Quercetin and Green Tea Extract Supplementation Downregulates Genes Related to Tissue Inflammatory Responses to a 12-Week High Fat-Diet in Mice
Abstract
1. Introduction
2. Materials and Methods
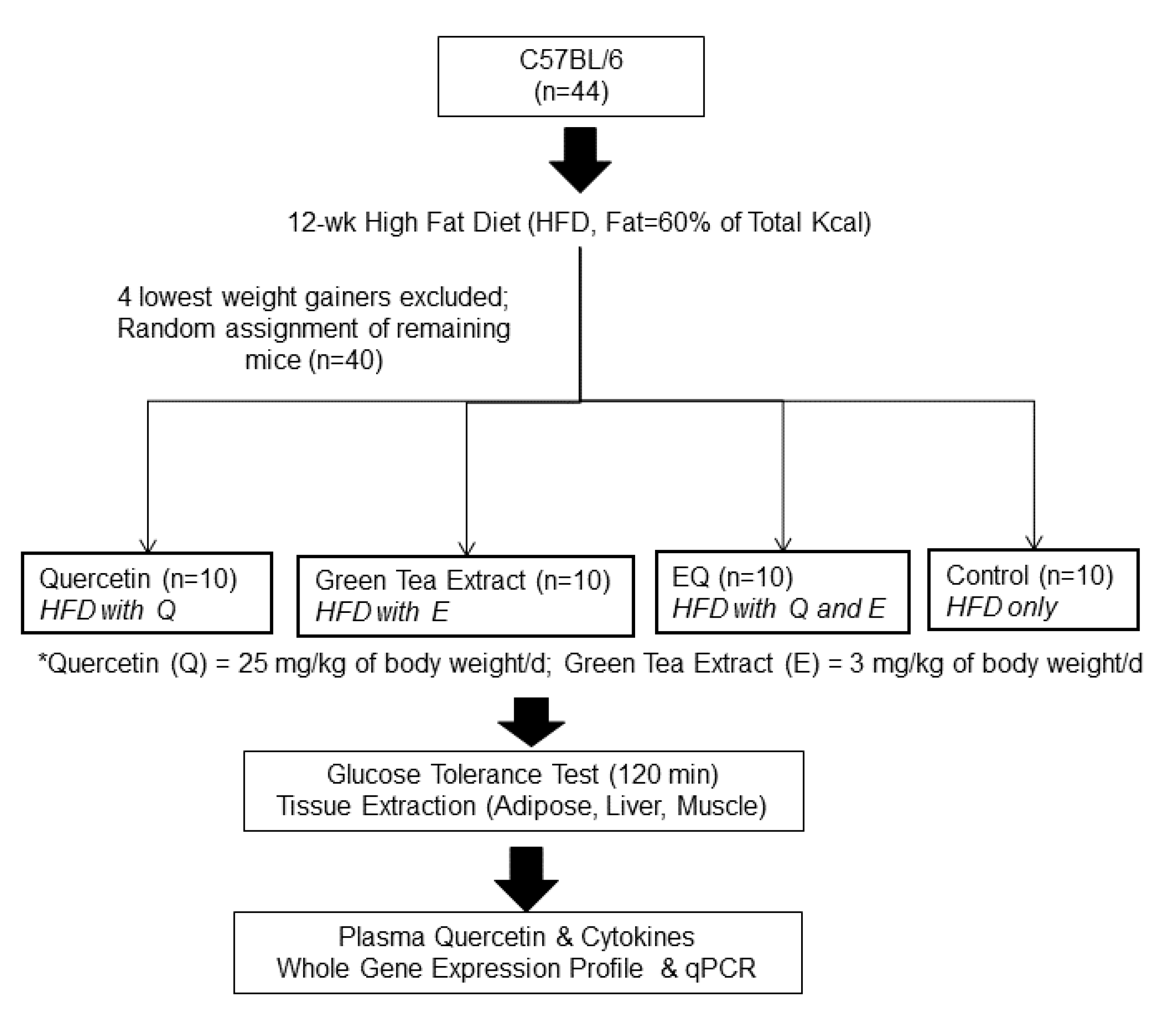
2.1. Animals and Experimental Design
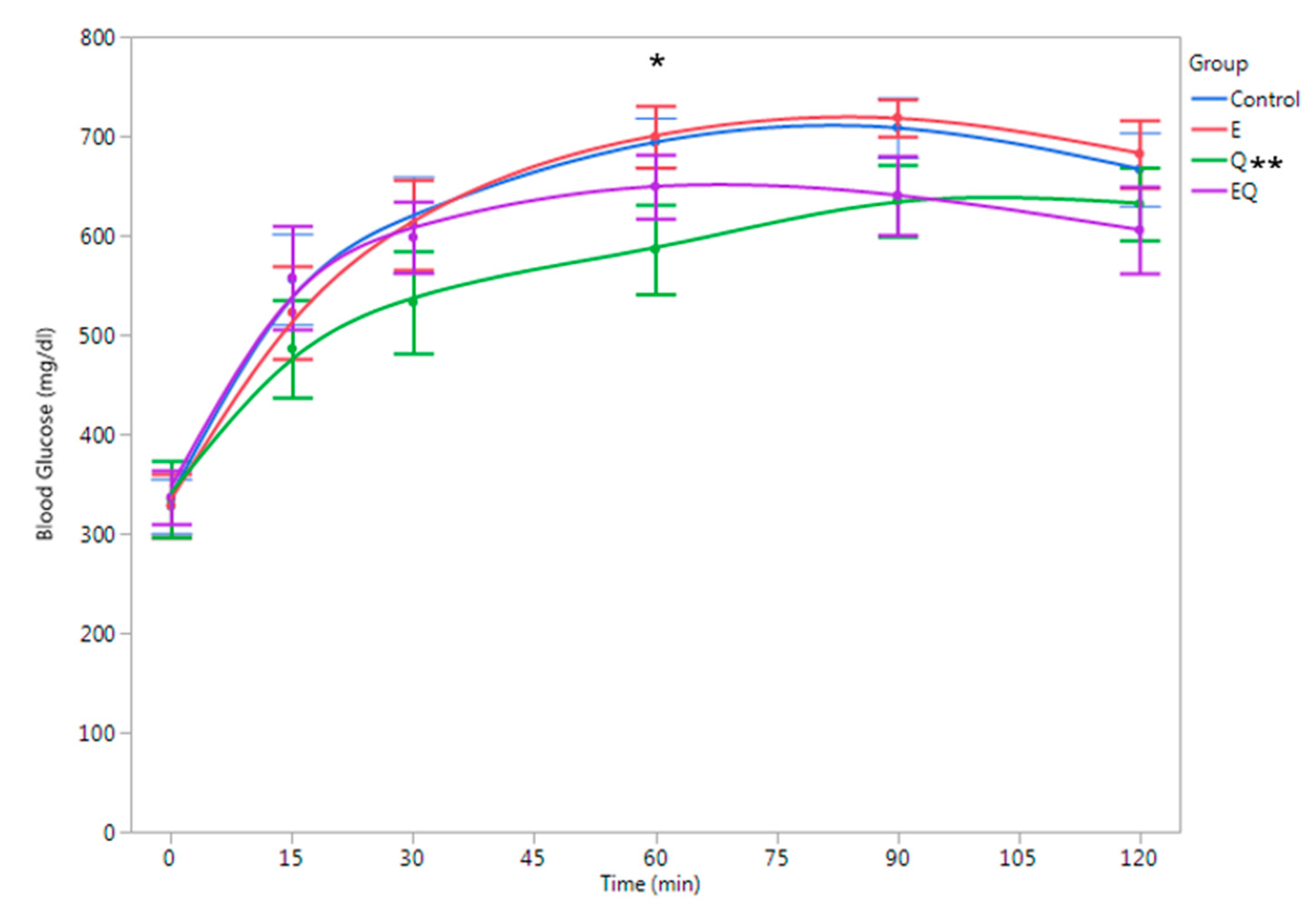
2.2. Glucose Tolerance Test and Blood and Tissue Collection
2.3. Biochemical Assays
2.4. Genomic Analysis
2.5. Statistical Analysis
3. Results
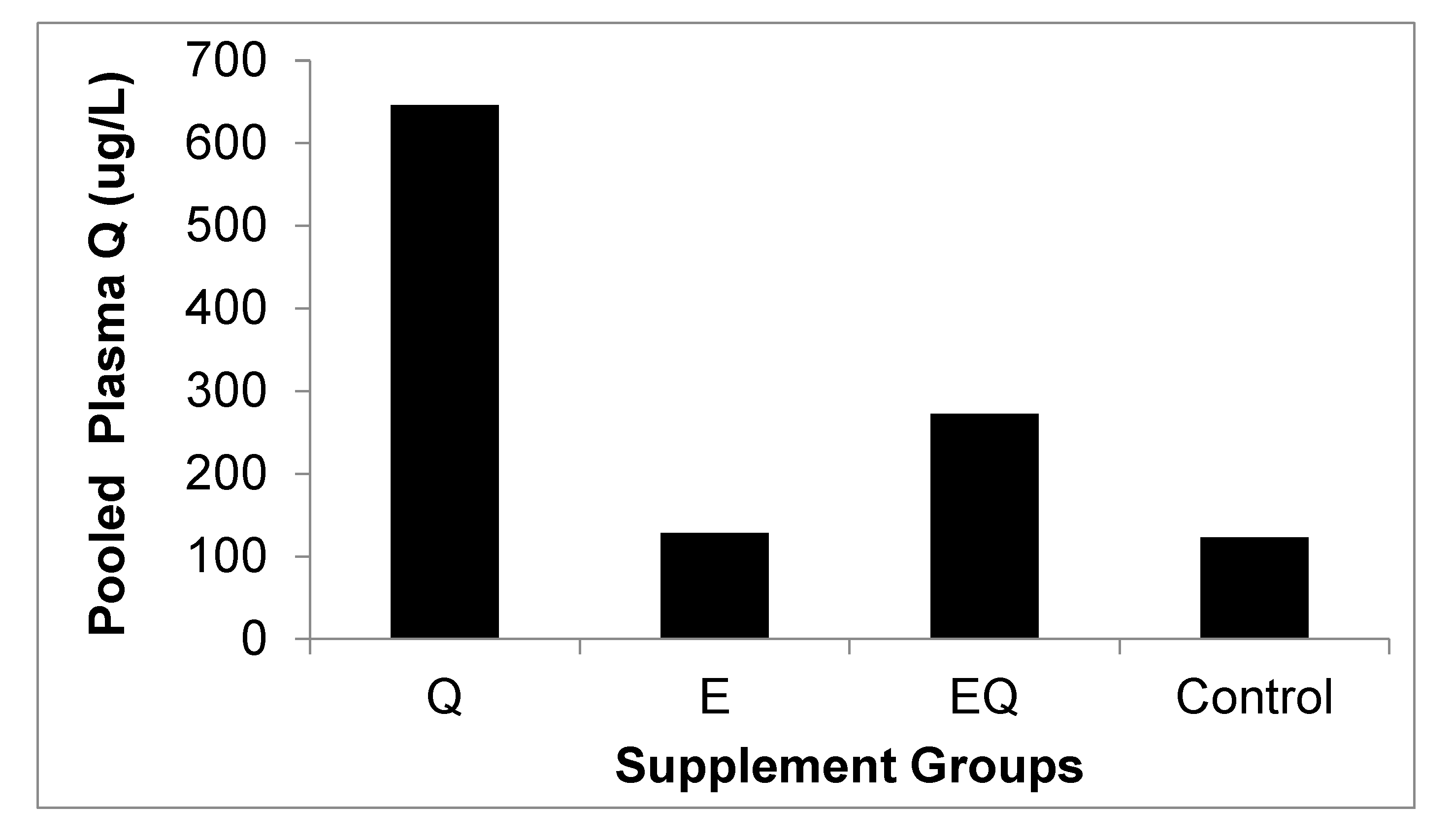
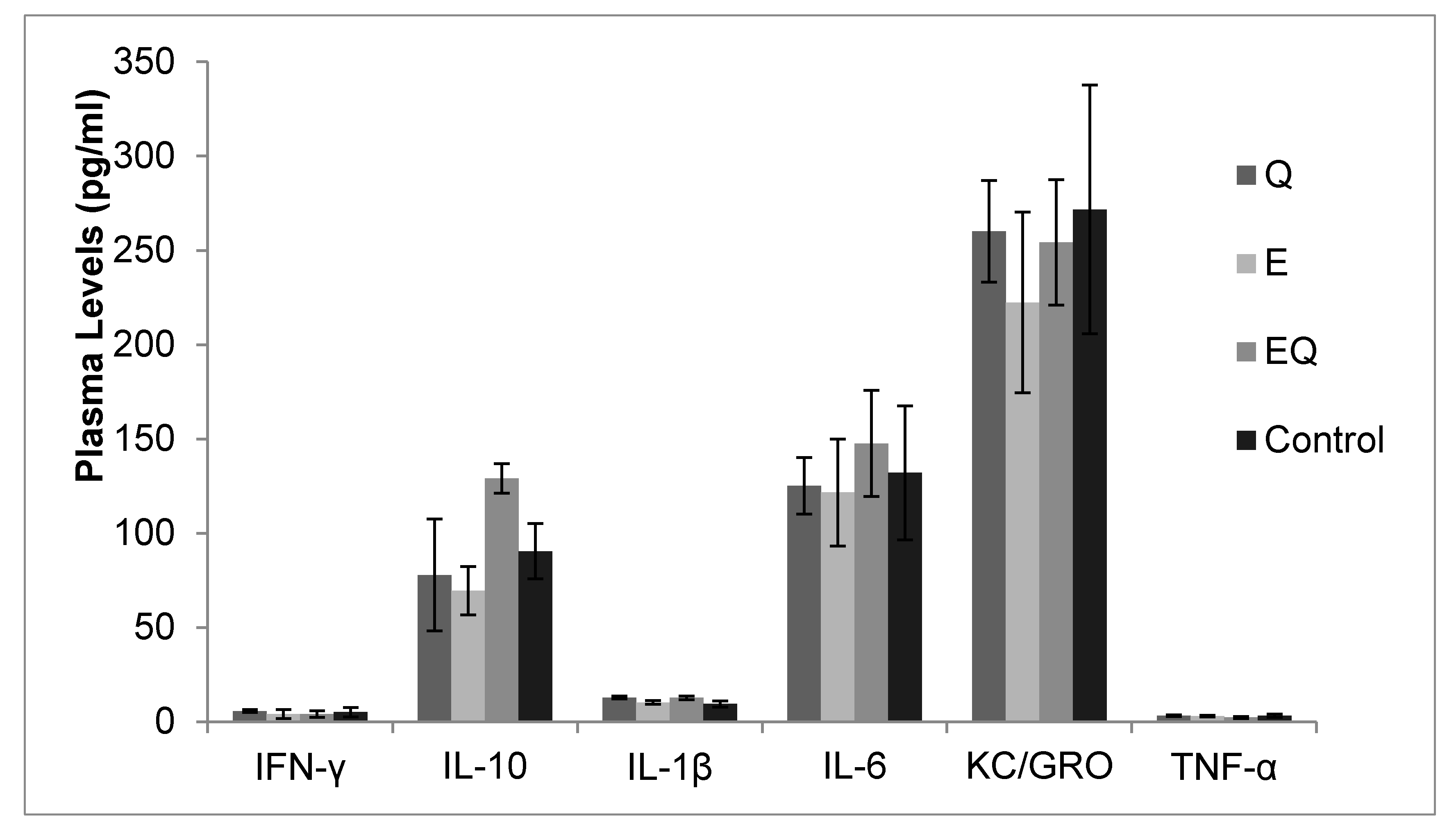
3.1. Body Mass and Biochemical Analysis
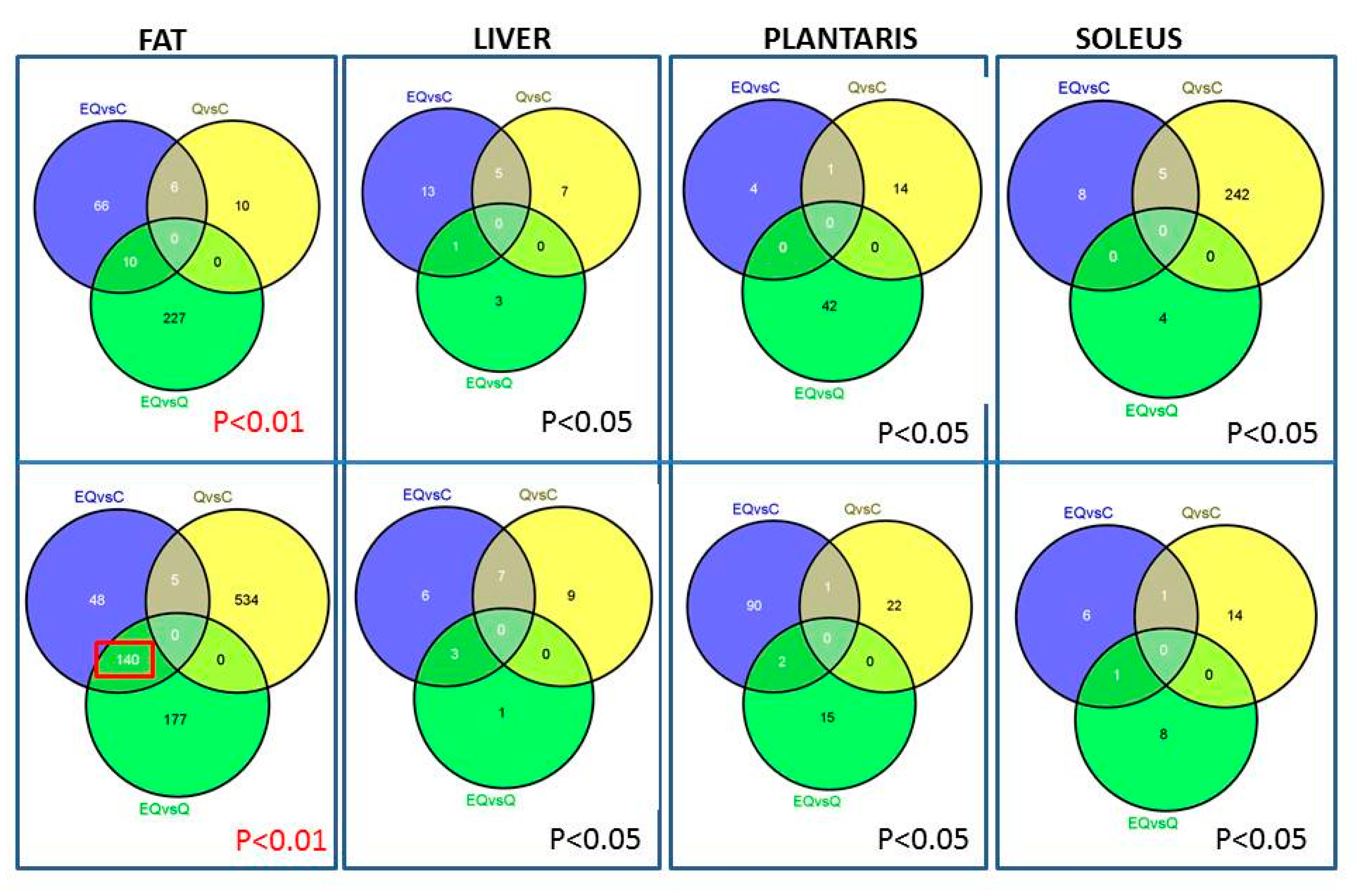
3.2. Genomic Analysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, C.Y. The effect of high-fat diet-induced pathophysiological changes in the gut on obesity: What should be the ideal treatment? Clin. Transl. Gastroenterol. 2013, 4, e39. [Google Scholar] [CrossRef] [PubMed]
- Cherniack, E.P. Polyphenols: Planting the seeds of treatment for the metabolic syndrome. Nutrition 2011, 27, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Siriwardhana, N.; Kalupahana, N.S.; Cekanova, M.; LeMieux, M.; Greer, B.; Moustaid-Moussa, N. Modulation of adipose tissue inflammation by bioactive food compounds. J. Nutr. Biochem. 2013, 24, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Parks, J.S.; Kang, H.W. Quercetin, a functional compound of onion peel, remodels white adipocytes to brown-like adipocytes. J. Nutr. Biochem. 2017, 42, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Kim, J. The effects of green tea on obesity and type 2 diabetes. Diabetes Metab. J. 2013, 37, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods. Available online: http://www.ARS.USDA.Gov/nutrientdata/flav (accessed on 15 May 2017).
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.C.; Martinez, K.; Xie, G.; Kennedy, A.; Bumrungpert, A.; Overman, A.; Jia, W.; McIntosh, M.K. Quercetin is equally or more effective than resveratrol in attenuating tumor necrosis factor-α-mediated inflammation and insulin resistance in primary human adipocytes. Am. J. Clin. Nutr. 2010, 92, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Overman, A.; Chuang, C.C.; McIntosh, M. Quercetin attenuates inflammation in human macrophages and adipocytes exposed to macrophage-conditioned media. Int. J. Obes. 2011, 35, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Comalada, M.; Ballester, I.; Bailon, E.; Sierra, S.; Xaus, J.; Galvez, J.; De Medina, F.S.; Zarzuelo, A. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: Analysis of the structure-activity relationship. Biochem. Pharmacol. 2006, 72, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.S.; Porawski, M.; Alonso, M.; Marroni, N.; Collado, P.S.; Gonzalez-Gallego, J. Quercetin decreases oxidative stress, NF-kβ activation, and iNOS overexpression in liver of streptozotocin-induced diabetic rats. J. Nutr. 2005, 135, 2299–2304. [Google Scholar] [PubMed]
- Comalada, M.; Camuesco, D.; Sierra, S.; Ballester, I.; Xaus, J.; Galvez, J.; Zarzuelo, A. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kβ pathway. Eur. J. Immunol. 2005, 35, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.P.; Mahajan, S.; Reynolds, J.L.; Aalinkeel, R.; Nair, H.; Schwartz, S.A.; Kandaswami, C. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-kβ system. Clin. Vaccine Immunol. 2006, 13, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.K.; Gao, J.; Zhu, D.N. Kaempferol and quercetin isolated from euonymus alatus improve glucose uptake of 3T3-L1 cells without adipogenesis activity. Life Sci. 2008, 82, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Yen, G.C. Induction of cell apoptosis in 3t3-L1 pre-adipocytes by flavonoids is associated with their antioxidant activity. Mol. Nutr. Food Res. 2006, 50, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Lee, H.; Kim, S.; Park, J.; Ha, T. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem. Biophys. Res. Commun. 2008, 373, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Lara-Guzman, O.J.; Tabares-Guevara, J.H.; Leon-Varela, Y.M.; Alvarez, R.M.; Roldan, M.; Sierra, J.A.; Londono-Londono, J.A.; Ramirez-Pineda, J.R. Proatherogenic macrophage activities are targeted by the flavonoid quercetin. J. Pharmacol. Exp. Ther. 2012, 343, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.K.; Soileau, J.L.; Ribnicky, D.; Wang, Z.Q.; Raskin, I.; Poulev, A.; Majewski, M.; Cefalu, W.T.; Gettys, T.W. Quercetin transiently increases energy expenditure but persistently decreases circulating markers of inflammation in C57BL/6 mice fed a high-fat diet. Metabolism 2008, 57, S39–S46. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, M.; Arsenovic-Ranin, N.; Stojic-Vukanic, Z.; Bufan, B.; Vucicevic, D.; Jancic, I. Quercetin ameliorates experimental autoimmune myocarditis in rats. J. Pharm. Pharm. Sci. 2010, 13, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.H.; Zhang, X.; Pan, Y.; Li, Y.C.; Kong, L.D. Allopurinol, quercetin and rutin ameliorate renal NLRP3 inflammasome activation and lipid accumulation in fructose-fed rats. Biochem. Pharmacol. 2012, 84, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Rivera, L.; Moron, R.; Sanchez, M.; Zarzuelo, A.; Galisteo, M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese zucker rats. Obesity 2008, 16, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.H.; Wang, C.; Li, J.M.; Zhang, D.M.; Kong, L.D. Allopurinol, rutin, and quercetin attenuate hyperuricemia and renal dysfunction in rats induced by fructose intake: Renal organic ion transporter involvement. Am. J. Physiol. Renal. Physiol. 2009, 297, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Kannappan, S.; Anuradha, C.V. Insulin sensitizing actions of fenugreek seed polyphenols, quercetin & metformin in a rat model. Indian J. Med. Res. 2009, 129, 401–408. [Google Scholar] [PubMed]
- Kobori, M.; Masumoto, S.; Akimoto, Y.; Oike, H. Chronic dietary intake of quercetin alleviates hepatic fat accumulation associated with consumption of a western-style diet in C57/BL6J mice. Mol. Nutr. Food Res. 2011, 55, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Liu, K.; Huang, F.; Guo, X.; Wang, M.; Liu, B. Opposite effects of quercetin, luteolin, and epigallocatechin gallate on insulin sensitivity under normal and inflammatory conditions in mice. Inflammation 2013, 36, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, S.; Zhao, A.; Wang, K.; Fan, Z.; Yang, H.; Liao, W.; Bao, S.; Zhao, L.; Zhang, Y.; et al. Transcriptomic and metabonomic profiling reveal synergistic effects of quercetin and resveratrol supplementation in high fat diet fed mice. J. Proteome Res. 2012, 11, 4961–4971. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.M.; Zhao, B.; Luo, T.; Kaiser, C.; Cavender, G.; Hamilton-Reeves, J.; Sullivan, D.K.; Shay, N.F. Consumption of quercetin and quercetin-containing apple and cherry extracts affects blood glucose concentration, hepatic metabolism, and gene expression patterns in obese C57/BL6J high fat-fed mice. J. Nutr. 2016, 146, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Cho, I.; Ahn, J.; Jeon, T.I.; Ha, T.Y. Quercetin reduces high-fat diet-induced fat accumulation in the liver by regulating lipid metabolism genes. Phytother. Res. 2013, 27, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Egert, S.; Bosy-Westphal, A.; Seiberl, J.; Kurbitz, C.; Settler, U.; Plachta-Danielzik, S.; Wagner, A.E.; Frank, J.; Schrezenmeir, J.; Rimbach, G.; et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009, 102, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Brull, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Muller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Naaf, S.; et al. Effects of a quercetin-rich onion skin extract on 24 h ambulatory blood pressure and endothelial function in overweight-to-obese patients with (pre-)hypertension: A randomised double-blinded placebo-controlled cross-over trial. Br. J. Nutr. 2015, 114, 1263–1277. [Google Scholar] [CrossRef] [PubMed]
- Heinz, S.A.; Henson, D.A.; Nieman, D.C.; Austin, M.D.; Jin, F. A 12-week supplementation with quercetin does not affect natural killer cell activity, granulocyte oxidative burst activity or granulocyte phagocytosis in female human subjects. Br. J. Nutr. 2010, 104, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Knab, A.M.; Shanely, R.A.; Jin, F.; Austin, M.D.; Sha, W.; Nieman, D.C. Quercetin with vitamin C and niacin does not affect body mass or composition. Appl. Physiol. Nutr. Metab. 2011, 36, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Knab, A.M.; Shanely, R.A.; Henson, D.A.; Jin, F.; Heinz, S.A.; Austin, M.D.; Nieman, D.C. Influence of quercetin supplementation on disease risk factors in community-dwelling adults. J. Am. Diet. Assoc. 2011, 111, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Heinz, S.A.; Henson, D.A.; Austin, M.D.; Jin, F.; Nieman, D.C. Quercetin supplementation and upper respiratory tract infection: A randomized community clinical trial. Pharmacol. Res. 2010, 62, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Rezvan, N.; Moini, A.; Janani, L.; Mohammad, K.; Saedisomeolia, A.; Nourbakhsh, M.; Gorgani-Firuzjaee, S.; Mazaherioun, M.; Hosseinzadeh-Attar, M.J. Effects of quercetin on adiponectin-mediated insulin sensitivity in polycystic ovary syndrome: A randomized placebo-controlled double-blind clinical trial. Horm. Metab. Res. 2017, 49, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Williamson, G. Quercetin lowers plasma uric acid in pre-hyperuricaemic males: A randomised, double-blinded, placebo-controlled, cross-over trial. Br. J. Nutr. 2016, 115, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Sae-tan, S.; Grove, K.A.; Lambert, J.D. Weight control and prevention of metabolic syndrome by green tea. Pharmacol. Res. 2011, 64, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.T.; Chang, T.W.; Lee, M.S.; Lin, J.K. Suppression of free fatty acid-induced insulin resistance by phytopolyphenols in C2C12 mouse skeletal muscle cells. J. Agric. Food Chem. 2012, 60, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Pournourmohammadi, S.; Grimaldi, M.; Stridh, M.H.; Lavallard, V.; Waagepetersen, H.S.; Wollheim, C.B.; Maechler, P. Epigallocatechin-3-gallate (EGCG) activates AMPK through the inhibition of glutamate dehydrogenase in muscle and pancreatic SS-cells: A potential beneficial effect in the pre-diabetic state? Int. J. Biochem. Cell Biol. 2017, 88, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Nishiumi, S.; Nagayasu, H.; Fukuda, I.; Yoshida, K.; Ashida, H. Epigallocatechin gallate promotes glut4 translocation in skeletal muscle. Biochem. Biophys. Res. Commun. 2008, 377, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.B.; Zhang, R.; Miao, S.; Gao, B.; Lu, Y.; Hui, S.; Li, L.; Shi, X.P.; Wen, A.D. Epigallocatechin-3-gallate ameliorates insulin resistance in hepatocytes. Mol. Med. Rep. 2017, 15, 3803–3809. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.Y.; Hong, C.G.; Suh, Y.S.; Cho, H.C.; Park, J.H.; Bae, J.H.; Park, W.K.; Han, J.; Song, D.K. Role of (−)-epigallocatechin-3-gallate in cell viability, lipogenesis, and retinol-binding protein 4 expression in adipocytes. Naunyn Schmiedebergs Arch. Pharmacol. 2010, 382, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Kim, C.T.; Kim, I.H.; Kim, Y. Inhibitory effects of green tea catechin on the lipid accumulation in 3T3-L1 adipocytes. Phytother. Res. 2009, 23, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.S.; Chung, C.S.; Lee, H.G.; Kim, T.G.; Choi, Y.J.; Cho, C.S. Inhibitory effect of (−)-epigallocatechin-3-gallate on lipid accumulation of 3T3-L1 cells. Obesity 2007, 15, 2571–2582. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, N.; Mochizuki, K.; Kameji, H.; Shimada, M.; Goda, T. (−)-epigallocatechin gallate enhances the expression of genes related to insulin sensitivity and adipocyte differentiation in 3T3-L1 adipocytes at an early stage of differentiation. Nutrition 2009, 25, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, D.; Zeng, R.; Xian, T.; Lu, Y.; Zeng, G.; Sun, Z.; Huang, B.; Huang, Q. Epigallocatechin-3-gallate inhibits adipogenesis through down-regulation of PPARγ and FAS expression mediated by PI3K-AKT signaling in 3T3-L1. Eur. J. Pharmacol. 2017, 795, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, J.T.; Pang, X.M.; Han, C.J.; Mao, J.J. Epigallocatechin-3-gallate inhibits angiotensin II and interleukin-6-induced C-reactive protein production in macrophages. Pharmacol. Rep. 2012, 64, 912–918. [Google Scholar] [CrossRef]
- Bose, M.; Lambert, J.D.; Ju, J.; Reuhl, K.R.; Shapses, S.A.; Yang, C.S. The major green tea polyphenol, (−)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J. Nutr. 2008, 138, 1677–1683. [Google Scholar] [PubMed]
- Cunha, C.A.; Lira, F.S.; Rosa Neto, J.C.; Pimentel, G.D.; Souza, G.I.; Da Silva, C.M.; De Souza, C.T.; Ribeiro, E.B.; Sawaya, A.C.; Oller do Nascimento, C.M.; et al. Green tea extract supplementation induces the lipolytic pathway, attenuates obesity, and reduces low-grade inflammation in mice fed a high-fat diet. Mediat. Inflamm. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Sampath, C.; Rashid, M.R.; Sang, S.; Ahmedna, M. Green tea epigallocatechin 3-gallate alleviates hyperglycemia and reduces advanced glycation end products via NRF2 pathway in mice with high fat diet-induced obesity. Biomed. Pharmacother. 2017, 87, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Szulinska, M.; Stepien, M.; Kregielska-Narozna, M.; Suliburska, J.; Skrypnik, D.; Bak-Sosnowska, M.; Kujawska-Luczak, M.; Grzymislawska, M.; Bogdanski, P. Effects of green tea supplementation on inflammation markers, antioxidant status and blood pressure in NaCl-induced hypertensive rat model. Food Nutr. Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Remely, M.; Ferk, F.; Sterneder, S.; Setayesh, T.; Roth, S.; Kepcija, T.; Noorizadeh, R.; Rebhan, I.; Greunz, M.; Beckmann, J.; et al. Egcg prevents high fat diet-induced changes in gut microbiota, decreases of DNA strand breaks, and changes in expression and DNA methylation of DNMT1 and MLH1 in C57BL/6J male mice. Oxidative Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Grove, K.A.; Lambert, J.D. Laboratory, epidemiological, and human intervention studies show that tea (camellia sinensis) may be useful in the prevention of obesity. J. Nutr. 2010, 140, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Hase, T.; Tokimitsu, I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity 2007, 15, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Hase, T.; Komine, Y.; Meguro, S.; Takeda, Y.; Takahashi, H.; Matsui, Y.; Inaoka, S.; Katsuragi, Y.; Tomitsu, J.; Shimasaki, H.; et al. Anti-obesity effects of tea catechins in humans. J. Oleo Sci. 2001, 50, 599–605. [Google Scholar] [CrossRef]
- Basu, A.; Sanchez, K.; Leyva, M.J.; Wu, M.; Betts, N.M.; Aston, C.E.; Lyons, T.J. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J. Am. Coll. Nutr. 2010, 29, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Du, M.; Sanchez, K.; Leyva, M.J.; Betts, N.M.; Blevins, S.; Wu, M.; Aston, C.E.; Lyons, T.J. Green tea minimally affects biomarkers of inflammation in obese subjects with metabolic syndrome. Nutrition 2011, 27, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.L.; Lane, J.; Coverly, J.; Stocks, J.; Jackson, S.; Stephen, A.; Bluck, L.; Coward, A.; Hendrickx, H. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: Randomized controlled trial. Br. J. Nutr. 2009, 101, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, S.; Sun, J.; Buys, N.; Jamshidi, A.; Nikbakht-Nasrabadi, E.; Khosravi-Boroujeni, H. Green tea catechins and blood pressure: A systematic review and meta-analysis of randomised controlled trials. Eur. J. Nutr. 2014, 53, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Onakpoya, I.; Spencer, E.; Heneghan, C.; Thompson, M. The effect of green tea on blood pressure and lipid profile: A systematic review and meta-analysis of randomized clinical trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhou, R.; Wang, B.; Yu, X.; Yang, X.; Liu, K.; Mi, M. Effect of green tea consumption on blood pressure: A meta-analysis of 13 randomized controlled trials. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Chiu, A.; Barone, M.K.; Avino, D.; Wang, F.; Coleman, C.I.; Phung, O.J. Green tea catechins decrease total and low-density lipoprotein cholesterol: A systematic review and meta-analysis. J. Am. Diet. Assoc. 2011, 111, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.X.; Xu, Y.L.; Li, S.H.; Liu, X.X.; Hui, R.; Huang, X.H. Green tea intake lowers fasting serum total and LDL cholesterol in adults: A meta-analysis of 14 randomized controlled trials. Am. J. Clin. Nutr. 2011, 94, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhou, R.; Wang, B.; Chen, K.; Shi, L.Y.; Zhu, J.D.; Mi, M.T. Effect of green tea on glucose control and insulin sensitivity: A meta-analysis of 17 randomized controlled trials. Am. J. Clin. Nutr. 2013, 98, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; Davis, J.M.; Dumke, C.L.; Gross, S.J.; Jenkins, D.P.; Murphy, E.A.; Carmichael, M.D.; Quindry, J.C.; McAnulty, S.R.; et al. Quercetin ingestion does not alter cytokine changes in athletes competing in the western states endurance run. J. Interferon Cytokine Res. 2007, 27, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; Davis, J.M.; Angela Murphy, E.; Jenkins, D.P.; Gross, S.J.; Carmichael, M.D.; Quindry, J.C.; Dumke, C.L.; Utter, A.C.; et al. Quercetin’s influence on exercise-induced changes in plasma cytokines and muscle and leukocyte cytokine mRNA. J. Appl. Physiol. 2007, 103, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; Maxwell, K.R.; Williams, A.S.; McAnulty, S.R.; Jin, F.; Shanely, R.A.; Lines, T.C. Effects of quercetin and EGCG on mitochondrial biogenesis and immunity. Med. Sci. Sports Exerc. 2009, 41, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; Gross, S.J.; Jenkins, D.P.; Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Dumke, C.L.; Utter, A.C.; McAnulty, S.R.; et al. Quercetin reduces illness but not immune perturbations after intensive exercise. Med. Sci. Sports Exerc. 2007, 39, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Irizarry, R.A.; Hobbs, B.; Collin, F.; Beazer-Barclay, Y.D.; Antonellis, K.J.; Scherf, U.; Speed, T.P. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 2003, 4, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Baldi, P.; Long, A.D. A bayesian framework for the analysis of microarray expression data: Regularized t-test and statistical inferences of gene changes. Bioinformatics 2001, 17, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Dam, V.; Sikder, T.; Santosa, S. From neutrophils to macrophages: Differences in regional adipose tissue depots. Obes. Rev. 2016, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Oh, D.Y.; Bandyopadhyay, G.; Li, D.; Xu, J.; McNelis, J.; Lu, M.; Li, P.; Yan, Q.; Zhu, Y.; et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 2012, 18, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Krause, B.R.; Hartman, A.D. Adipose tissue and cholesterol metabolism. J. Lipid Res. 1984, 25, 97–110. [Google Scholar] [PubMed]
- Parr, E.B.; Coffey, V.G.; Hawley, J.A. ‘Sarcobesity’: A metabolic conundrum. Maturitas 2013, 74, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Brill, M.J.; Diepstraten, J.; Van Rongen, A.; Van Kralingen, S.; Van den Anker, J.N.; Knibbe, C.A. Impact of obesity on drug metabolism and elimination in adults and children. Clin. Pharmacokinet. 2012, 51, 277–304. [Google Scholar] [CrossRef] [PubMed]
- Luster, A.D.; Alon, R.; Von Andrian, U.H. Immune cell migration in inflammation: Present and future therapeutic targets. Nat. Immunol. 2005, 6, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Cialdella-Kam, L.; Nieman, D.C.; Knab, A.M.; Shanely, R.A.; Meaney, M.P.; Jin, F.; Sha, W.; Ghosh, S. A mixed flavonoid-fish oil supplement induces immune-enhancing and anti-inflammatory transcriptomic changes in adult obese and overweight women-a randomized controlled trial. Nutrients 2016, 8, 277. [Google Scholar] [CrossRef] [PubMed]
- Bakker, G.C.; Van Erk, M.J.; Pellis, L.; Wopereis, S.; Rubingh, C.M.; Cnubben, N.H.; Kooistra, T.; Van Ommen, B.; Hendriks, H.F. An antiinflammatory dietary mix modulates inflammation and oxidative and metabolic stress in overweight men: A nutrigenomics approach. Am. J. Clin. Nutr. 2010, 91, 1044–1059. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Stahl, F.; Mutz, K.O.; Scheper, T.; Hahn, A.; Schuchardt, J.P. Different gene expression profiles in normo- and dyslipidemic men after fish oil supplementation: Results from a randomized controlled trial. Lipids Health Dis. 2012, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Skulas-Ray, A.C. Omega-3 fatty acids and inflammation: A perspective on the challenges of evaluating efficacy in clinical research. Prostaglandins Other Lipid Mediat. 2015, 116–117, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Heber, D.; Henning, S.M. Quercetin increased bioavailability and decreased methylation of green tea polyphenols in vitro and in vivo. Food Funct. 2012, 3, 635–642. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Glaeser, H.; Bujok, K.; Schmidt, I.; Fromm, M.F.; Mandery, K. Organic anion transporting polypeptides and organic cation transporter 1 contribute to the cellular uptake of the flavonoid quercetin. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.; Timmermann, B.N.; Hagenbuch, B. Interactions of green tea catechins with organic anion-transporting polypeptides. Drug Metab. Dispos. 2011, 39, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Henagan, T.M.; Lenard, N.R.; Gettys, T.W.; Stewart, L.K. Dietary quercetin supplementation in mice increases skeletal muscle PGC1alpha expression, improves mitochondrial function and attenuates insulin resistance in a time-specific manner. PLoS ONE 2014, 9, e89365. [Google Scholar] [CrossRef] [PubMed]
- Palermo, N.E.; Gianchandani, R.Y.; McDonnell, M.E.; Alexanian, S.M. Stress hyperglycemia during surgery and anesthesia: Pathogenesis and clinical implications. Curr. Diabetes Rep. 2016, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- Togashi, Y.; Shirakawa, J.; Okuyama, T.; Yamazaki, S.; Kyohara, M.; Miyazawa, A.; Suzuki, T.; Hamada, M.; Terauchi, Y. Evaluation of the appropriateness of using glucometers for measuring the blood glucose levels in mice. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gallego, J.; Garcia-Mediavilla, M.V.; Sanchez-Campos, S.; Tunon, M.J. Fruit polyphenols, immunity and inflammation. Br. J. Nutr. 2010, 104, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Mereles, D.; Hunstein, W. Epigallocatechin-3-gallate (EGCG) for clinical trials: More pitfalls than promises? Int. J. Mol. Sci. 2011, 12, 5592–5603. [Google Scholar] [CrossRef] [PubMed]
- Van Duynhoven, J.; Vaughan, E.E.; Jacobs, D.M.; Kemperman, R.A.; Van Velzen, E.J.J.; Gross, G.; Roger, L.C.; Possemiers, S.; Smilde, A.K.; Doré, J.; et al. Metabolic fate of polyphenols in the human superorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 4531–4538. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.J. Cytochrome P450 humanised mice. Hum. Genom. 2004, 1, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Graf, B.A.; Ameho, C.; Dolnikowski, G.G.; Milbury, P.E.; Chen, C.Y.; Blumberg, J.B. Rat gastrointestinal tissues metabolize quercetin. J. Nutr. 2006, 136, 39–44. [Google Scholar] [PubMed]
- Lambert, J.D.; Lee, M.J.; Lu, H.; Meng, X.; Hong, J.J.; Seril, D.N.; Sturgill, M.G.; Yang, C.S. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J. Nutr. 2003, 133, 4172–4177. [Google Scholar] [PubMed]
| Downregulated Pathways | Fat | Liver | Muscle | Comments |
|---|---|---|---|---|
| Steroid Biosynthesis | Q; EQ | Target of Statins | ||
| Phagocytosis/ leukocyte extravasation | EQ | Innate Immune Response | ||
| EIF2 signaling | Q; EQ | Stress Response | ||
| Mitochondrial dysfunction | Q | Associated with disease | ||
| eIF4/p70S6K signaling | Q; EQ | Insulin Signaling | ||
| Oxidative phosphorylation | Q; EQ | Energy Production | ||
| PPARα/RXRα activation | Q | Gene Expression |
| Description | Q | p | EQ | p | Description |
|---|---|---|---|---|---|
| Change | Change | ||||
| Adipose | |||||
| Ifngr1 | –0.28 | 0.340 | –0.18 | 0.659 | interferon gamma receptor 1 |
| Ifngr2 | –0.14 | 0.820 | –0.54 | 0.090 | interferon gamma receptor 2 |
| Il10ra | –0.36 | 0.550 | –0.63 | 0.194 | interleukin 10 receptor, alpha |
| Il10rb | –0.29 | 0.550 | –0.73 | 0.047 | interleukin 10 receptor, beta |
| Il1r1 | –060 | 0.037 | –0.53 | 0.087 | interleukin 1 receptor, type I |
| Il1rap | –0.10 | 0.908 | 0.10 | 0.916 | interleukin 1 receptor accessory protein |
| Il1rn | –0.31 | 0.783 | –1.22 | 0.052 | interleukin 1 receptor antagonist |
| Il6ra | –0.29 | 0.466 | 0.32 | 0.428 | interleukin 6 receptor, alpha |
| Il6st | –0.18 | 0.645 | –0.04 | 0.977 | interleukin 6 signal transducer |
| Cxcl1 | –0.17 | 0.679 | –0.11 | 0.873 | chemokine (C-X-C motif) ligand 1 |
| Tnfrsf1a | –0.47 | 0.196 | 0.99 | 0.188 | tumor necrosis factor receptor superfamily, member 1a |
| Tnfrsf1b | –0.33 | 0.565 | 0.20 | 0.046 | tumor necrosis factor receptor superfamily, member 1b |
| Soleus | |||||
| Ifngr1 | –0.27 | 0.423 | –0.15 | 0.775 | interferon gamma receptor 1 |
| Il10rb | –0.10 | 0.876 | 0.10 | 0.879 | interleukin 10 receptor, beta |
| Il6ra | –0.18 | 0.692 | –0.12 | 0.845 | interleukin 6 receptor, alpha |
| Il6st | –0.31 | 0.151 | –0.16 | 0.602 | interleukin 6 signal transducer |
| Tnfrsf1a | –0.42 | 0.162 | 0.21 | 0.968 | tumor necrosis factor receptor superfamily, member 1a |
| Liver | |||||
| Ifngr1 | –0.07 | 0.935 | –0.13 | 0.805 | interferon gamma receptor 1 |
| Ifngr2 | 0.15 | 0.678 | –0.05 | 0.961 | interferon gamma receptor 2 |
| Il10rb | 0.00 | 0.999 | –0.06 | 0.964 | interleukin 10 receptor, beta |
| Il1b | 0.07 | 0.985 | 0.07 | 0.986 | interleukin 1 beta |
| Il1r1 | 0.31 | 0.799 | 0.79 | 0.243 | interleukin 1 receptor, type I |
| Il1rap | –0.01 | 0.998 | 0.00 | 0.999 | interleukin 1 receptor accessory protein |
| Il1rn | 0.06 | 0.971 | 0.32 | 0.413 | interleukin 1 receptor antagonist |
| Il6ra | –0.24 | 0.550 | 0.55 | 0.055 | interleukin 6 receptor, alpha |
| Il6st | –0.07 | 0.929 | 0.12 | 0.801 | interleukin 6 signal transducer |
| Cxcl1 | 0.72 | 0.222 | 1.15 | 0.030 | chemokine (C-X-C motif) ligand 1 |
| Tnfrsf1a | –0.42 | 0.162 | 0.21 | 0.968 | tumor necrosis factor receptor superfamily, member 1a |
| Description | Q | E | EQ | Pathways |
|---|---|---|---|---|
| Adipose | ||||
| Srebf2 | 0.44 * | 0.57 | 0.82 | Sterol biosynthesis |
| Atf2 | 0.51 * | 0.83 | 1.10 | Transcriptional activator |
| Sirt1 | 0.40 * | 0.92 | 0.74 | Stress response |
| Soleus | ||||
| Srebf2 | 0.60 | 0.54 * | 0.62 * | Sterol biosynthesis |
| Pparag | 1.04 | 0.71 | 0.69 * | Fatty acid storage and Glucose metabolism |
| Scd1 | 0.44 | 0.97 | 0.40 * | Fatty Acid metabolism |
| Cd68 | 0.88 | 0.71 | 0.57 * | Promote phagocytosis and activation of macrophages |
| Atf2 | 1.23 | 0.62 * | 0.85 | Transcriptional activator |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cialdella-Kam, L.; Ghosh, S.; Meaney, M.P.; Knab, A.M.; Shanely, R.A.; Nieman, D.C. Quercetin and Green Tea Extract Supplementation Downregulates Genes Related to Tissue Inflammatory Responses to a 12-Week High Fat-Diet in Mice. Nutrients 2017, 9, 773. https://doi.org/10.3390/nu9070773
Cialdella-Kam L, Ghosh S, Meaney MP, Knab AM, Shanely RA, Nieman DC. Quercetin and Green Tea Extract Supplementation Downregulates Genes Related to Tissue Inflammatory Responses to a 12-Week High Fat-Diet in Mice. Nutrients. 2017; 9(7):773. https://doi.org/10.3390/nu9070773
Chicago/Turabian StyleCialdella-Kam, Lynn, Sujoy Ghosh, Mary Pat Meaney, Amy M. Knab, R. Andrew Shanely, and David C. Nieman. 2017. "Quercetin and Green Tea Extract Supplementation Downregulates Genes Related to Tissue Inflammatory Responses to a 12-Week High Fat-Diet in Mice" Nutrients 9, no. 7: 773. https://doi.org/10.3390/nu9070773
APA StyleCialdella-Kam, L., Ghosh, S., Meaney, M. P., Knab, A. M., Shanely, R. A., & Nieman, D. C. (2017). Quercetin and Green Tea Extract Supplementation Downregulates Genes Related to Tissue Inflammatory Responses to a 12-Week High Fat-Diet in Mice. Nutrients, 9(7), 773. https://doi.org/10.3390/nu9070773








