The Impact of Shiftwork on Skeletal Muscle Health
Abstract
:1. Introduction
1.1. Shiftwork
1.2. Skeletal Muscle Health
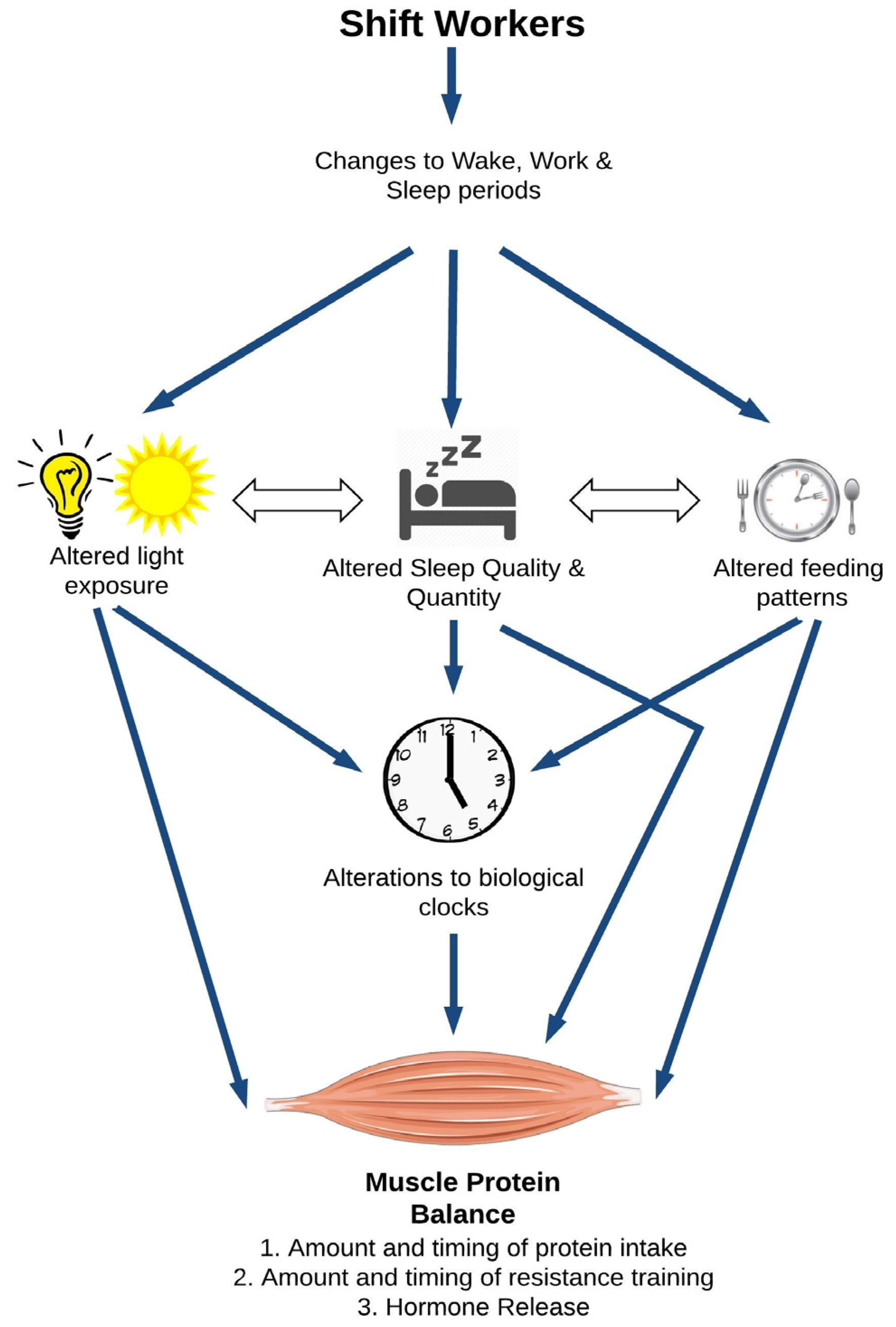
2. A Model for Shiftwork and Skeletal Muscle Health
2.1. Direct Effects of Circadian Disruption on Skeletal Muscle Tissue
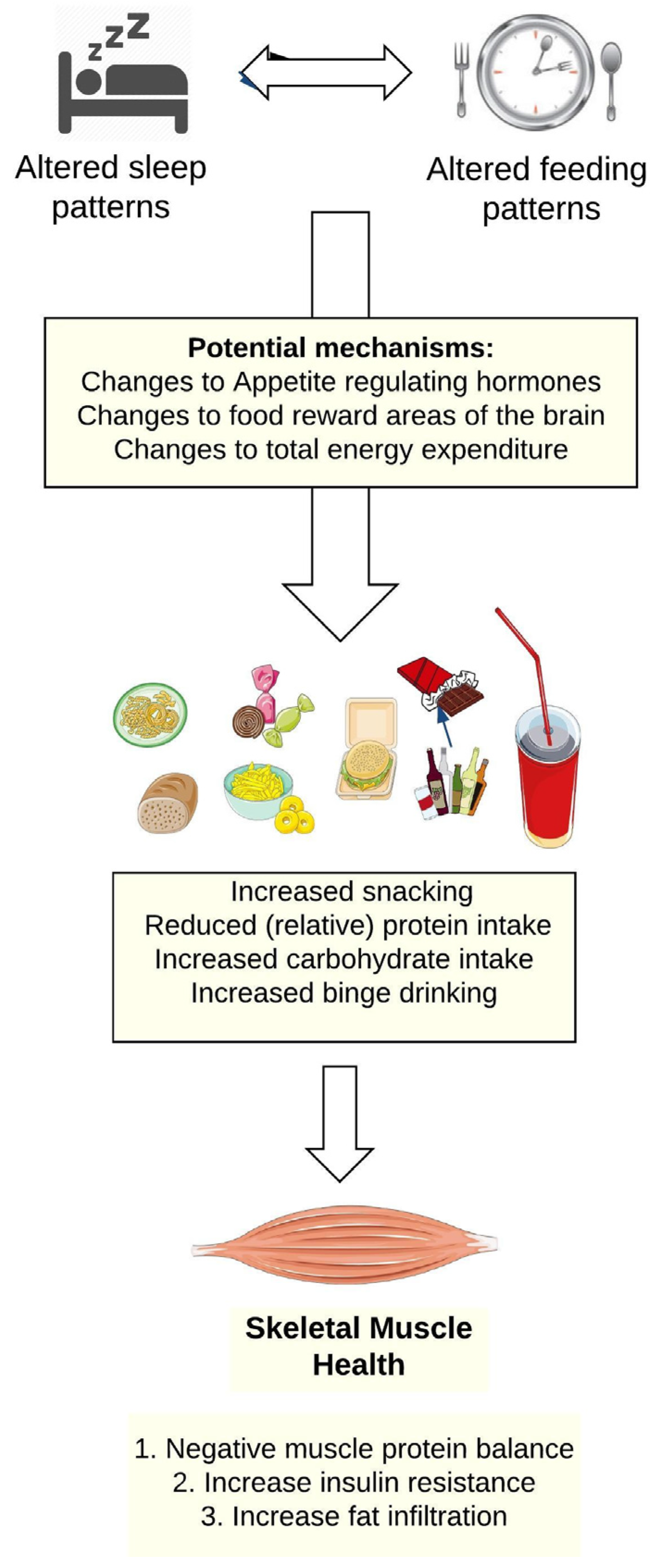
2.2. Shiftwork, Sleep Disruption, and Food and Beverage Choices
2.3. Shiftwork, Sleep Disruption, and Hormonal Changes
2.3.1. Testosterone
2.3.2. Insulin and Insulin-Like Growth Factor 1 (IGF-1)
2.3.3. Cortisol
3. Potential Countermeasures to Preserve Skeletal Muscle Health in Shiftworkers
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wright, K.P., Jr.; Bogan, R.K.; Wyatt, J.K. Shift work and the assessment and management of shift work disorder (swd). Sleep Med. Rev. 2013, 17, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Alterman, T.; Luckhaupt, S.E.; Calvert, G.M.; Dahlhamer, J.M.; Ward, B.W. Prevalence rates of work organization characteristics among workers in the U.S.: Data from the 2010 national health interview survey. Am. J. Ind. Med. 2013, 56, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Eurofound. Sixth European Working Conditions Survey. 2015. Available online: http://www.eurofound.europa.eu/surveys/data-visualisation/sixth-europeanworking-conditions-survey-2015 (accessed on 2 November 2016).
- Australian Bureau of Statistics. Australian Labour Market Statistics, October 2010. Available online: www.abs.gov.au (accessed on 2 November 2016).
- Ferguson, S.A.; Paterson, J.L.; Jay, S.M.; Hall, S.J.; Aisbett, B. On-call work: To sleep or not to sleep? It depends. Chronobiol. Int. 2016, 33, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Kecklund, G.; Axelsson, J. Health consequences of shift work and insufficient sleep. BMJ (Clin. Res. Ed.) 2016, 355, i5210. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, A.S.; Sigstad Lie, J.-A. Shift and night work and long working hours—A systematic review of safety implications. Scand. J. Work Environ. Health 2011, 37, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Bambra, C.; Whitehead, M.; Sowden, A.; Akers, J.; Petticrew, M. “A hard day’s night?” The effects of compressed working week interventions on the health and work-life balance of shift workers: A systematic review. J. Epidemiol. Community Health 2008, 62, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Harma, M. Workhours in relation to work stress, recovery and health. Scand. J. Work Environ. Health 2006, 32, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Yang, C.; Tong, X.Y.; Sun, H.L.; Cong, Y.J.; Yin, X.X.; Li, L.Q.; Cao, S.Y.; Dong, X.X.; Gong, Y.H.; et al. Shift work and diabetes mellitus: A meta-analysis of observational studies. Occup. Environ. Med. 2015, 72, U91. [Google Scholar] [CrossRef] [PubMed]
- Van Drongelen, A.; Boot, C.R.L.; Merkus, S.L.; Smid, T.; van der Beek, A.J. The effects of shift work on body weight change—A systematic review of longitudinal studies. Scand. J. Work Environ. Health 2011, 37, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Chen, W.; Wei, F.; Ying, M.; Wei, W.; Xie, X. Night-shift work increases morbidity of breast cancer and all-cause mortality: A meta-analysis of 16 prospective cohort studies. Sleep Med. 2015, 16, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Anothaisintawee, T.; Reutrakul, S.; van Cauter, E.; Thakkinstian, A. Sleep disturbances compared to traditional risk factors for diabetes development: Systematic review and meta-analysis. Sleep Med. Rev. 2015, 30, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Proper, K.I.; van de Langenberg, D.; Rodenburg, W.; Vermeulen, R.C.H.; van der Beek, A.J.; van Steeg, H.; van Kerkhof, L.W.M. The relationship between shift work and metabolic risk factors: A systematic review of longitudinal studies. Am. J. Prev. Med. 2016, 50, e147–e157. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Ralston, S.H.; Rizzoli, R. Emerging impact of skeletal muscle in health and disease. Calcif. Tissue Int. 2015, 96, 181–182. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Rustad, P.I.; Kolnes, A.J.; Lai, Y.C. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front. Physiol. 2011, 2, 112. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, B.B.; Phillips, S.M. Contractile and nutritional regulation of human muscle growth. Exerc. Sport Sci. Rev. 2003, 31, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Wolkow, A.; Ferguson, S.; Aisbett, B.; Main, L. Effects of work-related sleep restriction on acute physiological and psychological stress responses and their interactions: A review among emergency service personnel. Int. J. Occup. Med. Environ. Health 2015, 28, 183–208. [Google Scholar] [CrossRef] [PubMed]
- Vedaa, Ø.; Harris, A.; Bjorvatn, B.; Waage, S.; Sivertsen, B.; Tucker, P.; Pallesen, S. Systematic review of the relationship between quick returns in rotating shift work and health-related outcomes. Ergonomics 2016, 59, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bassel-Duby, R.; Olson, E.N. Signaling pathways in skeletal muscle remodeling. Annu. Rev. Biochem. 2006, 75, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M. Skeletal muscle metabolism during exercise in humans. Clin. Exp. Pharmacol. Physiol. 2000, 27, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.P. Molecular regulation of skeletal muscle mass. Clin. Exp. Pharmacol. Physiol. 2010, 37, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Short, K.R.; Nair, K.S. Muscle protein metabolism and the sarcopenia of aging. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, 119–127. [Google Scholar] [CrossRef]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J.; et al. Akt/mtor pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, A.A.; Tipton, K.D.; Bamman, M.M.; Wolfe, R.R. Resistance exercise maintains skeletal muscle protein synthesis during bed rest. J. Appl. Physiol. 1997, 82, 807–810. [Google Scholar] [PubMed]
- Rommel, C.; Bodine, S.C.; Clarke, B.A.; Rossman, R.; Nunez, L.; Stitt, T.N.; Yancopoulos, G.D.; Glass, D.J. Mediation of igf-1-induced skeletal myotube hypertrophy by pi(3)k/akt/mtor and pi(3)k/akt/gsk3 pathways. Nat. Cell Biol. 2001, 3, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Schroder, E.A.; Harfmann, B.D.; Zhang, X.; Srikuea, R.; England, J.H.; Hodge, B.A.; Wen, Y.; Riley, L.A.; Yu, Q.; Christie, A.; et al. Intrinsic muscle clock is necessary for musculoskeletal health. J. Physiol. 2015, 593, 5387–5404. [Google Scholar] [CrossRef] [PubMed]
- Harfmann, B.D.; Schroder, E.A.; Esser, K.A. Circadian rhythms, the molecular clock, and skeletal muscle. J. Biol. Rhythm. 2015, 30, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Lefta, M.; Wolff, G.; Esser, K.A. Circadian rhythms, the molecular clock, and skeletal muscle. Curr. Top. Dev. Biol. 2011, 96, 231–271. [Google Scholar] [PubMed]
- Miller, B.H.; McDearmon, E.L.; Panda, S.; Hayes, K.R.; Zhang, J.; Andrews, J.L.; Antoch, M.P.; Walker, J.R.; Esser, K.A.; Hogenesch, J.B.; et al. Circadian and clock-controlled regulation of the mouse transcriptome and cell proliferation. Proc. Natl. Acad. Sci. USA 2007, 104, 3342–3347. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.L.; Zhang, X.; McCarthy, J.J.; McDearmon, E.L.; Hornberger, T.A.; Russell, B.; Campbell, K.S.; Arbogast, S.; Reid, M.B.; Walker, J.R.; et al. Clock and bmal1 regulate myod and are necessary for maintenance of skeletal muscle phenotype and function. Proc. Natl. Acad. Sci. USA 2010, 107, 19090–19095. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Ma, K. Circadian clock regulation of skeletal muscle growth and repair. F1000Research 2016, 5, 1549. [Google Scholar] [CrossRef] [PubMed]
- Perrin, L.; Loizides-Mangold, U.; Skarupelova, S.; Pulimeno, P.; Chanon, S.; Robert, M.; Bouzakri, K.; Modoux, C.; Roux-Lombard, P.; Vidal, H.; et al. Human skeletal myotubes display a cell-autonomous circadian clock implicated in basal myokine secretion. Mol. Metab. 2015, 4, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Cedernaes, J.; Osler, M.E.; Voisin, S.; Broman, J.E.; Vogel, H.; Dickson, S.L.; Zierath, J.R.; Schioth, H.B.; Benedict, C. Acute sleep loss induces tissue-specific epigenetic and transcriptional alterations to circadian clock genes in men. J. Clin. Endocrinol. Metab. 2015, 100, E1255–E1261. [Google Scholar] [CrossRef] [PubMed]
- Nindl, B.C.; Leone, C.D.; Tharion, W.J.; Johnson, R.F.; Castellani, J.W.; Patton, J.F.; Montain, S.J. Physical performance responses during 72 h of military operational stress./performances physiques lors de 72 h d’operation militaire. Med. Sci. Sports Exerc. 2002, 34, 1814–1822. [Google Scholar] [CrossRef] [PubMed]
- Nedeltcheva, A.V.; Kilkus, J.M.; Imperial, J.; Schoeller, D.A.; Penev, P.D. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann. Intern. Med. 2010, 153, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Dattilo, M.; Antunes, H.K.; Medeiros, A.; Monico-Neto, M.; Souza Hde, S.; Lee, K.S.; Tufik, S.; de Mello, M.T. Paradoxical sleep deprivation induces muscle atrophy. Muscle Nerve 2012, 45, 431–433. [Google Scholar] [CrossRef] [PubMed]
- De Sa Souza, H.; Antunes, H.K.; Dattilo, M.; Lee, K.S.; Monico-Neto, M.; de Campos Giampa, S.Q.; Phillips, S.M.; Tufik, S.; de Mello, M.T. Leucine supplementation is anti-atrophic during paradoxical sleep deprivation in rats. Amino Acids 2016, 48, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Monico-Neto, M.; Antunes, H.K.; Lee, K.S.; Phillips, S.M.; Giampa, S.Q.; Souza Hde, S.; Dattilo, M.; Medeiros, A.; de Moraes, W.M.; Tufik, S.; et al. Resistance training minimizes catabolic effects induced by sleep deprivation in rats. Appl. Physiol. Nutr. Metab. 2015, 40, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Huang, F.; Wang, P.; Chen, C.; Zhu, G.; Chen, L.; Wu, G. Chronic sleep deprivation alters the myosin heavy chain isoforms in the masseter muscle in rats. Br. J. Oral Maxillofac. Surg. 2015, 53, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, A.; Ji, L.L.; Cirelli, C. Sleep deprivation and cellular responses to oxidative stress. Sleep 2004, 27, 27–35. [Google Scholar] [PubMed]
- Lucassen, E.A.; Coomans, C.P.; van Putten, M.; de Kreij, S.R.; van Genugten, J.H.; Sutorius, R.P.; de Rooij, K.E.; van der Velde, M.; Verhoeve, S.L.; Smit, J.W.; et al. Environmental 24-hr cycles are essential for health. Curr. Biol. 2016, 26, 1843–1853. [Google Scholar] [CrossRef] [PubMed]
- Motil, K.J.; Matthews, D.E.; Bier, D.M.; Burke, J.F.; Munro, H.N.; Young, V.R. Whole-body leucine and lysine metabolism: Response to dietary protein intake in young men. Am. J. Physiol. Endocrinol. Metab. 1981, 240, E712–E721. [Google Scholar]
- Paddon-Jones, D.; Rasmussen, B.B. Dietary protein recommendations and the prevention of sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Symons, T.B.; Sheffield-Moore, M.; Wolfe, R.R.; Paddon-Jones, D. Moderating the portion size of a protein-rich meal improves anabolic efficiency in young and elderly. J. Am. Diet. Assoc. 2009, 109, 1582–1586. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Robinson, M.J.; Fry, J.L.; Tang, J.E.; Glover, E.I.; Wilkinson, S.B.; Prior, T.; Tarnopolsky, M.A.; Phillips, S.M. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. 2008, 89, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Børsheim, E.; Tipton, K.D.; Wolf, S.E.; Wolfe, R.R. Essential amino acids and muscle protein recovery from resistance exercise. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E648–E657. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, F.; Liu, H.; Li, Y.; Liu, Y.; Kong, X.; Zhang, Y.; Deng, D.; Tang, Y.; Feng, Z.; et al. Nutritional and regulatory roles of leucine in muscle growth and fat reduction. Front. Biosci. (Landmark Ed.) 2015, 20, 796–813. [Google Scholar] [PubMed]
- Tipton, K.; Elliott, T.; Cree, M.; Wolf, S.E.; Sanford, A.; Wolfe, R.R. Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Med. Sci. Sports Exerc. 2004, 36, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Arne, L.; Moreno, C.; Holmbäck, U.; Lennernäs, M.; Tucker, P. Eating and shift work—Effects on habits, metabolism and performance. Scand. J. Work Environ. Health 2010, 36, 150–162. [Google Scholar]
- Nedeltcheva, A.V.; Kessler, L.; Imperial, J.; Penev, P.D. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J. Clin. Endocrinol. Metab. 2009, 94, 3242–3250. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Tasali, E.; Penev, P.; Van Cauter, E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 2004, 141, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Beebe, D.W.; Simon, S.; Summer, S.; Hemmer, S.; Strotman, D.; Dolan, L.M. Dietary intake following experimentally restricted sleep in adolescents. Sleep 2013, 36, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Spaeth, A.M.; Dinges, D.F.; Goel, N. Sex and race differences in caloric intake during sleep restriction in healthy adults. Am. J. Clin. Nutr. 2014, 100, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Markwald, R.R.; Melanson, E.L.; Smith, M.R.; Higgins, J.; Perreault, L.; Eckel, R.H.; Wright, K.P. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc. Natl. Acad. Sci. USA 2013, 110, 5695–5700. [Google Scholar] [CrossRef] [PubMed]
- Hogenkamp, P.S.; Nilsson, E.; Nilsson, V.C.; Chapman, C.D.; Vogel, H.; Lundberg, L.S.; Zarei, S.; Cedernaes, J.; Rångtell, F.H.; Broman, J.-E.; et al. Acute sleep deprivation increases portion size and affects food choice in young men. Psychoneuroendocrinology 2013, 38, 1668–1674. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.; Nilsson, E.; Nilsson, V.; Cedernaes, J.; Rångtell, F.; Vogel, H.; Dickson, S.; Broman, J.; Hogenkamp, P.; Schiöth, H.; et al. Acute sleep deprivation increases food purchasing in men. Obesity (Silver Spring, Md.) 2013, 21, E555–E560. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Wolfe, S.; Sy, M.; Shechter, A.; Hirsch, J. Sleep restriction increases the neuronal response to unhealthy food in normal-weight individuals. Int. J. Obes. (2005) 2014, 38, 411–416. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.-P.; McReynolds, A.; Trivedi, Z.B.; Roberts, A.L.; Sy, M.; Hirsch, J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am. J. Clin. Nutr. 2012, 95, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Gangwisch, J.E. Epidemiological evidence for the links between sleep, circadian rhythms and metabolism. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2009, 10, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Crispim, C.A.; Waterhouse, J.; Dâmaso, A.R.; Zimberg, I.Z.; Padilha, H.G.; Oyama, L.M.; Tufik, S.; de Mello, M.T. Hormonal appetite control is altered by shift work: A preliminary study. Metabolism 2011, 60, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, W.M.A.; Hublin, C.; Sallinen, M.; Härmä, M.; Hirvonen, A.; Porkka-Heiskanen, T. Prolonged sleep restriction affects glucose metabolism in healthy young men. Int. J. Endocrinol. 2010, 2010, 7. [Google Scholar] [CrossRef] [PubMed]
- Dorrian, J.; Heath, G.; Sargent, C.; Banks, S.; Coates, A. Alcohol use in shiftworkers. Accid. Anal. Prev. 2017, 99, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Dorrian, J.; Skinner, N. Alcohol consumption patterns of shiftworkers compared with dayworkers. Chronobiol. Int. 2012, 29, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, M.; Jokela, M.; Nyberg, S.T.; Madsen, I.E.H.; Lallukka, T.; Ahola, K.; Alfredsson, L.; Batty, G.D.; Bjorner, J.B.; Borritz, M.; et al. Long working hours and alcohol use: Systematic review and meta-analysis of published studies and unpublished individual participant data. BMJ (Clin. Res. Ed.) 2015, 350, g7772. [Google Scholar] [CrossRef] [PubMed]
- Parr, E.B.; Camera, D.M.; Areta, J.L.; Burke, L.M.; Phillips, S.M.; Hawley, J.A.; Coffey, V.G. Alcohol ingestion impairs maximal post-exercise rates of myofibrillar protein synthesis following a single bout of concurrent training. PLoS ONE 2014, 9, e88384. [Google Scholar] [CrossRef] [PubMed]
- Smiles, W.J.; Parr, E.B.; Coffey, V.G.; Lacham-Kaplan, O.; Hawley, J.A.; Camera, D.M. Protein coingestion with alcohol following strenuous exercise attenuates alcohol-induced intramyocellular apoptosis and inhibition of autophagy. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E836–E849. [Google Scholar] [CrossRef] [PubMed]
- Urban, R.J.; Bodenburg, Y.H.; Gilkison, C.; Foxworth, J.; Coggan, A.R.; Wolfe, R.R.; Ferrando, A. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am. J. Physiol. Endocrinol. Metab. 1995, 269, E820–E826. [Google Scholar]
- Zhao, W.; Pan, J.; Wang, X.; Wu, Y.; Bauman, W.A.; Cardozo, C.P. Expression of the muscle atrophy factor muscle atrophy f-box is suppressed by testosterone. Endocrinology 2008, 149, 5449–5460. [Google Scholar] [CrossRef] [PubMed]
- Wittert, G. The relationship between sleep disorders and testosterone in men. Asian J. Androl. 2014, 16, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Touitou, Y.; Motohashi, Y.; Reinberg, A.; Touitou, C.; Bourdeleau, P.; Bogdan, A.; Auzéby, A. Effect of shift work on the night-time secretory patterns of melatonin, prolactin, cortisol and testosterone. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 60, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Chatterton, R.J.; Dooley, S. Reversal of diurnal cortisol rhythm and suppression of plasma testosterone in obstetric residents on cal. J. Soc. Gynecol. Investig. 1999, 61, 50–54. [Google Scholar] [CrossRef]
- Jensen, M.A.; Hansen, A.M.; Kristiansen, J.; Nabe-Nielsen, K.; Garde, A.H. Changes in the diurnal rhythms of cortisol, melatonin, and testosterone after 2, 4, and 7 consecutive night shifts in male police officers. Chronobiol. Int. 2016, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Papantoniou, K.; Pozo, O.J.; Espinosa, A.; Marcos, J.; Castano-Vinyals, G.; Basagana, X.; Juanola Pages, E.; Mirabent, J.; Martin, J.; Such Faro, P.; et al. Increased and mistimed sex hormone production in night shift workers. Cancer Epidemiol. Biomark. Prev. 2015, 24, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Luboshitzky, R.; Zabari, Z.; Shen-Orr, Z.; Herer, P.; Lavie, P. Disruption of the nocturnal testosterone rhythm by sleep fragmentation in normal men. J. Clin. Endocrinol. Metab. 2001, 86, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.C.; Dorrian, J.; Liu, P.Y.; Van Dongen, H.P.A.; Wittert, G.A.; Harmer, L.J.; Banks, S. Impact of five nights of sleep restriction on glucose metabolism, leptin and testosterone in young adult men. PLoS ONE 2012, 7, e41218. [Google Scholar] [CrossRef] [PubMed]
- Leproult, R.; van Cauter, E. Effect of 1 week of sleep restriction on testosterone levels in young healthy menfree. JAMA 2011, 305, 2173–2174. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, J.; Ingre, M.; Akerstedt, T.; Holmbäck, U. Effects of acutely displaced sleep on testosterone. J. Clin. Endocrinol. Metab. 2005, 90, 4530–4535. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, G.; Mitrou, P.; Lambadiari, V.; Maratou, E.; Raptis, S.A. Insulin effects in muscle and adipose tissue. Diabetes Res. Clin. Pract. 2011, 93, S52–S59. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; de Fronzo, R.A. Pathogenesis of insulin resistance in skeletal muscle. J. Biomed. Biotechnol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.I.; Greene, N.P.; Dobson, J.P.; Wiggs, M.P.; Gasier, H.G.; Macias, B.R.; Shimkus, K.L.; Fluckey, J.D. Insulin resistance syndrome blunts the mitochondrial anabolic response following resistance exercise. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E466–E474. [Google Scholar] [CrossRef] [PubMed]
- Buxton, O.M.; Pavlova, M.; Reid, E.W.; Wang, W.; Simonson, D.C.; Adler, G.K. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes 2010, 59, 2126–2133. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.N.; Neylan, T.C.; Grunfeld, C.; Mulligan, K.; Schambelan, M.; Schwarz, J.-M. Subchronic sleep restriction causes tissue-specific insulin resistance. J. Clin. Endocrinol. Metab. 2015, 100, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Dattilo, M.; Antunes, H.K.M.; Medeiros, A.; Mônico Neto, M.; Souza, H.S.; Tufik, S.; de Mello, M.T. Sleep and muscle recovery: Endocrinological and molecular basis for a new and promising hypothesis. Med. Hypotheses 2011, 77, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Chennaoui, M.; Drogou, C.; Sauvet, F.; Gomez-Merino, D.; Scofield, D.E.; Nindl, B.C. Effect of acute sleep deprivation and recovery on insulin-like growth factor-i responses and inflammatory gene expression in healthy men. Eur. Cytokine Netw. 2014, 25, 52–57. [Google Scholar] [PubMed]
- Hayes, L.D.; Bickerstaff, G.F.; Baker, J.S. Interactions of cortisol, testosterone, and resistance training: Influence of circadian rhythms. Chronobiol. Int. 2010, 27, 675–705. [Google Scholar] [CrossRef] [PubMed]
- Hennig, J.; Kieferdorf, P.; Moritz, C.; Huwe, S.; Netter, P. Changes in cortisol secretion during shiftwork: Implications for tolerance to shiftwork? Ergonomics 1998, 41, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Charles, L.E.; Fekedulegn, D.; Burchfiel, C.M.; Hartley, T.A.; Andrew, M.E.; Violanti, J.M.; Miller, D.B. Shiftwork and diurnal salivary cortisol patterns among police officers. J. Occup. Environ. Med. 2016, 58, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Lac, G.; Chamoux, A. Biological and psychological responses to two rapid shiftwork schedules. Ergonomics 2004, 47, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.; Burch, J.; Violanti, J.; Burchfiel, C.; Fekedulegn, D.; Andrew, M.; Zhang, H.; Miller, D.B.; Héébert, J.R.; Vena, J.E. Shiftwork duration and the awakening cortisol response among police officers. Chronobiol. Int. J. Biol. Med. Rhythm Res. 2011, 28, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Golden, S.H.; Wand, G.S.; Malhotra, S.; Kamel, I.; Horton, K. Reliability of hypothalamic-pituitary-adrenal axis assessment methods for use in population-based studies. Eur. J. Epidemiol. 2011, 26, 525. [Google Scholar] [CrossRef] [PubMed]
- Bedrosian, T.A.; Galan, A.; Vaughn, C.A.; Weil, Z.M.; Nelson, R.J. Light at night alters daily patterns of cortisol and clock proteins in female siberian hamsters. J. Neuroendocrinol. 2013, 25, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Piovezan, R.D.; Abucham, J.; Dos Santos, R.V.T.; Mello, M.T.; Tufik, S.; Poyares, D. The impact of sleep on age-related sarcopenia: Possible connections and clinical implications. Ageing Res. Rev. 2015, 23, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Res, P.T.; Groen, B.; Pennings, B.; Beelen, M.; Wallis, G.A.; Gijsen, A.P.; Senden, J.M.; van Loon, L.J. Protein ingestion before sleep improves postexercise overnight recovery. Med. Sci. Sports Exerc. 2012, 44, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Atherton, P.J.; Selby, A.; Rankin, D.; Williams, J.; Smith, K.; Hiscock, N.; Rennie, M.J. Muscle protein synthetic responses to exercise: Effects of age, volume, and intensity. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 67, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Reilly, T.; Piercy, M. The effect of partial sleep deprivation on weight-lifting performance. Ergonomics 1994, 37, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.L.; Anderson-Hanley, C.; Arciero, P.J. Virtual and live social facilitation while exergaming: Competiveness moderates exercise intensity. J. Sport Exerc. Psychol. 2012, 34, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Anderson-Hanley, C.; Snyder, A.L.; Nimon, J.P.; Arciero, P.J. Social facilitation in virtual reality-enhanced exercise: Competitiveness moderates exercise effort of older adults. Clin. Interv. Aging 2011, 6, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Aisbett, B.; Wolkow, A.; Sprajcer, M.; Ferguson, S.A. “Awake, smoky, and hot”: Providing an evidence-base for managing the risks associated with occupational stressors encountered by wildland firefighters. Appl. Ergon. 2012, 43, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Blumert, P.A.; Crum, A.J.; Ernsting, M.; Volek, J.S.; Hollander, D.B.; Haff, E.E.; Haff, G.G. The acute effects of twenty-four hours of sleep loss on the performance of national-caliber male collegiate weightlifters. J. Strength Cond. Res. (Allen Press Publ. Serv. Inc.) 2007, 21, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Vincent, G.; Ferguson, S.A.; Tran, J.; Larsen, B.; Wolkow, A.; Aisbett, B. Sleep restriction during simulated wildfire suppression: Effect on physical task performance. PLoS ONE 2015, 10, e0115329. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aisbett, B.; Condo, D.; Zacharewicz, E.; Lamon, S. The Impact of Shiftwork on Skeletal Muscle Health. Nutrients 2017, 9, 248. https://doi.org/10.3390/nu9030248
Aisbett B, Condo D, Zacharewicz E, Lamon S. The Impact of Shiftwork on Skeletal Muscle Health. Nutrients. 2017; 9(3):248. https://doi.org/10.3390/nu9030248
Chicago/Turabian StyleAisbett, Brad, Dominique Condo, Evelyn Zacharewicz, and Séverine Lamon. 2017. "The Impact of Shiftwork on Skeletal Muscle Health" Nutrients 9, no. 3: 248. https://doi.org/10.3390/nu9030248
APA StyleAisbett, B., Condo, D., Zacharewicz, E., & Lamon, S. (2017). The Impact of Shiftwork on Skeletal Muscle Health. Nutrients, 9(3), 248. https://doi.org/10.3390/nu9030248





