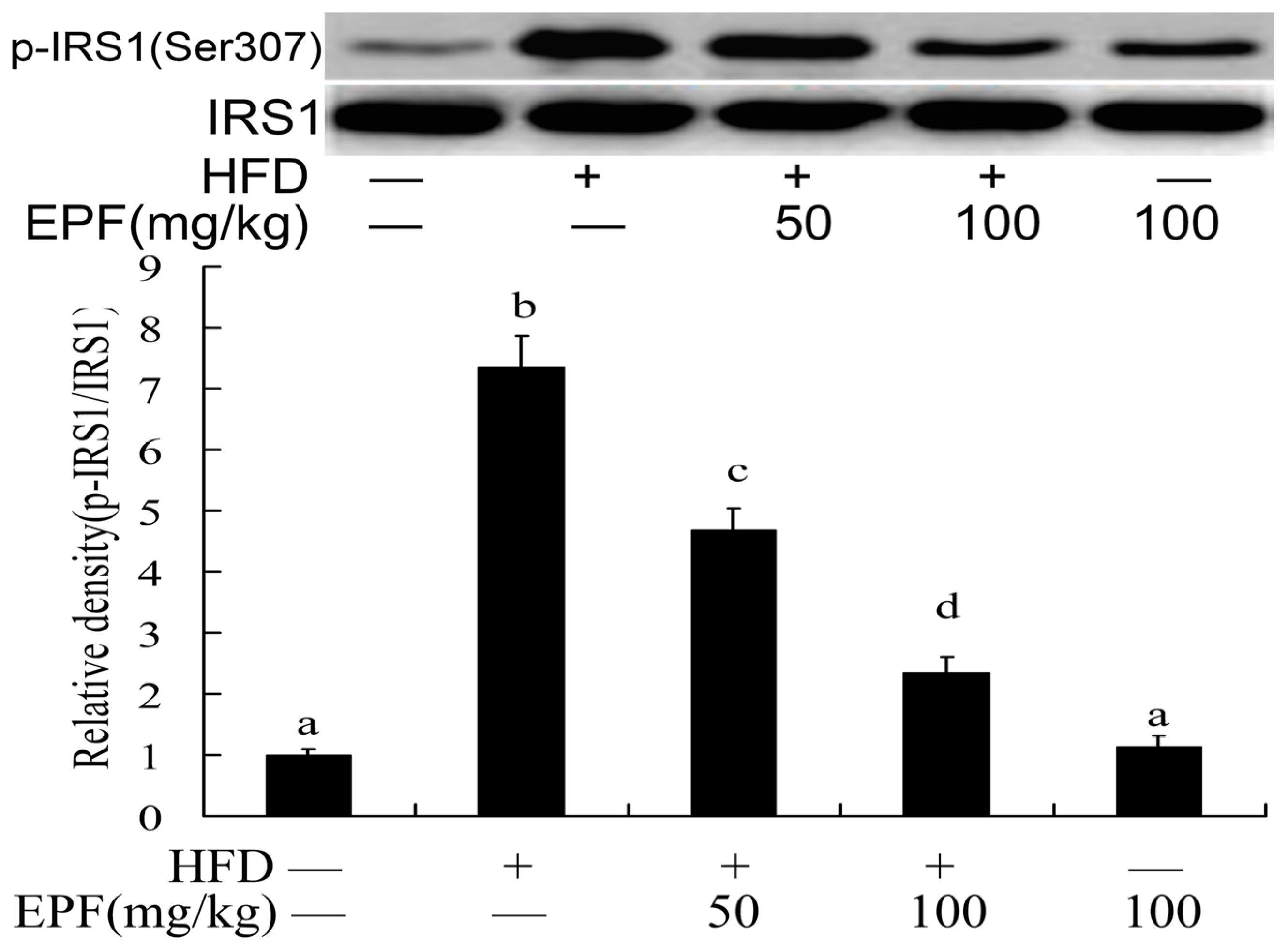
3.11. Hepatic Activation of IRS-1
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| AMPK | AMP-activated kinase |
| CPT1 | the carnitine palmitoyltransferase 1 |
| EPF | the extract from Paulownia fortunei flowers |
| FAS | fatty acid synthase |
| HFD | high fat diet |
| HMGCR | 3-hydroxy-3-methylglutaryl-CoA reductase |
| IRS-1 | insulin receptor substrate 1 |
| SREBP-1c | sterol regulatory element binding protein 1c |
References
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yki-Järvinen, H. Nutritional modulation of non-alcoholic fatty liver disease and insulin resistance. Nutrients 2015, 7, 9127–9138. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMP-activated protein kinase: A target for drugs both ancient and modern. Chem. Biol. 2012, 19, 1222–1236. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.T.; Kwon, D.Y.; Yoon, S.H. AMP-activated protein kinase: A potential target for the diseases prevention by natural occurring polyphenols. New Biotechnol. 2009, 26, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Firenzuoli, F.; Gori, L. Herbal medicine today: Clinical and research issues. Evid. Based Complement. Altern. Med. 2007, 4, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Šmejkal, K.; Svačinová, J.; Šlapetová, T.; Schneiderová, K.; Dall’Acqua, S.; Innocenti, G.; Závalová, V.; Kollár, P.; Chudík, S.; Marek, R.; et al. Cytotoxic activities of several geranyl-substituted flavanones. J. Nat. Prod. 2010, 73, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.Y.; Jin, X.; Tang, W.Z.; Wang, X.J.; Zhao, Y.X. New geranylated flavanones from the fruits of Paulownia catalpifolia Gong Tong with their anti-proliferative activity on lung cancer cells A549. Bioorg. Med. Chem. Lett. 2015, 25, 3686–3689. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Lee, C.; Lee, J.W.; Lee, D.; Kim, Y.; Hong, J.T.; Kim, J.S.; Kim, J.H.; Lee, M.K.; Hwang, B.Y. Geranylated Flavanones from Paulownia coreana and their inhibitory effects on nitric oxide production. Chem. Pharm. Bull. 2015, 63, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.K.; Curtis-Long, M.J.; Lee, K.H.; Kim, D.W.; Ryu, H.W.; Yuk, H.J.; Park, K.H. Geranylated flavonoids displaying SARS-CoV papain-like protease inhibition from the fruits of Paulownia tomentosa. Bioorg. Med. Chem. 2013, 21, 3051. [Google Scholar] [CrossRef] [PubMed]
- Navrátilová, A.; Nešuta, O.; Vančatová, I.; Čížek, A.; Varela-M, R.E.; López-Abán, J.; Villa-Pulgarin, J.A.; Mollinedo, F.; Muro, A.; Žemličková, H.; et al. C-Geranylated flavonoids from Paulownia tomentosa fruits with antimicrobial potential and synergistic activity with antibiotics. Pharm. Biol. 2016, 54, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.K.; Ryu, Y.B.; Curtis-Long, M.J.; Ryu, H.W.; Yuk, H.J.; Kim, D.W.; Kim, H.J.; Lee, W.S.; Park, K.H. Cholinestrase inhibitory effects of geranylated flavonoids from Paulownia tomentosa fruits. Bioorg. Med. Chem. 2012, 20, 2595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.F.; Li, C. Flavones from flowers of Paulownia fortunei. Zhongguo Zhong Yao Za Zhi 2008, 33, 2629–2632. [Google Scholar] [PubMed]
- Li, X.Q.; Zhang, P.F.; Duan, W.D.; Zhang, D.L.; Li, C. Studies on the chemical constituents from Flower of Paulownia fortunei. Zhong Yao Cai 2009, 32, 1227–1229. [Google Scholar] [PubMed]
- Liu, C.M.; Ma, J.Q.; Sun, Y.Z. Protective role of puerarin on lead-induced alterations of the hepatic glutathione antioxidant system and hyperlipidemia in rats. Food Chem. Toxicol. 2011, 49, 3119–3127. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Q.; Ding, J.; Zhao, H.; Liu, C.M. Puerarin attenuates carbon tetrachloride-induced liver oxidative stress and hyperlipidaemia in mouse by JNK/c-Jun/CYP7A1 pathway. Basic Clin. Pharmacol. Toxicol. 2014, 115, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Samad, N.B.; Debnath, T.; Jin, H.L.; Lee, B.R.; Park, P.J.; Lee, S.Y.; Lim, B.O. Antioxidant activity of Benincasa hispida seeds. J. Food Biochem. 2013, 37, 388–395. [Google Scholar] [CrossRef]
- Sakakibara, H.; Honda, Y.; Nakagawa, S.; Ashida, H.; Kanazawa, K. Simultaneous determination of all polyphenols in vegetables, fruits, and teas. J. Agric. Food Chem. 2003, 51, 571–581. [Google Scholar] [CrossRef] [PubMed]
- De Las Heras, N.; Valero-Muñoz, M.; Martín-Fernández, B.; Ballesteros, S.; López-Farré, A.; Ruiz-Roso, B.; Lahera, V. Molecular factors involved in the hypolipidemic- and insulin-sensitizing effects of a ginger (Zingiber officinale Roscoe) extract in rats fed a high-fat diet. Appl. Physiol. Nutr. Metab. 2017, 42, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.S.; Hong, S.W.; Yeon, S.H.; Kim, Y.M.; Um, K.A.; Kim, J.H.; Kim, H.J.; Chang, K.C.; Park, S.W. Magnolia of officinalis attenuates free fatty acid-induced lipogenesis via AMPK phosphorylation in hepatocytes. J. Ethnopharmacol. 2014, 157, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Porto, L.C.; da Silva, J.; Ferraz, A.B.; Ethur, E.M.; Porto, C.D.; Marroni, N.P.; Picada, J.N. The antidiabetic and antihypercholesterolemic effects of an aqueous extract from pecan shells in wistar rats. Plant Foods Hum. Nutr. 2015, 70, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Naowaboot, J.; Wannasiri, S.; Pannangpetch, P. Morin attenuates hepatic insulin resistance in high-fat-diet-induced obese mice. J. Physiol. Biochem. 2016, 72, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Dai, G.; Zheng, X. Mulberry anthocyanin extract ameliorates insulin resistance by regulating PI3K/AKT pathway in HepG2 cells and db/db mice. J. Nutr. Biochem. 2016, 36, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Sudhakara, G.; Mallaiah, P.; Sreenivasulu, N.; Sasi Bhusana Rao, B.; Rajendran, R.; Saralakumari, D. Beneficial effects of hydro-alcoholic extract of Caralluma fimbriata against high-fat diet-induced insulin resistance and oxidative stress in Wistar male rats. J. Physiol. Biochem. 2014, 70, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Ragab, S.M.M.; Elghaffar, S.K.A.; El-Metwally, T.H.; Badr, G.; Mahmoud, M.H. Effect of a high fat, high sucrose diet on the promotion of non-alcoholic fatty liver disease in male rats: The ameliorative role of three natural compounds. Lipids Health Dis. 2015, 14, 83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Hartnett, S.; Sample, A.; Schnack, S.; Li, Y. High fat diet induced alterations of atrial electrical activities in mice. Am. J. Cardiovasc. Dis. 2016, 6, 1–9. [Google Scholar] [PubMed]
- Kochi, T.; Shimizu, M.; Terakura, D.; Baba, A.; Ohno, T.; Kubota, M.; Shirakami, Y.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. Non-alcoholic steatohepatitis and preneoplastic lesions develop in the liver of obese and hypertensive rats: Suppressing effects of EGCG on the development of liver lesions. Cancer Lett. 2014, 342, 60–69. [Google Scholar] [CrossRef] [PubMed]
- El-Domiaty, M.M.; Wink, M.; Abdel Aal, M.M.; Abou-Hashem, M.M.; Abd-Alla, R.H. Antihepatotoxic activity and chemical constituents of Buddleja asiatica Lour. Z. Naturforsch. C 2009, 64, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Zhou, Z.; Strappe, P.; Blanchard, C. A comparison of RS4-type resistant starch to RS2-type resistant starch in suppressing oxidative stress in high-fat-diet-induced obese rats. Food Funct. 2017, 8, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.Y.; Jung, U.J.; Park, T.; Yun, J.W.; Choi, M.S. Luteolin attenuates hepatic steatosis and insulin resistance through the interplay between the liver and adipose tissue in mice with diet-induced obesity. Diabetes 2015, 64, 1658–1669. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Cho, Y.Y.; Choi, M.S. Apigenin ameliorates dyslipidemia, hepatic steatosis and insulin resistance by modulating metabolic and transcriptional profiles in the liver of high-fat diet-induced obese mice. Nutrients 2016, 8, 305. [Google Scholar] [CrossRef] [PubMed]
- Hoek-van den Hil, E.F.; van Schothorst, E.M.; van der Stelt, I.; Swarts, H.J.; van Vliet, M.; Amolo, T.; Vervoort, J.J.; Venema, D.; Hollman, P.C.; Rietjens, I.M.; et al. Direct comparison of metabolic health effects of the flavonoids quercetin, hesperetin, epicatechin, apigenin and anthocyanins in high-fat-diet-fed mice. Genes Nutr. 2015, 10, 469. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jin, H.; Oh, S.Y.; Ji, G.E. Anti-obese effects of two Lactobacilli and two Bifidobacteria on ICR mice fed on a high fat diet. Biochem. Biophys. Res. Commun. 2016, 480, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, W.; Zhao, J.; Wang, J.; Qu, W. Hypolipidaemic and hepatoprotective effects of ethanolic and aqueous extracts from Asparagus officinalis L. by-products in mice fed a high-fat diet. J. Sci. Food Agric. 2010, 90, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.J.; Jegal, J.; Ahn, J.; Kim, J.; Yang, M.H. Anti-obesity effect of Dioscorea oppositifolia extract in high-fat diet induced obese mice and its chemical characterization. Biol. Pharm. Bull. 2016, 39, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, Y.; Liu, C.; Xu, D.; Zhang, R.; Cheng, Y.; Pan, Y.; Huang, C.; Chen, Y. Luteolin alleviates alcoholic liver disease induced by chronic and binge ethanol feeding in mice. J. Nutr. 2014, 144, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Jayanthy, G.; Roshana Devi, V.; Ilango, K.; Subramanian, S.P. Rosmarinic acid mediates mitochondrial biogenesis in insulin resistant skeletal muscle through activation of AMPK. J. Cell. Biochem. 2017, 118, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Wu, W.; Shi, J.; Liang, H.; Yin, W.; Chen, Y.; Tang, S.; Cao, S.; Cai, M.; Shen, S.; et al. Role for sterol regulatory element binding protein-1c activation in mediating skeletal muscle insulin resistance via repression of rat insulin receptor substrate-1 transcription. Diabetologia 2014, 57, 592–602. [Google Scholar] [CrossRef] [PubMed]


| Compound | Content (mg/g Dry Weight) |
|---|---|
| Apigenin | 16.63 ± 0.01 |
| Luteolin | 7.16 ± 0.04 |
| Rutin | 1.87 ± 0.02 |
| Luteolin 7-O-glucoside | 9.26 ± 0.02 |
| Kaempferol 3-O-glucoside | 5.17 ± 0.01 |
| Apigenin 7-O-glucoside | 4.13 ± 0.03 |
| Quercetin 3-O-glucoside | 3.95 ± 0.04 |
| Parameter | SND | HFD | HFD + EPF (50 mg/kg) | HFD + EPF (100 mg/kg) | SND + EPF (100 mg/kg) |
|---|---|---|---|---|---|
| Body weight gain (g) | 10.26 ± 1.93 a | 17.85 ± 3.12 b | 13.67 ± 3.04 c | 12.27 ± 2.71 c | 10.09 ± 2.18 a |
| Food intake (g/day) | 4.82 ± 0.15 a | 3.93 ± 0.22 b | 3.41 ± 0.13 c | 3.42 ± 0.29 c | 4.79 ± 0.24 a |
| Calorie intake (kcal/g/day) | 14.94 ± 0.47 a | 20.04 ± 1.12 b | 17.39 ± 0.66 c | 17.44 ± 1.48 c | 14.85 ± 0.75 a |
| Parameter | SND | HFD | HFD + EPF (50 mg/kg) | HFD + EPF (100 mg/kg) | SND + EPF (100 mg/kg) |
|---|---|---|---|---|---|
| ALT (U/L) | 28.32 ± 1.38 a | 51.91 ± 2.16 b | 42.36 ± 1.83 c | 36.51 ± 2.11 d | 28.43 ± 2.07 a |
| AST (U/L) | 42.86 ± 2.14 a | 60.97 ± 1.83 b | 44.73 ± 3.21 c | 43.68 ± 2.54 c | 42.89 ± 3.19 a |
| Glucose (mM) | 7.65 ± 0.48 a | 15.38 ± 2.05 b | 11.12 ± 1.25 c | 9.93 ± 1.01 d | 7.68 ± 0.27 a |
| Insulin (mU/L) | 3.38 ± 0.17 a | 5.45 ± 1.32 b | 4.26 ± 0.42 c | 3.82 ± 0.24 d | 3.39 ± 0.23 a |
| HOMA-IR | 1.16 ± 0.02 a | 3.73 ± 0.04 b | 2.11 ± 0.05 c | 1.69 ± 0.03 d | 1.16 ± 0.01 a |
| TC (mM) | 2.92 ± 0.21 a | 7.06 ± 0.34 b | 5.27 ± 0.19 c | 4.46 ± 0.32 d | 2.91 ± 0.16 a |
| TG (mM) | 0.83 ± 0.11 a | 1.57 ± 0.14 b | 1.19 ± 0.12 c | 0.97 ± 0.09 d | 0.82 ± 0.14 a |
| HDL (mM) | 1.59 ± 0.15 a | 1.36 ± 0.13 b | 1.49 ± 0.11 c | 1.53 ± 0.12 a | 1.59 ± 0.21 a |
| LDL (mM) | 0.41 ± 0.06 a | 2.63 ± 0.21 b | 1.67 ± 0.13 c | 1.54 ± 0.17 c | 0.40 ± 0.09 a |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Ma, J.; Sun, J.; Cheng, C.; Feng, Z.; Jiang, H.; Yang, W. Flavonoid-Rich Extract of Paulownia fortunei Flowers Attenuates Diet-Induced Hyperlipidemia, Hepatic Steatosis and Insulin Resistance in Obesity Mice by AMPK Pathway. Nutrients 2017, 9, 959. https://doi.org/10.3390/nu9090959
Liu C, Ma J, Sun J, Cheng C, Feng Z, Jiang H, Yang W. Flavonoid-Rich Extract of Paulownia fortunei Flowers Attenuates Diet-Induced Hyperlipidemia, Hepatic Steatosis and Insulin Resistance in Obesity Mice by AMPK Pathway. Nutrients. 2017; 9(9):959. https://doi.org/10.3390/nu9090959
Chicago/Turabian StyleLiu, Chanmin, Jieqiong Ma, Jianmei Sun, Chao Cheng, Zhaojun Feng, Hong Jiang, and Wei Yang. 2017. "Flavonoid-Rich Extract of Paulownia fortunei Flowers Attenuates Diet-Induced Hyperlipidemia, Hepatic Steatosis and Insulin Resistance in Obesity Mice by AMPK Pathway" Nutrients 9, no. 9: 959. https://doi.org/10.3390/nu9090959
APA StyleLiu, C., Ma, J., Sun, J., Cheng, C., Feng, Z., Jiang, H., & Yang, W. (2017). Flavonoid-Rich Extract of Paulownia fortunei Flowers Attenuates Diet-Induced Hyperlipidemia, Hepatic Steatosis and Insulin Resistance in Obesity Mice by AMPK Pathway. Nutrients, 9(9), 959. https://doi.org/10.3390/nu9090959





