Intake of Marine-Derived Omega-3 Polyunsaturated Fatty Acids and Mortality in Renal Transplant Recipients
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Renal Transplant Characteristics
2.3. Dietary Assessment
2.4. Measurements
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Association between Intake of Marine-Derived n-3 PUFA with Clinical Baseline Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BSA | Body surface area |
| CI | Confidence interval |
| CV | Cardiovascular |
| DHA | Docosahexaenoic acid |
| EPA | Eicosapentaenoic acid |
| EPA-DHA | Eicosapentaenoic acid plus Docosahexaenoic acid |
| FFQ | Food frequency questionnaire |
| HDL | High-density lipoprotein |
| HR | Hazard Ratio |
| HbA1C | Glycated hemoglobin |
| hs-CRP | High-sensitive C-reactive protein |
| IQR | Interquartile range |
| kJ | Kilojoule |
| LDL | Low-density lipoprotein |
| n-3 PUFA | Omega-3 polyunsaturated fatty acids |
| RTR | Renal transplant recipients |
| SQUASH | Short QUestionnaire to ASsess Health-enhancing physical activity |
References
- Schippers, H.; Kalff, M.W. Cost comparison haemodialysis and renal transplantation. HLA 1976, 7, 86–90. [Google Scholar] [CrossRef]
- Laupacis, A.; Keown, P.; Pus, N.; Krueger, H.; Ferguson, B.; Wong, C.; Muirhead, N. A study of the quality of life and cost-utility of renal transplantation. Kidney Int. 1996, 50, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Jofre, R.; Lopez-Gomez, J.M.; Moreno, F.; Sanz-Guajardo, D.; Valderrabano, F. Changes in quality of life after renal transplantation. Am. J. Kidney Dis. 1998, 32, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.A.; Ashby, V.B.; Milford, E.L.; Ojo, A.O.; Ettenger, R.E.; Agodoa, L.Y.C.; Held, P.J.; Port, F.K. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med. 1999, 341, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Oniscu, G.C.; Brown, H.; Forsythe, J.L. Impact of cadaveric renal transplantation on survival in patients listed for transplantation. J. Am. Soc. Nephrol. 2005, 16, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Chkhotua, A.; Pantsulaia, T.; Managadze, L. The quality of life analysis in renal transplant recipients and dialysis patients. Georgian Méd. News 2011, 11, 10–17. [Google Scholar] [PubMed]
- Tonelli, M.; Wiebe, N.; Knoll, G.; Bello, A.; Browne, S.; Jadhav, D.; Klarenbach, S.; Gill, J. Systematic review: Kidney transplantation compared with dialysis in clinically relevant outcomes. Am. J. Transplant. 2011, 11, 2093–2109. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, M.; Ichikawa, Y.; Yoshiya, K.; Isotani, S.; Higuchi, A.; Nagano, S.; Arakawa, S.; Hamami, G.; Matsumoto, O.; Kamidono, S. Assessment of health-related quality of life in renal transplant and hemodialysis patients using the SF-36 health survey. Urology 2000, 56, 201–206. [Google Scholar] [CrossRef]
- Oterdoom, L.H.; de Vries, A.P.; van Ree, R.M.; Gansevoort, R.T.; van Son, W.J.; van der Heide, J.J.H.; Navis, G.; de Jong, P.E.; Gans, R.O.; Bakker, S.J. N-terminal pro-B-type natriuretic peptide and mortality in renal transplant recipients versus the general population. Transplantation 2009, 87, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Schankel, K.; Robinson, J.; Bloom, R.D.; Guerra, C.; Rader, D.; Joffe, M.; Rosas, S.E. Determinants of coronary artery calcification progression in renal transplant recipients. Am. J. Transplant. 2007, 7, 2158–2164. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.; Excell, L.; Livingston, B. ANZDATA Registry Report; Thirty-Third Report; Australia and New Zealand Dialysis and Transplant Registry: Adelaide, Australia, 2010. [Google Scholar]
- Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; Howard, B.; et al. Diet and lifestyle recommendations revision 2006. Circulation 2006, 114, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.B.; Jrgensen, K.A.; Christensen, J.H. OPACH Study Group. N-3 fatty acids as secondary prevention against cardiovascular events in patients who undergo chronic hemodialysis: A randomized, placebo-controlled intervention trial. Clin. J. Am. Soc. Nephrol. 2006, 1, 780–786. [Google Scholar]
- Friedman, A.N.; Yu, Z.; Tabbey, R.; Denski, C.; Tamez, H.; Wenger, J.; Thadhani, R.; Li, Y.; Watkins, B.A. Inverse relationship between long-chain n-3 fatty acids and risk of sudden cardiac death in patients starting hemodialysis. Kidney Int. 2013, 83, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Homan van der Heide, J.J.; Bilo, H.; Tegzess, A.M.; Donker, A. The effects of dietary supplementation with fish oil on renal function in cyclosporine-treated renal transplant recipients. Transplantation 1990, 49, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Homan van der Heide, J.J.; Bilo, H.; Donker, J.M.; Wilmink, J.M.; Tegzess, A.M. Effect of dietary fish oil on renal function and rejection in cyclosporine-treated recipients of renal transplants. N. Engl. J. Med. 1993, 329, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Berthoux, F.C.; Guerin, C.; Burgard, G.; Berthoux, P.; Alamartine, E. One-year randomized controlled trial with omega-3 fatty acid-fish oil in clinical renal transplantation. Transplant. Proc. 1992, 24, 2578–2582. [Google Scholar] [PubMed]
- Psota, T.L.; Gebauer, S.K.; Kris-Etherton, P. Dietary omega-3 fatty acid intake and cardiovascular risk. Am. J. Cardiol. 2006, 98, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Kromhout, D.; Bosschieter, E.B.; Coulander, C.D.L. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N. Engl. J. Med. 1985, 312, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Kromhout, D.; Feskens, E.J.; Bowles, C.H. The protective effect of a small amount of fish on coronary heart disease mortality in an elderly population. Int. J. Epidemiol. 1995, 24, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Dolecek, T.A.; Grandits, G. Dietary polyunsaturated fatty acids and mortality in the Multiple Risk Factor Intervention Trial (MRFIT). World Rev. Nutr. Diet. 1991, 66, 205–216. [Google Scholar] [PubMed]
- Hu, F.B.; Bronner, L.; Willett, W.C.; Stampfer, M.J.; Rexrode, K.M.; Albert, C.M.; Hunter, D.; Manson, J.E. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA 2002, 287, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sasaki, S.; Amano, K.; Kesteloot, H. Fish consumption and mortality from all causes, ischemic heart disease, and stroke: An ecological study. Prev. Med. 1999, 28, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Burr, M.L.; Gilbert, J.F.; Holliday, R.M.; Sweetnam, P.M.; Elwood, P.C.; Deadman, N.M. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: Diet and reinfarction trial (DART). Lancet 1989, 334, 757–761. [Google Scholar] [CrossRef]
- GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-prevenzione trial. Lancet 1999, 354, 447–455. [Google Scholar]
- Eide, I.A.; Jenssen, T.; Hartmann, A.; Diep, L.M.; Dahle, D.O.; Reister, A.V.; Bjerve, K.S.; Christensen, J.H.; Schmidt, E.B. The association between marine n-3 polyunsaturated fatty acid levels and survival after renal transplantation. Clin. J. Am. Soc. Nephrol. 2015, 10, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Andy, K.H.L.; Karen, J.M.; Matthew, A.R.; Margaret, B.F. Fish oil for kidney transplant recipients. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef]
- Pranger, I.G.; Gruppen, E.G.; van den Berg, E.; Soedamah-Muthu, S.S.; Navis, G.; Gans, R.O.; Muskiet, F.A.; Kema, I.P.; Joosten, M.M.; Bakker, S.J. Intake of n-3 fatty acids and long-term outcome in renal transplant recipients: A post hoc analysis of a prospective cohort study. Br. J. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, E.; Pasch, A.; Westendorp, W.H.; Navis, G.; Brink, E.J.; Gans, R.O.; van Goor, H.; Bakker, S.J. Urinary sulfur metabolites associate with a favorable cardiovascular risk profile and survival benefit in renal transplant recipients. J. Am. Soc. Nephrol. 2014, 25, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Wendel-Vos, G.W.; Schuit, A.J.; Saris, W.H.; Kromhout, D. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J. Clin. Epidemiol. 2003, 56, 1163–1169. [Google Scholar] [CrossRef]
- Feunekes, G.I.; Van Staveren, W.A.; De Vries, J.H.; Burema, J.; Hautvast, J.G. Relative and biomarker-based validity of a food-frequency questionnaire estimating intake of fats and cholesterol. Am. J. Clin. Nutr. 1993, 58, 489–496. [Google Scholar] [PubMed]
- Stichting, N. Nederlands Voedingsstoffen Bestand: NEVO Tabel 2006, Dutch Nutrient Database; Voorlichtingsbureau voor de voeding: The Hague, The Netherlands, 2006. [Google Scholar]
- Du Bois, D.; Du Bois, E.F. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989, 5, 303–311. [Google Scholar] [PubMed]
- Van den Berg, E.; Geleijnse, J.M.; Brink, E.J.; van Baak, M.A.; van der Heide, J.J.H.; Gans, R.O.; Navis, G.; Bakker, S.J. Sodium intake and blood pressure in renal transplant recipients. Nephrol. Dial. Transplant. 2012, 27, 3352–3359. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Peelen, L.M.; Corpeleijn, E.; van der Schouw, Y.T.; Stolk, R.P.; Spijkerman, A.M.; Moons, K.G.; Navis, G.; Bakker, S.J.; Beulens, J.W. Prediction models for risk of developing type 2 diabetes: Systematic literature search and independent external validation study. BMJ 2012, 345, e5900. [Google Scholar] [CrossRef] [PubMed]
- Shabir, S.; Jham, S.; Harper, L.; Ball, S.; Borrows, R.; Sharif, A. Validity of glycated haemoglobin to diagnose new onset diabetes after transplantation. Transplant. Int. 2013, 26, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Christoulas, D.; Kastritis, E.; Katodritou, E.; Pouli, A.; Michalis, E.; Papassotiriou, I.; Dimopoulos, M.A. The chronic kidney disease epidemiology collaboration cystatin C (CKD-EPI-CysC) equation has an independent prognostic value for overall survival in newly diagnosed patients with symptomatic multiple myeloma; is it time to change from MDRD to CKD-EPI-CysC equations? Eur. J. Haematol. 2013, 91, 347–355. [Google Scholar] [PubMed]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1228S. [Google Scholar]
- Eide, I.A.; Jenssen, T.; Hartmann, A.; Diep, L.M.; Dahle, D.O.; Reisaeter, A.V.; Bjerve, K.S.; Christensen, J.H.; Schmidt, E.B.; Svensson, M. Plasma levels of marine n-3 polyunsaturated fatty acids and renal allograft survival. Nephrol. Dial. Transplant. 2016, 31, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castillo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 2013, 38, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 93, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia–hyperaminoacidaemia in healthy young and middle-aged men and women. Clin. Sci. 2011, 121, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Rodacki, C.L.; Rodacki, A.L.; Pereira, G.; Naliwaiko, K.; Coelho, I.; Pequito, D.; Fernandes, L.C. Fish-oil supplementation enhances the effects of strength training in elderly women. Am. J. Clin. Nutr. 2012, 95, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Reidy, P.T.; Konopka, A.R.; Hinkley, J.M.; Suer, M.K.; Harber, M.P. The effect of feeding during recovery from aerobic exercise on skeletal muscle intracellular signaling. Int. J. Sport. Nutr. Exerc. Metab. 2014, 24, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Van Norren, K.; Kegler, D.; Argiles, J.M.; Luiking, Y.; Gorselink, M.; Laviano, A.; Arts, K.; Faber, J.; Jansen, H.; van der Beek, E.M.; et al. Dietary supplementation with a specific combination of high protein, leucine, and fish oil improves muscle function and daily activity in tumour-bearing cachectic mice. Br. J. Cancer 2009, 100, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Gingras, A.A.; White, P.J.; Chouinard, P.Y.; Julien, P.; Davis, T.A.; Dombrowski, L.; Couture, Y.; Dubreuil, P.; Myre, A.; Bergeron, K.; et al. Long-chain omega-3 fatty acids regulate bovine whole-body protein metabolism by promoting muscle insulin signalling to the Akt-mTOR-S6K1 pathway and insulin sensitivity. J. Physiol. 2007, 579, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Spitale, R.C.; Cheng, M.Y.; Chun, K.A.; Gorell, E.S.; Munoz, C.A.; Kern, D.G.; Wood, S.M.; Knaggs, H.E.; Wulff, J.; Beebe, K.D. Differential effects of dietary supplements on metabolomic profile of smokers versus non-smokers. Genome Med. 2012, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Murff, H.J.; Tindle, H.A.; Shrubsole, M.J.; Cai, Q.; Smalley, W.; Milne, G.L.; Swift, L.L.; Ness, R.M.; Zheng, W. Smoking and red blood cell phospholipid membrane fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2016, 112, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Pawlosky, R.J.; Hibbeln, J.R.; Salem, N. Compartmental analyses of plasma n-3 essential fatty acids among male and female smokers and nonsmokers. J. Lipid. Res. 2007, 48, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.D.; Frei, B.; Longmire, A.W.; Gaziano, J.M.; Lynch, S.M.; Shyr, Y.; Strauss, W.E.; Oates, J.A.; Roberts, L.J. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers—Smoking as a cause of oxidative damage. N. Engl. J. Med. 1995, 332, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Seet, R.C.; Lee, C.J.; Loke, W.M.; Huang, S.H.; Huang, H.; Looi, W.F.; Chew, E.S.; Quek, A.M.; Lim, E.C.; Halliwell, B. Biomarkers of oxidative damage in cigarette smokers: Which biomarkers might reflect acute versus chronic oxidative stress? Free Radic. Biol. Med. 2011, 50, 1787–1793. [Google Scholar] [CrossRef] [PubMed]
- Koenig, W.; Sund, M.; Frohlich, M.; Lowel, H.; Hutchinson, W.L.; Pepys, M.B. Refinement of the association of serum C-reactive protein concentration and coronary heart disease risk by correction for within-subject variation over time: The MONICA Augsburg studies, 1984 and 1987. Am. J. Epidemiol. 2003, 158, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Danesh, J.; Wheeler, J.G.; Hirschfield, G.M.; Eda, S.; Eiriksdottir, G.; Rumley, A.; Lowe, G.D.; Pepys, M.B.; Gudnason, V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N. Engl. J. Med. 2004, 350, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
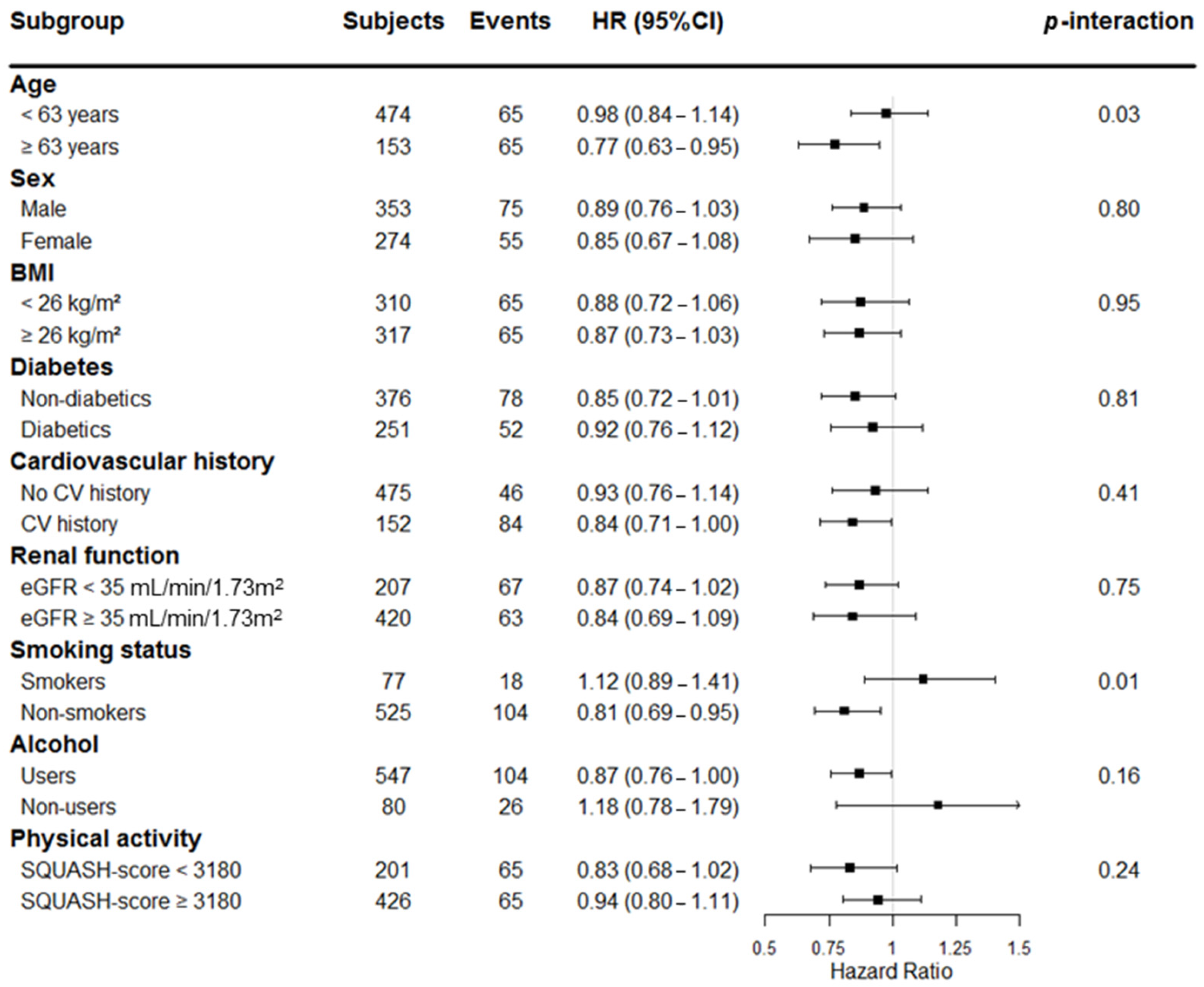
| Clinical Variables | All Patients | EPA-DHA Intake (100 mg/Day) | |
|---|---|---|---|
| Std.β | p | ||
| No. of patients | 627 | - | - |
| DHA, mg/day | 60 (28–129) | - | - |
| EPA, mg/day | 39 (13–85) | - | - |
| EPA-DHA, mg/day | 102 (42–215) | - | - |
| Demographics | |||
| Age, years | 53 ± 13 | 0.13 | 0.001 |
| Ethnicity (caucasian), n (%) | 625 (99.7) | 0.03 | 0.46 |
| Sex (male), n (%) | 353 (56.3) | 0.04 | 0.39 |
| Body mass index, kg/m2 | 26.6 ± 4.7 | 0.08 | 0.04 |
| Body surface area, m2 | 1.9 ± 0.2 | 0.01 | 0.78 |
| Cardiovascular history, n (%) | 251 (40.0) | 0.04 | 0.28 |
| Renal transplantation characteristics | |||
| Pre-emptive transplantation, n (%) | 102 (16.3) | 0.003 | 0.93 |
| Time between transplantation and baseline, years | 5.7 (2.0–12.2) | −0.002 | 0.95 |
| Hemodynamic parameters | |||
| Systolic blood pressure, mmHg | 136 ± 17 | 0.001 | 0.98 |
| Diastolic blood pressure, mmHg | 83 ± 11 | 0.03 | 0.41 |
| Mean arterial pressure, mmHg | 108 ± 15 | 0.02 | 0.71 |
| Heart rate, beats per minute | 69 ± 12 | 0.01 | 0.78 |
| Antihypertensives, n (%) | 552 (88.0) | −0.04 | 0.34 |
| Renal function parameters | |||
| Creatinine, umol/L | 123 (99–159) | 0.005 | 0.91 |
| Cystatine-C, mg/L | 1.7 (1.3–2.2) | −0.06 | 0.13 |
| eGFR, mL/min/1.73 m2 | 45 ± 19 | −0.01 | 0.83 |
| Proteinuria ≥0.5 g/day, n (%) | 139 (22.2) | −0.02 | 0.55 |
| Glucose homeostasis | |||
| Glucose, mmol/L | 5.3 (4.8–6.0) | 0.06 | 0.14 |
| HbA1C, % | 5.8 (5.5–6.2) | 0.05 | 0.27 |
| Diabetes, n (%) | 152 (24.2) | 0.02 | 0.60 |
| Antidiabetic medication, n (%) | 98 (15.6) | 0.02 | 0.67 |
| Serum parameters | |||
| Albumin, g/L | 43.0 ± 3.0 | −0.02 | 0.67 |
| hs-CRP, mg/L | 1.6 (0.7–4.5) | 0.06 | 0.13 |
| Lipids | |||
| Total cholesterol, mmol/L | 5.1 ± 1.1 | 0.08 | 0.06 |
| LDL cholesterol, mmol/L | 3.0 ± 0.9 | 0.06 | 0.13 |
| HDL cholesterol, mmol/L | 1.3 (1.1–1.7) | 0.07 | 0.10 |
| Triglycerides, mmol/L | 1.7 (1.2–2.3) | −0.01 | 0.75 |
| Statin use, n (%) | 333 (53.1) | 0.02 | 0.57 |
| Health lifestyle | |||
| Current smoker, n (%) | 77 (12.3) | −0.008 | 0.85 |
| Alcohol consumers, n (%) | 547 (87.2) | −0.14 | <0.001 |
| Physical activity, intensity × hours | 5250 (2400–8160) | 0.03 | 0.41 |
| Total energy intake, kJ/day | 8756 (7224–10,636) | - | - |
| Model | EPA-DHA Intake, 100 mg/Day | |
|---|---|---|
| HR (95% CI) | p | |
| All-cause mortality | ||
| Model 1 | 0.87 (0.77–0.99) | 0.03 |
| Model 2 | 0.85 (0.75–0.97) | 0.02 |
| Model 3 | 0.87 (0.77–1.00) | 0.04 |
| Model 4 | 0.87 (0.76–0.99) | 0.03 |
| Model 5 | 0.84 (0.73–0.96) | 0.01 |
| Model 6 | 0.85 (0.74–0.97) | 0.02 |
| CV mortality | ||
| Model 1 | 0.85 (0.69–1.05) | 0.13 |
| Model 2 | 0.83 (0.68–1.02) | 0.08 |
| Model 3 | 0.86 (0.70–1.07) | 0.18 |
| Model 4 | 0.84 (0.68–1.03) | 0.10 |
| Model 5 | 0.81 (0.64–1.01) | 0.06 |
| Model 6 | 0.82 (0.65–1.03) | 0.08 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes Neto, A.W.; Sotomayor, C.G.; Pranger, I.G.; Van den Berg, E.; Gans, R.O.B.; Soedamah-Muthu, S.S.; Navis, G.J.; Bakker, S.J.L. Intake of Marine-Derived Omega-3 Polyunsaturated Fatty Acids and Mortality in Renal Transplant Recipients. Nutrients 2017, 9, 363. https://doi.org/10.3390/nu9040363
Gomes Neto AW, Sotomayor CG, Pranger IG, Van den Berg E, Gans ROB, Soedamah-Muthu SS, Navis GJ, Bakker SJL. Intake of Marine-Derived Omega-3 Polyunsaturated Fatty Acids and Mortality in Renal Transplant Recipients. Nutrients. 2017; 9(4):363. https://doi.org/10.3390/nu9040363
Chicago/Turabian StyleGomes Neto, António W., Camilo G. Sotomayor, Ilse G. Pranger, Else Van den Berg, Rijk O. B. Gans, Sabita S. Soedamah-Muthu, Gerjan J. Navis, and Stephan J. L. Bakker. 2017. "Intake of Marine-Derived Omega-3 Polyunsaturated Fatty Acids and Mortality in Renal Transplant Recipients" Nutrients 9, no. 4: 363. https://doi.org/10.3390/nu9040363
APA StyleGomes Neto, A. W., Sotomayor, C. G., Pranger, I. G., Van den Berg, E., Gans, R. O. B., Soedamah-Muthu, S. S., Navis, G. J., & Bakker, S. J. L. (2017). Intake of Marine-Derived Omega-3 Polyunsaturated Fatty Acids and Mortality in Renal Transplant Recipients. Nutrients, 9(4), 363. https://doi.org/10.3390/nu9040363






