Vitamin D Decreases Serum VEGF Correlating with Clinical Improvement in Vitamin D-Deficient Women with PCOS: A Randomized Placebo-Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Interventions and Blood Collection
2.3. Assays of All Measured Hormones, 25OH-D and VEGF
2.4. Clinical Parameters
2.5. Statistical Analysis
3. Results
3.1. Demographics and Changes in Serum 25OH-D Levels
3.2. Changes in PCOS Clinical and Biochemical Parameters Following Vitamin D Supplementation
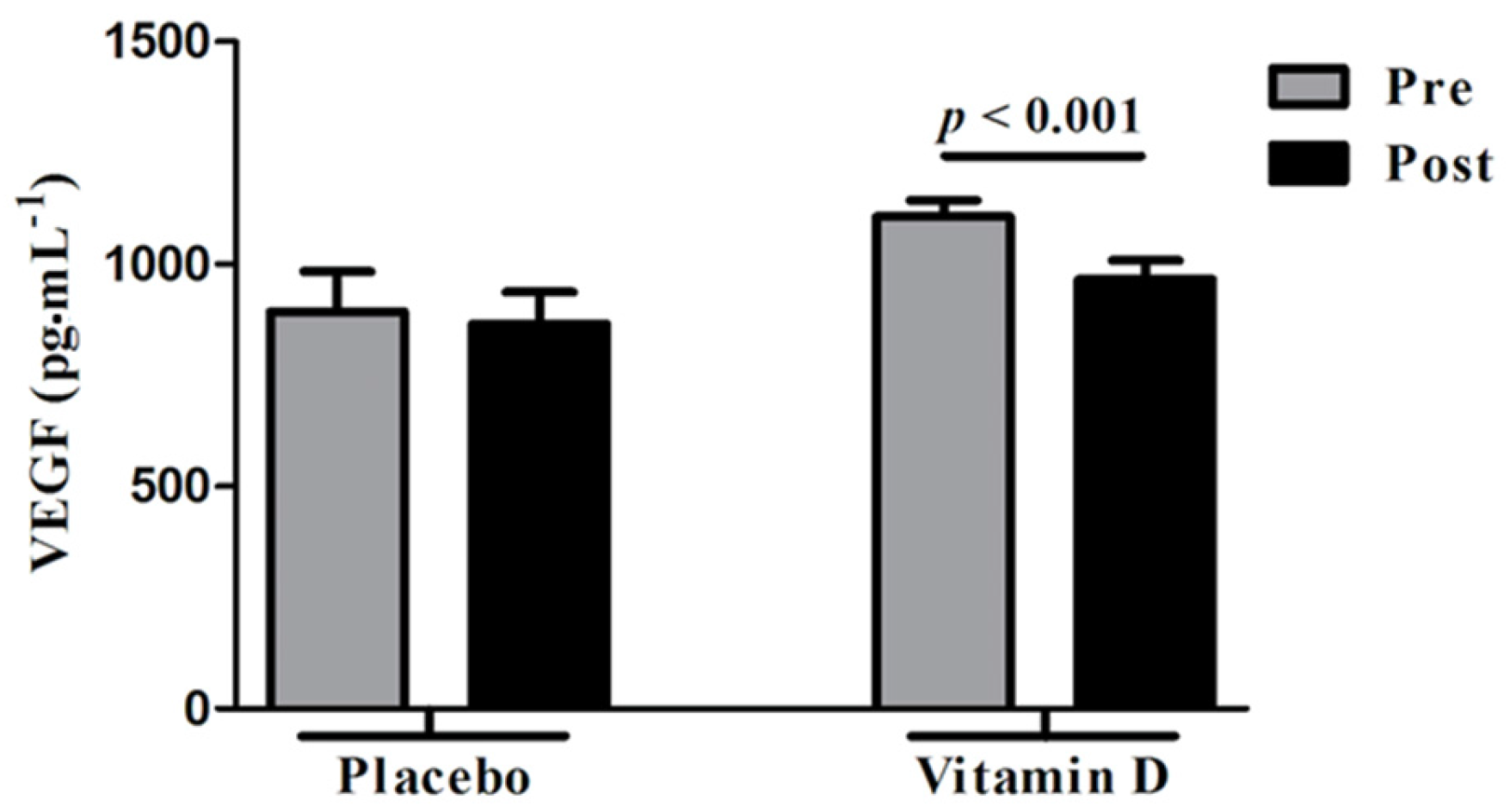
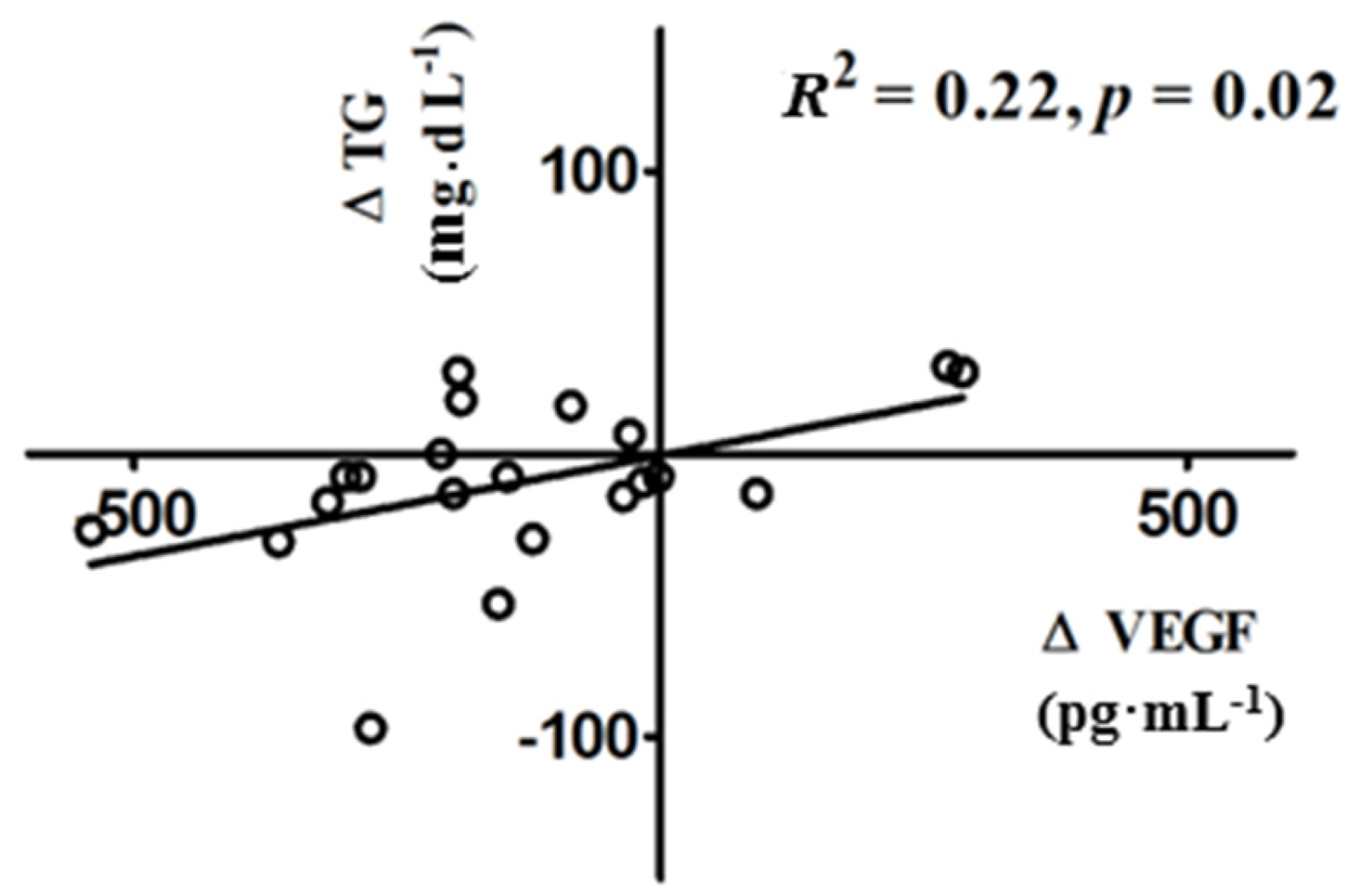
3.3. Changes in VEGF Following Vitamin D Supplementation
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bozdag, G.; Mumusoglu, S.; Zengin, D.; Karabulut, E.; Yildiz, B.O. The prevalence and phenotypic features of polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 2016, 31, 2841–2855. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, M.O.; Dumesic, D.A.; Chazenbalk, G.; Azziz, R. Polycystic ovary syndrome: Etiology, pathogenesis and diagnosis. Nat. Rev. Endocrinol. 2011, 7, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Setji, T.L.; Brown, A.J. Polycystic ovary syndrome: Update on diagnosis and treatment. Am. J. Med. 2014, 127, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Tal, R.; Seifer, D.B.; Arici, A. The emerging role of angiogenic factor dysregulation in the pathogenesis of polycystic ovarian syndrome. Semin. Reprod. Med. 2015, 33, 195–207. [Google Scholar] [PubMed]
- Tischer, E.; Mitchell, R.; Hartman, T.; Silva, M.; Gospodarowicz, D.; Fiddes, J.C.; Abraham, J.A. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J. Biol. Chem. 1991, 266, 11947–11954. [Google Scholar] [PubMed]
- Lei, J.; Jiang, A.; Pei, D. Identification and characterization of a new splicing variant of vascular endothelial growth factor: VEGF183. Biochim. Biophys. Acta 1998, 1443, 400–406. [Google Scholar] [CrossRef]
- Poltorak, Z.; Cohen, T.; Sivan, R.; Kandelis, Y.; Spira, G.; Vlodavsky, I.; Keshet, E.; Neufeld, G. VEGF145, a secreted vascular endothelial growth factor isoform that binds to extracellular matrix. J. Biol. Chem. 1997, 272, 7151–7158. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Davis-Smyth, T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997, 18, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, J.; Campbell, S.; Pittrof, R.; Kyei-Mensah, A.; Shaker, A.; Jacobs, H.S.; Tan, S.L. Ovarian stromal blood flow in women with polycystic ovaries—A possible new marker for diagnosis? Hum. Reprod. 1995, 10, 1992–1996. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.A.; Wu, M.H.; Cheng, Y.C.; Li, C.H.; Chang, F.M. Quantification of Doppler signal in polycystic ovary syndrome using three-dimensional power Doppler ultrasonography: A possible new marker for diagnosis. Hum. Reprod. 2002, 17, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Kamat, B.R.; Brown, L.F.; Manseau, E.J.; Senger, D.R.; Dvorak, H.F. Expression of vascular permeability factor/vascular endothelial growth factor by human granulosa and theca lutein cells. Role in corpus luteum development. Am. J. Pathol. 1995, 146, 157–165. [Google Scholar] [PubMed]
- Agrawal, R.; Sladkevicius, P.; Engmann, L.; Conway, G.S.; Payne, N.N.; Bekis, J.; Tan, S.L.; Campbell, S.; Jacobs, H.S. Serum vascular endothelial growth factor concentrations and ovarian stromal blood flow are increased in women with polycystic ovaries. Hum. Reprod. 1998, 13, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Artini, P.G.; Monti, M.; Matteucci, C.; Valentino, V.; Cristello, F.; Genazzani, A.R. Vascular endothelial growth factor and basic fibroblast growth factor in polycystic ovary syndrome during controlled ovarian hyperstimulation. Gynecol. Endocrinol. 2006, 22, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.R.; Gomez, R.; Simon, C.; Garcia-Velasco, J.A.; Pellicer, A. Targeting the vascular endothelial growth factor system to prevent ovarian hyperstimulation syndrome. Hum. Reprod. Update 2008, 14, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Li, H.W.; Brereton, R.E.; Anderson, R.A.; Wallace, A.M.; Ho, C.K. Vitamin D deficiency is common and associated with metabolic risk factors in patients with polycystic ovary syndrome. Metabolism 2011, 60, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.; Haselhorst, U.; Tan, S.; Quadbeck, B.; Schmidt, M.; Roesler, S.; Janssen, O.E. Low serum 25-hydroxyvitamin D concentrations are associated with insulin resistance and obesity in women with polycystic ovary syndrome. Exp. Clin. Endocrinol. Diabetes 2006, 114, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Pal, L.; Berry, A.; Coraluzzi, L.; Kustan, E.; Danton, C.; Shaw, J.; Taylor, H. Therapeutic implications of vitamin D and calcium in overweight women with polycystic ovary syndrome. Gynecol. Endocrinol. 2012, 28, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Selimoglu, H.; Duran, C.; Kiyici, S.; Ersoy, C.; Guclu, M.; Ozkaya, G.; Tuncel, E.; Erturk, E.; Imamoglu, S. The effect of vitamin D replacement therapy on insulin resistance and androgen levels in women with polycystic ovary syndrome. J. Endocrinol. Investig. 2010, 33, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Lv, C.; Yuan, Q.; Wang, Q. Levels of serum 25(OH)VD3, HIF-1, VEGF, vWf, and IGF-1 and their correlation in type 2 diabetes patients with different urine albumin creatinine ratio. J. Diabetes Res. 2016, 2016, 1925424. [Google Scholar] [CrossRef] [PubMed]
- Simo, R.; Sundstrom, J.M.; Antonetti, D.A. Ocular Anti-VEGF therapy for diabetic retinopathy: The role of VEGF in the pathogenesis of diabetic retinopathy. Diabetes Care 2014, 37, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shoshan, M.; Amir, S.; Dang, D.T.; Dang, L.H.; Weisman, Y.; Mabjeesh, N.J. 1,25-dihydroxyvitamin D3 (Calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Mol. Cancer Ther. 2007, 6, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Gruber, H.E.; Hoelscher, G.; Ingram, J.A.; Chow, Y.; Loeffler, B.; Hanley, E.N., Jr. 1,25(OH)2-vitamin D3 inhibits proliferation and decreases production of monocyte chemoattractant protein-1, thrombopoietin, VEGF, and angiogenin by human annulus cells in vitro. Spine 2008, 33, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Irani, M.; Seifer, D.B.; Grazi, R.V.; Julka, N.; Bhatt, D.; Kalgi, B.; Irani, S.; Tal, O.; Lambert-Messerlian, G.; Tal, R. Vitamin D supplementation decreases TGF-β1 bioavailability in PCOS: A randomized placebo-controlled trial. J. Clin. Endocrinol. Metab. 2015, 100, 4307–4314. [Google Scholar] [CrossRef] [PubMed]
- Rotterdam, E. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, B.; Guler, T.; Akbulut, M.; Oztekin, O.; Sariiz, G. 1-α, 25-dihydroxyvitamin D3 regresses endometriotic implants in rats by inhibiting neovascularization and altering regulation of matrix metalloproteinase. Postgrad. Med. 2014, 126, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Li, W.; Zhao, Q.; Ma, L.; Zhu, J. The impact of 1,25-dihydroxy vitamin D3 on the expressions of vascular endothelial growth factor and transforming growth factor-beta(1) in the retinas of rats with diabetes. Diabetes Res. Clin. Pract. 2012, 98, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Tulandi, T.; Saleh, A.; Morris, D.; Jacobs, H.S.; Payne, N.N.; Tan, S.L. Effects of laparoscopic ovarian drilling on serum vascular endothelial growth factor and on insulin responses to the oral glucose tolerance test in women with polycystic ovary syndrome. Fertil. Steril. 2000, 74, 585–588. [Google Scholar] [CrossRef]
- Tal, R.; Seifer, D.B.; Grazi, R.V.; Malter, H.E. Follicular fluid placental growth factor is increased in polycystic ovarian syndrome: Correlation with ovarian stimulation. Reprod. Biol. Endocrinol. 2014, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Artini, P.G.; Ruggiero, M.; Parisen Toldin, M.R.; Monteleone, P.; Monti, M.; Cela, V.; Genazzani, A.R. Vascular endothelial growth factor and its soluble receptor in patients with polycystic ovary syndrome undergoing IVF. Hum. Fertil. 2009, 12, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Frantz, G.; LeCouter, J.; Dillard-Telm, L.; Pham, T.; Draksharapu, A.; Giordano, T.; Peale, F. Differential expression of the angiogenic factor genes vascular endothelial growth factor (VEGF) and endocrine gland-derived VEGF in normal and polycystic human ovaries. Am. J. Pathol. 2003, 162, 1881–1893. [Google Scholar] [CrossRef]
- Irani, M.; Merhi, Z. Role of vitamin D in ovarian physiology and its implication in reproduction: A systematic review. Fertil. Steril. 2014, 102, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.F.; Aal, D.E.; Darwish, A.M.; Meki, A.R. Evaluation of the impact of laparoscopic ovarian drilling on Doppler indices of ovarian stromal blood flow, serum vascular endothelial growth factor, and insulin-like growth factor-1 in women with polycystic ovary syndrome. Fertil. Steril. 2003, 79, 938–941. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irani, M.; Seifer, D.B.; Grazi, R.V.; Irani, S.; Rosenwaks, Z.; Tal, R. Vitamin D Decreases Serum VEGF Correlating with Clinical Improvement in Vitamin D-Deficient Women with PCOS: A Randomized Placebo-Controlled Trial. Nutrients 2017, 9, 334. https://doi.org/10.3390/nu9040334
Irani M, Seifer DB, Grazi RV, Irani S, Rosenwaks Z, Tal R. Vitamin D Decreases Serum VEGF Correlating with Clinical Improvement in Vitamin D-Deficient Women with PCOS: A Randomized Placebo-Controlled Trial. Nutrients. 2017; 9(4):334. https://doi.org/10.3390/nu9040334
Chicago/Turabian StyleIrani, Mohamad, David B. Seifer, Richard V. Grazi, Sara Irani, Zev Rosenwaks, and Reshef Tal. 2017. "Vitamin D Decreases Serum VEGF Correlating with Clinical Improvement in Vitamin D-Deficient Women with PCOS: A Randomized Placebo-Controlled Trial" Nutrients 9, no. 4: 334. https://doi.org/10.3390/nu9040334
APA StyleIrani, M., Seifer, D. B., Grazi, R. V., Irani, S., Rosenwaks, Z., & Tal, R. (2017). Vitamin D Decreases Serum VEGF Correlating with Clinical Improvement in Vitamin D-Deficient Women with PCOS: A Randomized Placebo-Controlled Trial. Nutrients, 9(4), 334. https://doi.org/10.3390/nu9040334




